Immunome Research
Open Access
ISSN: 1745-7580
ISSN: 1745-7580
Review Article - (2021)
Silicone has been widely used over the last two decades, mainly for aesthetic purposes. Growing data show that local free/liquid silicone injections, used as body filler, are possibly related to the onset of adverse effects, with autoimmune and inflammatory features. Several reactions have been described, some of which can be classified as ASIA-Autoimmune/Inflammatory Syndrome Induced by Adjuvants. Clinical presentations range from local inflammatory reactions to full-blown systemic autoimmune diseases. These cases share the similar chronological, clinical and histopathological features, as well as treatment rationale. The link between silicone breast implants and autoimmune disorders are still under debate, but local liquid silicone injections adverse effects are sporadically reported. The aim of this review is to identify common features of autoimmune and inflammatory adverse effects of local liquid silicone injections.
Silicone injection; Body filler injection; Adjuvant; ASIA; Shoenfeld’s syndrome; Autoimmune disease; Inflammatory disease; Granulomatosis, Siliconosis; Free liquid silicone)
ANA: Anti-Nuclear Antibodies; ASIA: Autoimmune/Inflammatory Syndrome Induced by Adjuvants; HA: Hyaluronic Acid; HLA: Human Leukocyte Antigen; SBI: Silicone Breast Implants
Silicone use as a biomaterial for aesthetic purposes dates back as early as the 1940’s, initially for breast implants in Japan [1]. Known and possibly related and reported adverse effects of Silicone Breast Implants (SBI) include autoimmune, inflammatory, rheumatic disorders, and even onco-hematological manifestations; regrouped under the term of “siliconosis” [2–6].
As differs to silicone prosthesis, there are vast types of chemical formulas for “free silicone” that are being used for medical as well as aesthetic fillers. Free liquid silicone is an aesthetic agent for dermal filling (subcutaneous or dermal injection). The first injections were used for breast augmentation on the 1960’s until reports of adverse effects appeared in the United States [1]. In 2009, the American Society of Aesthetic and Plastic Surgery confirmed that more than 10 million patients in the USA alone had had biomaterial filler injections including silicone, along with hyaluronic acid, collagen, polymethylmethacrylate and other dermal fillers [7,8]. Acute adverse effects such as treatment-site reactions (pain, swelling, edema, infection, necrosis) and migration of the injected material (acute pneumonitis, organ failure) are common adverse of liquid silicone effects and have been well described, but late- onset autoimmune/inflammatory adverse effects are starting to be increasingly reported [9].
Silicone has been reported to be able to induce diverse immune reactions despite good techniques and good quality material. Various immulogic and pathogenic hypothesis concerning the silicone induced inflammatory reaction have been presented [10]. In 2011, Shoenfeld and Agmon-Levin coined the term “ASIA – Autoimmune/inflammatory syndrome induced by adjuvants” as an entity that incorporates diverse autoimmune conditions induced by the exposure to various adjuvants [11]. Furthermore, silicone is now known to act as an adjuvant [1,12].
With this literature review, we aim to summarize findings concerning the onset of inflammatory/autoimmune reactions related to local free silicone injections, their different clinical presentations, treatment options, and common risk factors.
Silicones are a broad family of synthetic organosiloxane polymers containing a repeating silicon-oxygen backbone with organic side groups attached via carbon-silicon bonds. Silicone as a biomaterial must not be confused with silicon (14Si), which is the chemical element. Depending on their structure, they are classified as elastomers (solids), gels or liquids. Silicone is used in its different forms for various approved medical indications, but also as an off- label therapeutic, aesthetical and cosmetic purpose.
Silicone breast implants are composed of a silicone elastomer shell surrounding a viscous silicone gel, whereas subcutaneous dermal injections are performed with silicone oil. The oil microdroplets cause a local inflammatory fibrotic reaction which then turns into a capsule [13]. This liquid form is known as “dimethicone” or “dimethylpolysiloxane” and is approved by the American Food and Drug Administration (FDA) only for its medical use in the retina, yet is also widely used for soft-tissue injections. Indeed, subcutaneous silicone injections allows numerous cosmetic treatments, such as soft-tissue augmentation, correction of facial age-related wrinkles or acne scarring [9]. Liquid silicone has several advantageous biologic qualities. It is injectable, allegedly biologically inert and non-carcinogenic, has an ability to provide long-lasting cosmetic corrections, and above all is inexpensive [14]. It is actually the most inert and permanent filler material compared with Hyaluronic Acid (HA), polymethylmethacrylate and collagen [1,15]. The ideal presentation of injectable silicone, so called “Medical-Grade Silicone” (MGS), is a pure and sterile preparation with a constant viscosity of 360 centigrade [9]. It is often injected with a microdroplet technique at one-month intervals, which allows for regular follow-up from the physician. Regarding all these bioqualities, it is questioned whether silicone actually is such an optimal and inert material in its free liquid form.
In the 1980’s, several authors concluded that only minor and transient problems occur if pure silicone is properly used. However, since then, growing data suggested that inflammatory reactions might develop many years after injection despite good techniques and good quality materials. Most of these adverse reactions are local [9,14,16–20], but systemic reactions are also being reported [1,16,21,22].
Granulomatous reaction is the most commonly described immunologic and histologic finding associated with late-onset inflammatory reactions secondary to silicone injections. Most of the granulomas form within the first 12 months after injection [1,14]. A granuloma is a small nodular inflammatory lesion containing grouped mononuclear phagocytes, caused by infectious or non- infectious agents. In the case of a foreign-body induced granuloma, the phagocytes are modified macrophages with multinucleated giant cells, usually surrounded by lymphocytes. Granulomas can clinically appear as subcutaneous nodules, and potentially occur three weeks to several decades post injection [14,18]. These lesions can be either local or located at a distance from the injection site. The silicone can invade deep muscle tissue and trigger fibrosis, lymphadenopathy and granuloma formation. It can also migrate through the tissue layers [16]. The migration of injected silicone may occur over time, leading to particles and nodular granulomas accumulation, at sites far removed from the points of injection [14].
Different hypothesis concerning the immunologic mechanisms of the inflammatory reaction due to silicone injection have been suggested. It has been demonstrated that medical grade silicone acts more as an adjuvant than as a direct antigen-triggering T-cell response [1]. An adjuvant is a substance that enhances the immune response at either the cellular or the humoral level, preferably without triggering one antigen on its own [12]. The adjuvant effect is achieved via several mechanisms involving both the innate and adaptive immune pathways. Such mechanisms can include an increased local reaction in the site of injection by mimicking danger signals; a progressive release of the antigen, which enables a longer exposure to the antigen-presenting cells. The adjuvant also promotes the translocation of antigens to the lymph nodes where they are recognized by T-cells. Furthermore, it may induce the release of inflammatory cytokines and interacts with toll- like receptors and NOD-like receptors [16]. Another mechanism explaining the adjuvancy of silicone could be its hydrophobicity, as it triggers adsorption of plasma proteins on its surface, which then triggers the immune system [14].
It has been demonstrated that Peripheral Blood Mononucleated Cells (PBMC) from individuals who suffered late-onset adverse inflammatory reactions due to medical grade silicone injections presented higher basal levels of Il-6 than PBMC from healthy individuals. On the other hand, those individuals showed no presence of memory CD4+ T cells against silicone. In vitro results demonstrated that medical grade silicone was able to induce an inflammatory response by production of Il-6 and TNF-alpha. These in vivo and in vitro results prove that silicone induces an inflammatory immune response acting as an adjuvant rather than an antigen [1].
The underlying pathophysiology is yet to be fully understood. Some authors suggest that a second trigger, such as late infection, trauma, or denatured proteins can activate the T-cells (particularly Th1 and Th17 cells) that have previously absorbed silicone, which release pro-inflammatory cytokines such as TNF-alpha. The particular role of certain memory “homing” innate cells, mainly macrophages or CD123+ plasmacytoid dendritic cells could also explain the delay in the onset of the abnormal immune responses when a second, non-specific trigger appears [1]. The activation of the immune system triggered by the adjuvants is normally self-limited, but might go out of control in genetically susceptible subjects, in whom the production of autoantibodies is enhanced [16,23]. However, it is still unclear whether the immune system is responding to the silicone itself or to additives such as fumed silica, an aggregate of silica and platinum. It has indeed been shown in animal models to be highly immunogenic [14].
Local skin biopsies of the foreign material filled-in area can show either an specific inflammatory infiltrate, or specific to a particular disorder such as sarcoidosis or vasculitis [1], or a histopathological pattern linked to silicone use [15,19,20]. Granulomatous reaction to silicone has been well described. The histological aspect is characteristic, and is even useful to identify which dermal filler was used whenever there is a doubt, whether the patient cannot remember or when there is no written information about it. Indeed, hyaluronic acid, collagen or polymethylmethacrylate each have specific inflammatory histological patterns and can be differentiated by an experienced physician [24]. The histopathological pattern differs depending on the kind of silicone used. Oil and gel silicone, more commonly employed for facial dermal filling, show characteristic interstitial silicone vacuoles and empty cystic spaces of various sizes in-between collagen bundles, realizing the classic “Swiss cheese pattern”, with surrounding inflammatory infiltrate made of macrophages with foamy cytoplasm, histiocytes, and lymphocytes. Smaller vacuoles usually are intracellular, when larger vacuoles are extracellular, and the foreign material classically does not exhibit birefringence under polarized light microscopy [19]. The findings of this histological aspect are consistent throughout the different cases reported, adding credibility to the silicone implication in the inflammatory response.
Despite the ability of granulomas to wall off silicone, silicone may “leak” and migrate to regional or distant lymph nodes and travel to other parts of the body, where it can cause adverse immunologic phenomena.
In 2011, “Autoimmune/inflammatory syndrome induced by adjuvants”, also known as Shoenfeld’s syndrome, was presented by Shoenfeld, as an umbrella entity [11]. This syndrome incorporates five immune-mediated conditions induced by the exposure to agents with adjuvant characteristics: siliconosis, post-vaccination phenomena, macrophagic myofasciitis syndrome, Gulf War syndrome and sick building syndrome. More than 500 cases of ASIA syndrome have been reported by physicians from the whole world, validated by experts and officially registered [25,26]. Siliconosis summarizes all kinds of different symptoms such as abnormal fatigue, body aches, morning stiffness, impaired cognition, sicca syndrome, paresthesia and non-specific autoantibodies secondary to silicone implants [5].
ASIA diagnostic criteria according to Shoenfeld’s proposal
Major criteria: Variable latency time ranging from months to years, External stimulus exposure (infection, vaccine, silicone) before clinical signs, Injected paraffin or silicone act as an adjuvant, Appearance of typical clinical manifestations, Foreign body-type granuloma present in the injected or implanted area, Myalgia, myositis or muscle weakness, Foreign body-type granuloma may be seen in drainage lymph nodes, Arthralgia and/or arthritis, Presence of antibodies, Chronic fatigue, unrefreshing sleep or sleep disturbances, Symptoms may disappear after the implant material removal, Neurological manifestations, Infection and cancer have to be ruled out in analyzed tissue, Cognitive impairment, memory loss, Pyrexia, dry mouth, Removal of inciting agent induces improvement, Typical biopsy of involved organs.
Minor criteria: The appearance of autoantibodies or antibodies directed at adjuvant, other clinical symptoms (e.g. irritable bowel syndrome), Specific HLA (i.e. HLA DRB1, HLA DBQ1), Evolvement into an autoimmune disease (i.e. multiple sclerosis, systemic sclerosis).
A new set of criteria have been brought up by Alijotas-Reig but are still awaiting validation. Those new criteria include a minimal latency time, biological features such as hypergammaglobulinaemia, high angiotensine convertase and lactate dehydrogenase, and low complement levels.
There is growing evidence in the literature of local free silicone injections inducing ASIA-related symptoms. Vera-Lastra, presented a new model of ASIA referring to 50 patients who underwent unlicensed cosmetic injections of foreign substances including fluid silicone and developed autoimmune diseases linked to adjuvant [22]. Alijotas-Reig, published an analysis of a Spanish prospective cohort of 45 patients suffering from late-onset non-infectious inflammatory or autoimmune disorders related to bioimplants (biomaterials injections or prostheses). The inclusion criteria were minimum 3 months latency, at least one local symptom and one systemic complaint. They then looked up for autoimmune diseases using the usual criteria and looked for ASIA syndrome cases using Shoenfeld’s criteria. Among the 45 patients, 23 had skin fillers which 20 were medical-grade silicone. No patient had been injected with vaccines or reported clinical infections after the injection. The authors suggest that a possible second trigger could be a local surgery attempt to remove the foreign material or a second filler material. Fourteen out of the 20 patients who received medical grade silicone evolved towards an autoimmune or granulomatous disease. Two cases among the 45 patients had exclusively skin filler injections with medical-grade silicone. They both presented with angioedema and skin nodules. The skin biopsy found a chronic inflammation pattern for one and a granulomatous reaction for the other. They both had elevated antinuclear antibodies. One of them had elevated anti-thyroperoxydase related to an autoimmune thyroiditis. The other had an arthritis associated to low complement and elevated rheumatoid factors. They also tested patients for HLA class I and II: 10 out of 25 patients tested positive for HLA-B8 and HLA-DRB1*03 haplotype. Alijotas-Reig, also proceeded in doing a review of the literature on biomaterials and ASIA. They concluded that silicone injections are associated with diverse autoimmune systemic diseases, mainly Sjögren’s syndrome, rheumatoid arthritis, adult Still disease, Sharp disease, eosinophilic fasciitis, overlap Sjögren’s syndrome/systemic lupus erythematosus, inflammatory myopathy, and fibromyalgia [1].
The described late-onset adverse effects range from local to systemic reactions. Local events consist mainly in inflammatory lesions inducing recurrent facial edemas, granulomatosis, nodular reactions or even ulcers [9,17–19,27–30]. We remind that foreign body-type granuloma present in the injected area is one of the major criteria of Shoenfeld’s ASIA criteria. Sanchis-Bielsa, reported a series of 15 cases of granuloma formation from cosmetic injections. Nine of these 15 cases had been caused by silicone, successfully treated with systemic steroids [31]. Wang reported a case of a 63-year-old woman who presented gradual onset of nodularity, swelling, pain in the upper lip and nasolabial folds and glabella, which represent the silicone injection sites from five years earlier. The ultrasonography and computed tomography scan imagery uncovered the silicone in her tissues and the cytology of a fine-needle aspiration showed a reactive inflammatory process [17]. The same authors published a 10-year review from 15 articles from the PubMed database of patients presenting granuloma formation secondary to cosmetic facial silicone injection. The histological pattern was used to prove the presence of silicone. The latency between the injection and the clinical symptoms spanned from 5 months to several decades. The clinical presentations were diverse: subcutaneous nodules, angioedema, disfigured nose with thickening and lichenification, leonine face [14]. Requena, reported four female patients, ranging from 65 to 75-year-old, presenting with recurrent episodes of unilateral facial edema lasting one to three weeks, with a history of silicone injections for dermal filling. All four patients responded to an immunosuppressive therapy of oral corticosteroids. Histology showed silicone deposits and a granulomatous infiltrate in all four cases [19]. Ulcers linked to local lower limbs and buttocks silicone injections have also been described, after excluding any vascular or infectious differential diagnosis [18].
Ryu, have reported other cases of silicone lymphadenopathy and of distant soft tissue masses related to silicone leakage and migration in a retrospective study of patients with a history of breast implant or liquid silicone injections. The distant silicone granulomas, mainly axillary and cervical, and the silicone lymphadenopathy are supposedly due to the migration of the oil droplets of silicone. In those cases of distant silicone-related manifestations, imaging studies including ultrasonography, computed tomography scan and magnetic resonance imaging have proven useful to witness the presence of silicone [27]. It would be interesting to study more precisely the occurring of ASIA criteria in patients presenting with late onset granulomatous reactions related to silicone injections.
Two patients with a history of silicone injections who initially presented with severe symptomatic hypercalcemia were also reported. The hypercalcemia was either proven to be related to a granulomatous reaction [16] or was highly suspected [32]. In both cases, the more likely causes of hypercalcemia were ruled out before granulomatous disease was suspected. Both patients were treated with prednisone in addition to the symptomatic treatment of hypercalcemia, which normalized the calcium levels. However, the incidence of hypercalcemia in cosmetic injection-induced granulomas is currently unknown.
Above all, late-onset systemic inflammatory or autoimmune reactions with or without initial local reactions are being observed with growing interest. Andreu-Barasoain, report a 58-year- old patient who developed systemic sclerosis three years after undergoing silicone facial injection. However, they do not propose any proof of the association between silicone and systemic sclerosis, other than the temporal relationship. Oral prednisone treatment was followed by a quick clinical improvement [21]. Alijotas-Reig, in their ASIA related to biomaterials review, treated 23 patients who presented with ASIA symptoms (according to Shoenfeld’s criteria), associated to local silicone injections [1].
Local symptoms similar to the ones mentioned (i.e. edema, angioedema, skin induration, plaques, panniculitis, swelling/ tender nodules) and systemic symptoms (fever, myalgia, arthralgia or arthritis, chronic fatigue, sicca syndrome) were used as inclusion criteria. Histology, when performed, disclosed either chronic inflammation, with granulomatous reaction, and sometimes specific autoimmune diseases (sarcoidosis, scleroderma). Treatment with corticosteroids, other immunosuppressive or immunomodulative drugs and tacrolimus in refractory cases relieved symptoms in almost all patients ,Courtesy of Prof. Haik J, Dr. Harats and Dr. Quiros, [1] (Figure 1).
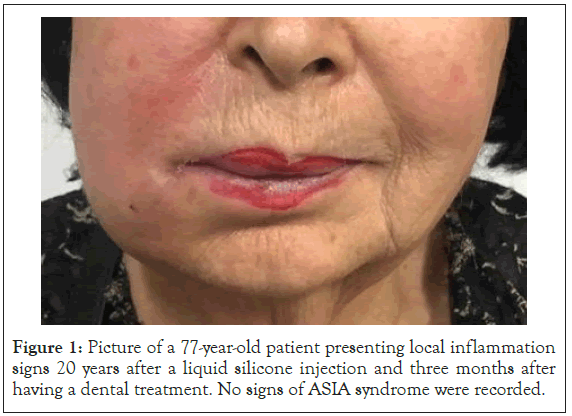
Figure 1: Picture of a 77-year-old patient presenting local inflammation signs 20 years after a liquid silicone injection and three months after having a dental treatment. No signs of ASIA syndrome were recorded.
ASIA manifestations occur at least three months after the initial adjuvant stimulation, according to Shoenfeld’s ASIA criteria. However, the clinical implications often appear long after the silicone injection, ranging from three months to several decades in some studies. Pointing to a positive diagnosis and proving the silicone injection liability is hereby more difficult, with a longer time loss until diagnosis, estimated to be in average of 20 months [1]. Patients can also fail to report having performed a dermal filling therapy, or do not remember which agent was used during the procedure.
The ASIA refers to a condition of autoimmune diseases due to hyperstimulation of the immune system with adjuvants. In this respect, the ASIA is similar to the Undefined Connective Tissue Disease (UCTD) [33].
Localized clinical forms as seen in Andre, and Requena, case reports [15,19] can be mistaken with orofacial granulomatosis, cellulitis, idiopathic angioedema, or Melkersson-Rosenthal syndrome. Siliconomas can be mistaken with liposarcomas. Sub-cutaneous granulomas have to be distinguished from non-inflammatory nodules resulting from the heterogeneous diffusion of the filler in the tissue without any granulomatous reaction. However, the histological presentation of silicone-induced inflammatory reaction with the characteristic “Swiss cheese pattern”, put together with a detailed patient history, proves to be quite specific regarding silicone- related immunological adverse events. Vacuolated macrophages can also mimic lipoblasts and liposarcomas histologically [14].
There is no consensus regarding the treatment of silicone granulomas. In some patients, granulomas will resolve spontaneously without treatment [9,14]. Surgical excision is a good option for localized granulomas. However, for deeper localizations, after silicone or granuloma migration, removing of the silicone is not recommended. Silicon clinically evades the structures in the area of injection and therefore cannot be removed completely. The surgery, when possible, is difficult and has a high risk of scarring, or of having an atrophic or hypertrophic outcome, as well as deformation of aesthetic contour of aesthetic units of the face, and aesthetic line violations by the surgical cuts, or removal of volume from the excised area containing the silicone or silicone granuloma. Furthermore, the effects of removal of foreign material are not systematically related to clinical improvement, and do not ensure a preventive procedure from recurring [1,19].
The more common medical treatments include systemic and/or local steroids, hydroxychloroquine, Non-Steroid Anti-Inflammatory Drugs (NSAIDs), minocycline, 5-fluorouracil, and isotretinoin, in intent to lower the overall steroid intake. More specific treatment modalities include imiquimod and etanercept (anti-TNFa) [14]. Some authors specify using antibiotic therapy, considering the possibility of a low-grade chronic infection lying in the silicone surrounding biofilm. However, no infection sign was reported, and the authors assessed their doubts regarding the effectiveness of antibiotic therapy [20]. Alijotas-Reig, treated 23 patients who presented with different forms of ASIA related to local silicone injections. The treatment always included prednisone, at a mean dose of 12.5 mg/day (ranging from 5 to 80 mg/day), combined with hydroxychloroquine, allopurinol, NSAIDs or antihistamines. The cases that remained refractory to the aforementioned drugs were treated with tacrolimus, with no resistance to be found. With this therapeutic schedule, almost all of their patients were symptom- free, and 70 % of them remained symptom-free up to two years after treatment removal. Their recommendation regarding characterized autoimmune disease (i.e. systemic lupus erythematosus, systemic sclerosis, Sjogren syndrome …) that might have appeared during the evolution is to treat according to the defined guidelines specific of each disease [1]. If a collection of fluid is seen in ultrasound imaging, then a “wash and drain system” can be installed, in purpose of drainage, and to avoid surgical incision, as described by Goldan [29,30]. In practice, according to the clinical manifestation local antibiotic as well as systemic antibiotic administration can be combined. Empiric systemic antibiotic treatment should be selected based on the suspected pathogen. The overall effectiveness of immunomodulative and/or immunosuppressive therapy may lead to treatment recommendations regarding late-onset adverse events of local dermal silicone injection. Lately, attempts for removal of fillers with various lasers were reported [34].
A non-medical grade silicone, too superficial injections, under- qualified and/or non-licensed practitioners are proven risk factors of acute adverse event. The American FDA actually never approved medical silicone for dermal filling and aesthetic purposes; leading to it being used off-label under sometimes-doubtful circumstances. However, many studies have shown that the use of medical-grade silicone, with proper microdroplet injection techniques and conducted by a practiced medical physician may reduce acute and long-term adverse effects and overall risk for the patients. Silicone, being a non-biodegradable dermal filler, is more prone to late- onset adverse reactions than other biodegradable fillers (hyaluronic acid, collagen). Permanent fillers are also linked to more severe complications and a higher need of secondary surgery [35].
ASIA, as a multi-factorial pathology, also has some genetic involvement. In the Shoenfeld et al., ASIA criteria list, HLA DRB1 and DBQ1 are categorized as minor criteria. Furthermore, three HLA haplotypes are hypothesized to be linked to autoimmune diseases, HLADR2DQ6, DR4DQ8 and DR3DQ2 [36]. Alijotas- Reig, specifies that the low prevalence of autoimmune features regarding the overall use of silicone and other biomaterials could point to a predisposing genetic background. In unpublished results, they show that HLA-B*8 and HLA-DRB1*03 haplotypes combinations represent a specific risk marker for late-onset adverse reactions to biofillers and ASIA [1,37]. If confirmed by further studies, genetic involvement in ASIA and other inflammatory and immune-related complications could prove useful to determine personal risk, and predictive testing could be applied.
Further studies showed that repeated injections of silicone did not increase the risk of developing ASIA, however it was a risk of presenting with more severe and chronic symptomatology. In the Alijotas-Reig, patient cohort, lactate dehydrogenase and angiotensin convertase were shown to be elevated, signifying continuous macrophage activation related to adjuvant stimulus. Such biological signs and predisposing genetic factors can therefore be linked to a higher risk of developing ASIA. A closer follow-up of asymptomatic patients but presenting signs of macrophagic activation could be considered to prevent the occurring of autoimmune diseases [1]. Colafrancesco, in 2014 elaborated the theory that in genetically predisposed hosts, the effects of diverse molecules acting as a trigger may evolve into more generalized inflammatory disorders that may present as ASIA syndrome. They based their theory on epidemiological, pathogenic, serological and clinical manifestation similarities between Sjögren’s syndrome and ASIA [38].
In 2001, Bigata reported a case of a 30 year-old woman who presented a sudden swelling of the lips and the nasolabial folds with multiple subcutaneous nodules where she had been injected liquid silicone 8 months earlier. Interestingly, a week prior the swelling she suffered from a flu-like syndrome. The biopsy found a granulomatous infiltrate without any sign of infection. The blood tests and the chest radiographs were normal. Neither NSAIDs nor steroid injections were efficient, and the symptoms spontaneously disappeared after 3 years. The authors considered the viral infection as an immunologic-cross reaction trigger who could explain why the reaction was unpredictable and occurred lately [9].
Silicone is amongst the most widely used injectable substances for cosmetic purposes. These injections are so far not approved by the FDA because of the acute and chronic risks that are being more and more described. Although silicone is supposedly inert, silicone formulations can be highly heterogeneous, necessitating some standardization in the way silicone is manufactured. Furthermore, some unlicensed practitioners provide silicone injections under doubtful circumstances. Local and distant granulomatous reactions have been pathologically proven to be induced by silicone in patients with history of local injections. Further studies including research of ASIA criteria on these local inflammatory reactions would be interesting. Silicone is also highly suspected to be responsible for numerous systemic chronic inflammatory diseases from the ASIA syndrome spectrum. This theory relies on the hypothesis of a non- antigen specific immune response where silicone would act like an adjuvant, in genetically predisposed patients. It could be interesting to identify more precisely the genotype of patients presenting ASIA- features related to silicone injections, in order to identify patients at a higher risk of either developing or exacerbating an autoimmune condition. Inflammatory aspects, as macrophagic activation syndrome could also be analyzed in asymptomatic patients who underwent silicone injections. Genetic and biological features, if confirmed by further studies, could prove useful for predictive testing in patients exposed to silicone regarding the risk of ASIA/ autoimmune diseases. Further investigations should be held in purpose to seek for the incidence of ASIA syndrome in the overall population, to compare it to the silicone-injected population and to compare it to other biofillers.
Citation: Thompson MR, Guérin A, Borba V, Haik J, Harats M, Quiros-Lim HE, et al. (2021) The Downside of Beauty: ASIA Syndrome Associated with Local Silicone injections: A Literature Review. Immunome Res. 16:7391.
Received: 11-Dec-2020 Accepted: 25-Dec-2020 Published: 01-Jan-2021 , DOI: 10.35248/1745-7580.21.s4.7391
Copyright: © 2021 Thompson MR, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.