
Journal of Clinical and Cellular Immunology
Open Access
ISSN: 2155-9899

ISSN: 2155-9899
Research - (2020)Volume 11, Issue 3
Dysbiosis in the gut microbiota has been observed in a various autoimmune disease, including SLE, which could cause a leaky gut, triggering an immune response, and thus worsening autoimmune disease expression. In our current studies, we hypothesized that increasing dietary fiber would create a healthy microbiota environment in the gut, leading to decreased leakiness of the gut and to decreased disease expression in and NZB/NZW female lupus-like mice. NZB/NZW mice were placed on standardized high fiber (HF 30%) or low fiber (LF 0.4%) for 12 weeks beginning at 20 weeks of age. Mice were assessed as they aged for various parameters of disease including proteinuria and anti-dsDNA antibody production. Alteration of the microbiota and short chain fatty acid (SCFA) levels were also assessed. At 36 weeks-of-age, the mice were euthanized, and we assessed occlusion protein expression, splenocyte profiles, and kidney tissue. We found as the mice aged, their body weights, anti-dsDNA antibody levels, and proteinuria were not significantly different between the groups. Similarly, there were no significant differences in SCFA levels. Regarding the microbiota, Chlostridiales bacteria were consistently increased in the HF treated mice compared to the LF treated mice. Furthermore, as the mice aged, disease progression as assessed by - spleen weight, immune cell profiles, proteinuria, dsDNA levels, and kidney pathology, was not different between the HF and LF treated groups. Taken together, these results indicate that in the NZB/W female lupus mouse model, a HF diet may alter the microbiota but does not influence disease progression.
Lupus nephritis (LN); Systemic lupus erythematosus (SLE); Fiber Diet therapy; Occlusion Proteins; Short Chain Fatty Acids (SCFAs); B-cell, Germinal center (GC)
Systemic lupus erythematosus (SLE) is a chronic autoimmune disease characterized by inflammation in various organs of the body, presence of anti-double stranded DNA (dsDNA) in the blood, IgG and C3 deposition in the kidneys resulting in – glomerulonephritis and unless therapeutic intervention is initiated may lead to end stage renal disease. Circulating autoantigens in patients with lupus contain more IgG associated with autoantibody production and complement activation [1,2]. In lupus patients, C3 and IgG deposition in the kidney is often observed and signals glomerular deterioration [3]. Germinal centers are specialized structures in the secondary lymphoid organs, such as the spleen, which generate high-affinity antibodies required for the humoral immune response [4]. In lupus, autoantibodies are thought to be formed in germinal centers. Treatment for SLE is limited to immunosuppressants, which have been shown to have severe negative side effects.
Several recent studies, including our reports, have suggested that the gut microbiota may help maintain tissue homeostasis in the body and decrease a leaky gut which may trigger immune dysfunction [5-8]. We have shown symbiotic gut bacteria reduces disease severity in MRL/lpr mice [9]. In SLE, a lower abundance of these symbiotes has been described, specifically, lower Firmicutes/Bacteroidetes ratio [10,11]. It has also been shown that lupus mice display decreased mucosal barrier function causing the leakage of proinflammatory cytokines which can cause distant inflammation as well as increased serum dsDNA [12]. Symbiotic microorganisms can enhance the intestinal barrier function, however, pointed strategies to increase the populations of these microorganisms remain limited [13].
Epigenetic factors such as diet has been shown to have a profound impact on SLE expression. Diets such as those rich in Vitamin D, Vitamin C, Vitamin E, Vitamin A, and/or omega-3 polyunsaturated fatty acids have been shown to reduce the onset or prevalence of lupus flares [14-17]. High fat diets have been shown to accelerate renal injury and chronic inflammation as well as decreased endothelial function [18]. One particular area of interest, which has been studied very little up to this point, is the effect of dietary fiber intake on SLE. Dietary fiber has many different classifications based on factors such as solubility, viscosity, and fermentability. Many of the fibers consumed in diets don ’ t participate in fecal bulking, but rather are metabolized by the gut microbiota producing metabolites such as SCFAs [19]. Thus, by increasing the fiber intake in NZB/W F1 mice, the energy source for the gut microorganisms will increase, leading to an increase in the gut flora.
We have previously shown that increasing the amount of lactobacillus in the gut significantly increased the mRNA levels of several tight junction proteins. In these present studies we sought to determine if a high fiber diet would increase symbiotic bacteria species and decrease the permeability of the gut leading to decreased disease pathology [20].
Mice
NZBW F1/J (NZB/W female mice; stock number 100008) mice were purchased from The Jackson Laboratory (Bar Harbor, ME). Mice were bred and maintained in a specific-pathogen-free facility according to the requirements of the Institutional Animal Care and Use Committee at Virginia Polytechnic Institute and State University. These strains mimic human SLE in several ways [21,22]. Modified American Institute of Nutrition (AIN) low fiber (0.4% Fiber) and high fiber (30% Fiber) diets were purchased from Dyets, Inc in Bethlehem, Pennsylvania. These diets are comparable; however, the high fiber diet contains slightly fewer calories due to increased cellulose content.
Microbiota sampling and analysis
Fecal samples were collected every 2 weeks and held in -80°C until being processed. The DNA was then extracted from the feces according to the protocol used by Mu et.al [9]. Sample homogenization and cell lysis was aided by mixing 0.1-g portions of samples with 0.1-mm sterile zirconia beads using a bead-beater (BioSpec Mini-Beadbeater-16) for 45 s. DNA was extracted by a phenol-chloroform method [23]. The V4 region (ca. 252 bp) of 16S rRNA gene was PCR amplified with 515F and 12-base GoLay barcoded 806R primers [24].The purified amplicons were sequenced bidirectionally (paired-end 150 bp) on an Illumina MiSeq at Argonne National Laboratory.
Transcript levels of tight junction proteins
Upon sacrificing the mice, the intestines were harvested. Intestinal epithelial cells were homogenized, and total RNA was extracted using an RNeasy Plus Mini Kit (Qiagen) following the manufacturer’s protocol. DNA was removed by digestion with RNase-free DNase I (Qiagen). Reverse transcriptase was performed by using iScript cDNA synthesis Kit (Bio-Rad). qPCR was performed with iTaq Universal SYBR Green Supermix (Bio- Rad) and ABI 7500 fast Real-Time PCR System (Applied Biosystems). Primer sequences for mouse Cldn1, Cldn2, Occludin, ZO1, and IL18 using Villin as the housekeeping gene.
Renal function
Urine was collected every 2 weeks and tested for proteinuria using Uristix4 dipstick. When the mice were euthanized, the kidneys were fixed in formalin for 24 hours, paraffin embedded, sectioned, and stained with periodic acid-Schiff (PAS) at the Histopathology Laboratory at Virginia Maryland Regional College of Veterinary Medicine. Slides were read with an Olympus BX43 microscope. All the slides were scored blindly by a certified veterinary pathologist (Dr. Miranda Vieson). Glomerular lesions were graded on a scale of 0 to 4 as a measure of mesangial cellularity and matrix, thickness of glomerular basement membrane, and endocapillary hypercellularity.
Cell Isolation and flow cytometry
Isolation cells from kidney tissue: We minced kidneys into 1-2mm pieces with a blade and digested kidney tissue with RPMI1640 medium containing collagenase D, Dnase I and HEPES for 30mins in cell culture hood at 37°C. Then, we smashed and passed digested kidney tissue through 70um strainer and collected cell suspension for flow staining.
Cells were blocked by anti-CD16/32 for 10 minutes on ice and then incubated with surface antibody mixtures for 15 minutes on ice in dark. Cells were then washed twice in PBS and preceded with intracellular staining following the instruction of Foxp3 staining kit (Thermofisher, eBioscience). FACS antibodies used are anti-mouse CD3-FITC, CD4-APC, CD8-PE, PD1-BV510, CXCR5-APC-Cy7, CD19-APC, CD138-BV421, GL7-FITC, IgD-PE, CD45-APC-Cy7, CD3-FITC, CD4-PerCp- Cy5.5, CD8-BV510, CD25-BV421, Foxp3-PE, RORyt-APC.
Statistical analysis
For the comparison of two groups, t test was used. Results were considered statistically significant if p < 0.05. All analyses were done using Prism GraphPad software.
Diet composition and body weight
At 20 weeks-of age, NZB/W female mice were place on either a HF or LF diet (Table 1). The diets were similar in most nutrients and vitamins except the HF diet contained 300 g/Kg cellulose while the LF diet contained 4 mg/kg. Additionally, the HF diet contained 147 g/Kg corn starch while the LF diet contained 443.4 g/Kg corn starch. This led to overall calorie content in the HF diet of 2860 kcal/kg while the LF diet had 3925 kcal/kg. With the diet calorie count differences, the HF diet treated mice showed consistently lower body weights than the animals on the LF diet although it was not statistically significant (Figure 1). In both groups the body weight decreased along the same trend throughout the course of the experiment. The mice lost weight as a result of normal disease progression [25].
| Ingredient g/kg | HF (30%) | LF (0.04%) |
|---|---|---|
| Casein | 200 | 200 |
| L-Cystine | 3 | 3 |
| Sucrose | 100 | 100 |
| Cornstarch | 147 | 443.4 |
| Dyetrose | 132 | 132 |
| Soybean Oil | 70 | 70 |
| t-Butylhydroquinone | 0.014 | 0 |
| Cellulose | 300 | 4 |
| Mineral Mix #210025 | 35 | 35 |
| Vitamin Mix #310025 | 10 | 10 |
| Choline Bitartrate | 2.5 | 3 |
| Calories (kcal/kg) | 2860 | 3925 |
Abbrevations: HF: High Fiber; NF: Normal Fiber; LF: Low Fiber
Table 1: Diet Composition as reported by Dyets. Inc.
Proteinuria and glomerular disease
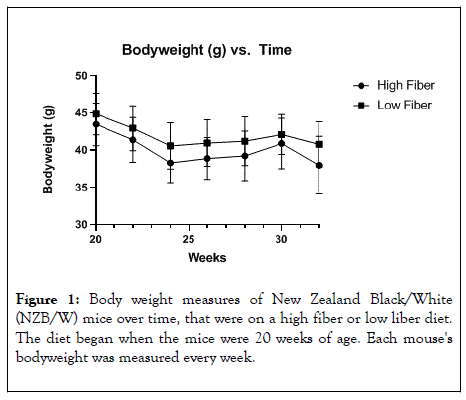
Figure 1: Body weight measures of New Zealand Black/White (NZB/W) mice over time, that were on a high fiber or low liber diet. The diet began when the mice were 20 weeks of age. Each mouse's bodyweight was measured every week.
As a measure for renal disease, we determined the proteinuria as the animals aged (Figure 2). In both the HF and LF treatment groups, proteinuria increased as the animals aged. There was no significant difference in proteinuria between the two groups. At the termination of the experiment, the kidneys were removed, and they were imbedded in parafilm, sectioned, and stained with PAS stain (Figure 3). The kidney sections were scored by a board-certified pathologist blinded to the treated groups. When the scores were tabulated, both the LF and HF treatment groups showed similar pathologic finding of glomerulonephritis that were typical for an NZB/W mouse at 32 weeks-of age.
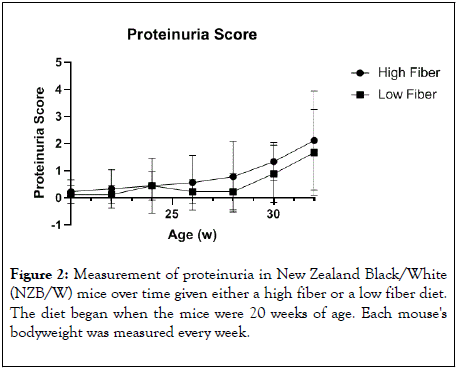
Figure 2: Measurement of proteinuria in New Zealand Black/White (NZB/W) mice over time given either a high fiber or a low fiber diet. The diet began when the mice were 20 weeks of age. Each mouse's bodyweight was measured every week.
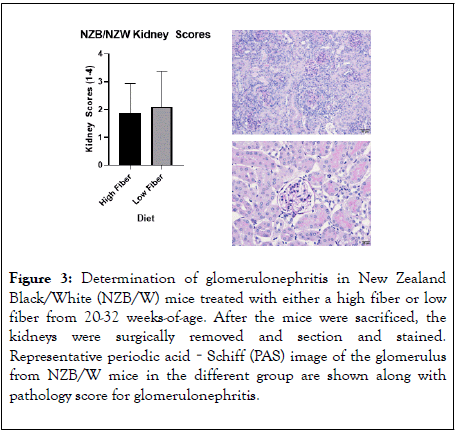
Figure 3: Determination of glomerulonephritis in New Zealand Black/White (NZB/W) mice treated with either a high fiber or low fiber from 20-32 weeks-of-age. After the mice were sacrificed, the kidneys were surgically removed and section and stained. Representative periodic acid‐Schiff (PAS) image of the glomerulus from NZB/W mice in the different group are shown along with pathology score for glomerulonephritis.
IgG and C3 deposits in kidney
After euthanasia, the kidneys were removed and placed in OCT media, sectioned and stained with IgG and C3 antibodies. Both the HF and LF groups showed strong fluorescence and similar levels of IgG and C3 kidney deposition (Figure 4). However, the LF treated group showed noticeably more C3 and IgG glomerular deposition. C3 deposits, from complement dysregulation, in the glomeruli lead to glomerular inflammation [26,27].
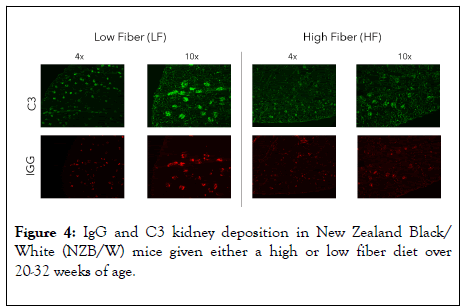
Figure 4: IgG and C3 kidney deposition in New Zealand Black/ White (NZB/W) mice given either a high or low fiber diet over 20-32 weeks of age.
SCFA levels
To assess the level of fiber fermentation occurring in the gut short chain fatty acid composition of the microbiota, fecal pellets were assessed by mass spectrometry at 32 weeks-of age (Figure 5). As measures of SCFA composition, we examined the levels of acetic acid, propionic acid, buryric acid and heptanic acid. Interestingly, propionic acid and butyric acid were significantly increased in the low fiber treatment group. The metabolites butyrate and propionate have been shown to have an effect on the function and differentiation of T-cells [28]. Acetic Acid and Heptanoic acid were also increased in the LF treated group although this was not statistically significant.
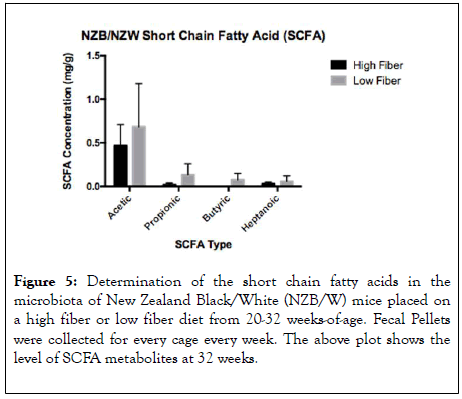
Figure 5: Determination of the short chain fatty acids in the microbiota of New Zealand Black/White (NZB/W) mice placed on a high fiber or low fiber diet from 20-32 weeks-of-age. Fecal Pellets were collected for every cage every week. The above plot shows the level of SCFA metabolites at 32 weeks.
Occlusion protein measures
Occlusion proteins, or tight junction proteins, play an essential role in the function of the intestinal barrier. Claudins make up a large part of the tight junction protein complexes which greatly affect the permeability of the epithelia [29]. Claudin1 (Cldn1) acts to prevent diffusion of small molecules through tight junction proteins [29]. Claudin 2 acts through calciumindependent cell-adhesion activity and is responsible for tight junction obliteration of the intercellular space [29,30]. Occludin regulates paracellular permeability and functions to promote cellular adhesion when tight junctions are lacking [29]. ZO-1, also called tight junction protein 1, limits the transport of substances through the paracellular space [29]. IL-18 is a proinflammatory cytokine which is involved in the cell immune response [29]. The integrity and rigidity of this barrier is crucial for proper function, as a leaky gut can contribute to a severe immune response. In our studies, we showed that the LF group expressed higher levels of all the studied occlusion proteins (Figure 6). There was a statistical difference between groups in Cldn1, Cldn2, and IL18.
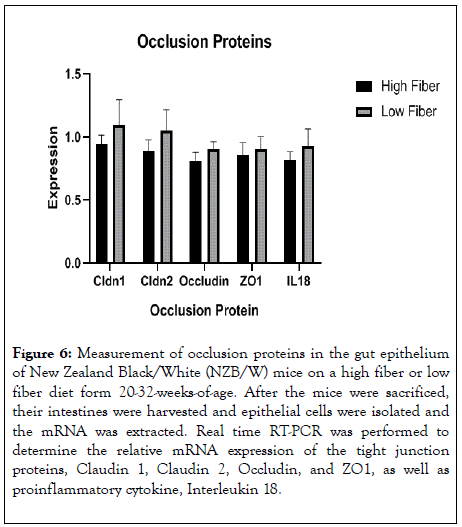
Figure 6: Measurement of occlusion proteins in the gut epithelium of New Zealand Black/White (NZB/W) mice on a high fiber or low fiber diet form 20-32-weeks-of-age. After the mice were sacrificed, their intestines were harvested and epithelial cells were isolated and the mRNA was extracted. Real time RT-PCR was performed to determine the relative mRNA expression of the tight junction proteins, Claudin 1, Claudin 2, Occludin, and ZO1, as well as proinflammatory cytokine, Interleukin 18.
Microbiota abundance
Mice, like humans, cannot digest insoluble fibers such as cellulose. However, some symbiotic bacteria in the colon can thrive on cellulose and produce short-chain fatty acids (SCFAs) including acetate, butyrate and propionate. A recent study showed significant upregulation of Lachnospiraceae and colonic production of propionate in immunodeficient mice fed a high cellulose diet [31], but the effects of dietary cellulose on the gut microbiota of lupus-prone mice had not been reported. Here, we show significant changes of gut microbiota comparing a 30% cellulose (HF) diet to a 0.04% cellulose (LF) diet. The HF diet led to a significantly more diverse gut microbiota (Figure 7A), and the bacterial compositions resulting from different diet formulations separated well on the unweighted Principal Coordinate Analysis (PCoA) plot (Figure 7B). On the class level, Verrucomicrobiae increased over time in both HF and LF diet treatments, with a delayed upregulation in the HF group (Figure 7C). The class Verrucomicrobiae (under the phylum Verrucomicrobia) includes a single bacterium Akkermansia muciniphila, which is a producer of acetate and propionate [32].
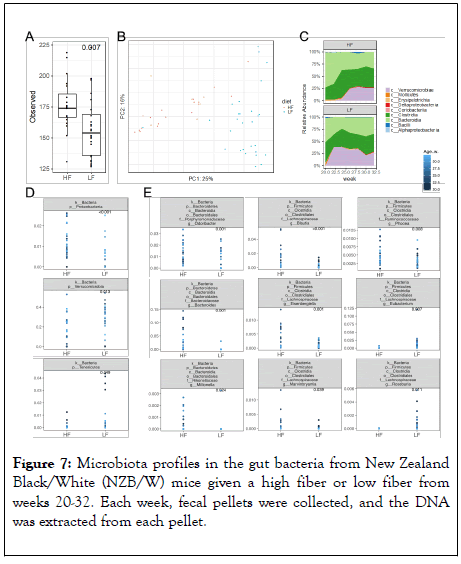
Figure 7: Microbiota profiles in the gut bacteria from New Zealand Black/White (NZB/W) mice given a high fiber or low fiber from weeks 20-32. Each week, fecal pellets were collected, and the DNA was extracted from each pellet.
The significantly lower abundance of Verrucomicrobia with HF diet (Figure 7D) is thus consistent with the moderate decrease of acetate and a significantly reduced level of propionate with this diet (Figure 5). Moreover, the phylum Proteobacteria significantly increased, whereas the phylum Tenericutes significantly decreased, with HF diet compared to LF diet (Figure 7D). Some taxa of Proteobacteria are butyrate producers [33]. Several genera in the family Lachnospiraceae were significantly increased with the high-cellulose diet as reported previously [31], including Blautia, Eisenbergiella, and Marvinbryantia (Figure 7E), which are known acetate producers [34]. The decrease of acetogen A. muciniphila and increases of these Lachnospiraceae taxa with HF diet may have neutralized each other leading to similar levels of fecal acetate between the two diet formulations (Figure 5). Two genera in the family Lachnospiraceae, namely Eubacterium and Roseburia that are butyrate producers [33], significantly decreased with HF diet (Figure 7E), consistent with a significantly reduced level of fecal butyrate with this treatment (Figure 5). Furthermore, several genera were shown to increase with HF diet including Odoribacter (family Porphyromonadaceae), Bacteroides (family Bacteroidaceae), Millionella (family Rikenellaceae), and Phocea (family Ruminococcaceae) (Figure 7E). The family Ruminococcaceae has many butyrate producers [33], but their relative abundance was low (less than 2%). Taken together, these results identified differential regulations of Verrucomicrobia (A. muciniphila; relative abundance of up to 40%) and two taxa in the family Lachnospiraceae (Eubacterium and Roseburia; relative abundance of up to 13%) that may have contributed to the significant differences in the levels of fecal propionate and butyrate, respectively.
Splenic measures and cell profile
Lupus disease is marked by the formation of germinal centers. Increased spleen weight is highly associated with germinal center formation. In our studies, there was no significant difference in spleen weight between HF and LF groups (Figure 8). To assess further the splenic profiles, we preformed flow cytometry (Figure 9). As lymphocytes become activated and differentiate, they can have profound effects on immune function. In our analysis of splenic cell profiles, we found that there was no significant difference in the amount of CD8+ T-cells between the HF and the LF treated mice. Additionally, there was no difference in the number of Foxp3+ CD25+ CD4+ T regulatory cells which are specific for self-antigens and have been show to develop in situations marked by chronic inflammation [35]. There were also no significant differences in CD4+ T cells which drive the T cell mediated immune response resulting in inflammation in the central nervous system [36]. Also, CD4+ Foxp3+ T cells were found in similar quantities between groups. These cells have been found to hinder effective immune responses against cancer cells [37]. There was also no significant difference in CD4+Foxp3+CD25-, a major T regulatory cell in the gut, which have been shown to have suppressive action [38]. Another inflammatory mediator, CD4+RORGT+ T helper cells, were shown to be present in not significantly different between the HF and LF groups [39].
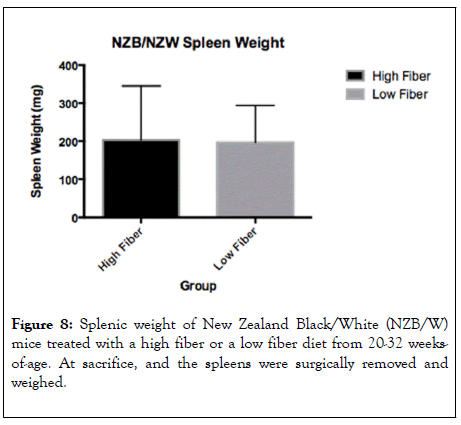
Figure 8: Splenic weight of New Zealand Black/White (NZB/W) mice treated with a high fiber or a low fiber diet from 20-32 weeksof- age. At sacrifice, and the spleens were surgically removed and weighed.
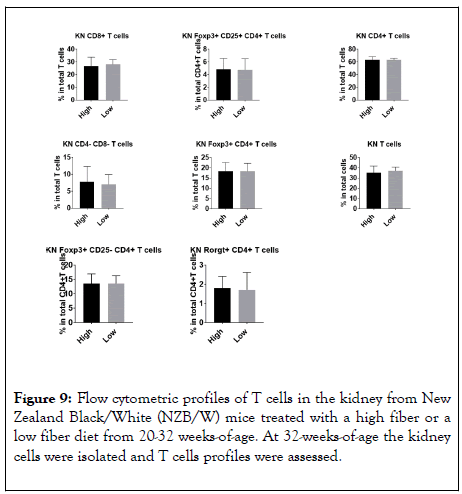
Figure 9: Flow cytometric profiles of T cells in the kidney from New Zealand Black/White (NZB/W) mice treated with a high fiber or a low fiber diet from 20-32 weeks-of-age. At 32-weeks-of-age the kidney cells were isolated and T cells profiles were assessed.
Similarly, there was no significant difference in the quantity of germinal center B cells, T-follicular T-cells, or plasma cells between the two groups (Figure 10).
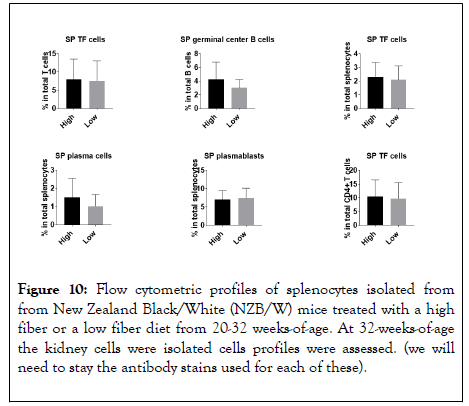
Figure 10: Flow cytometric profiles of splenocytes isolated from from New Zealand Black/White (NZB/W) mice treated with a high fiber or a low fiber diet from 20-32 weeks-of-age. At 32-weeks-of-age the kidney cells were isolated cells profiles were assessed. (we will need to stay the antibody stains used for each of these).
The severity of autoimmune diseases such as Multiple Sclerosis (MS), rheumatoid arthritis (RA), and Systemic Lupus Erythematosus (SLE) have been shown to be affected by various diet composition changes. In these diseases, the immune system is chronically inflamed and dysbiosis in the gut microbiome can lead to inflammation and increased intestinal permeability [40]. Leakage of proinflammatory cytokines can cause autoantibody production and inflammation in sites away from the intestines. In this study, we tested the hypothesis that a high fiber diet would alter the gut microbiota, decrease the gut permeability in the NZB/NZW lupus female mice, and limit disease severity. In autoimmunity deficient uptake of soluble dietary fiber has been shown to either directly cause dysregulation of the immune response or indirectly through changes in the microbiota [41]. Additionally, dietary fiber metabolites butyrate and propionate, which are SCFAs, have been shown to affect the differentiation and function of T-cells [28]. In our investigations, we found that there was a marked difference in gut flora composition between the two groups as a result of dietary fiber intake. The relative mRNA expression of various occlusion proteins and the DNA expression of two strains shown to promote SLE disease attenuation were significantly different between groups. However, common disease markers such as proteinurea levels, germinal center formation, IgG and C3 deposition, and glomerular scores were not statistically different. This is a surprising result because differences in gut microbiota as a result of diet changes has been shown to affect disease expression in NZB/W F1 mice.
One such diet being a calorie restricted diet which has been correlated to changes in disease expression. NZB/W F1 mice restricted to less calories had markedly reduced serum levels of the glycoprotein 70 (gp70) [42]. High serum levels of gp70 have been correlated with increased levels of immune complexes and development of lupus nephritis [43]. Another diet shown to have an effect on autoimmunity is a high fat diet. It has been shown that NZB/W F1 mice given a diet enriched in saturated and unsaturated fats showed reduced natural killer cell activity, reduced mitogenic response to T cell mitogens, and enhanced response to the B cell mitogen LPS [44]. In our studies we found no differences in T cell or B cell profiles. The mitogen LPS induces expansion of B cells and their proliferation and activation of macrophages [45]. Dietary vitamin D supplementation was also correlated with reduced disease expression. Specifically, a randomized 3 month study of dietary vitamin D supplementation showed significantly reduced levels of free serum double stranded DNA and reduced recurrence of arthritis [16]. Another study administered supplemental vitamins C and E which showed decreased lipid peroxidation when compared to the control groups, but unaffected endothelial function [17]. Lipid peroxidation introduces harmful free radicals into circulation and have been shown to be present in significantly increased levels in patients with SLE [46]. Contrary to these studies however, despite dietary differences leading to marked differences in the gut microbiome, the overall expression of disease in NZB/W F1 mice was unchanged.
The mucosal barrier bacteria composition affects both proinflammatory and anti-inflammatory mechanisms both at the intestines and at distant sites [47]. However, our studies show despite altering the bacterial composition of the mucosal barrier, the gut permeability was not affected leading to similar disease expression in both groups. Taken together, we found that we were able to alter the microbiome in NZB/W mice by altering the dietary fiber. This resulted in altered SCFA levels and differences in endothelial tight junction proteins. However, these differences did not correlate with decreased parameters of lupus disease development including, lymphocyte proliferation, immune complex deposition and renal disease. In the NZB/W female mouse, many factors contribute to disease progression, dietary fiber intake may not play a substantial role in renal disease.
Citation: Panther E, Puig XC, Ren J, Liao X, Swartwout B, Vieson M, et al. (2020) The Effect of Dietary Fiber Intake on Systemic Lupus Erythematosus (SLE) Disease in NZB/W Lupus Mice. J Clin Cell Immunol. 11:590. doi: 10.35248/2155-9899.20.11:590.
Received: 22-Apr-2020 Accepted: 06-May-2020 Published: 12-May-2020 , DOI: 10.35248/2155-9899.20.11.590
Copyright: © 2020 Panther E, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.