Journal of Women's Health Care
Open Access
ISSN: 2167-0420
ISSN: 2167-0420
Research Article - (2024)Volume 13, Issue 3
Accepted treatments for female sexual dysfunction (FSD) are currently limited to psychological, hormonal, surgical, and psychopharmacologic interventions. There are no FDA-approved pharmaceutical interventions that directly affect the female genitalia to improve female sexual function other than prasterone for dyspareunia. Botulinum neurotoxin type A (BoNT) has been shown to improve erectile function in men by increasing arterial blood flow and possibly by increasing parasympathetic tone. In all previous studies in women, BoNT has only been used to improve sexual function by either attenuation of sensation or of muscle contraction and never to accentuate sensation, desire, orgasm, lubrication, or satisfaction directly. This pilot study was undertaken to test the effects, if any, of the clitoral injection of BoNT on female sexual function. The female sexual function index (FSFI) was used as a measure. Our data indicate that the treatment enhances sexual function in women in multiple domains—including desire, arousal, lubrication, orgasm, satisfaction, and overall sexual function. The benefits to overall sexual function improved by an additional 50% when the BoNT was combined with platelet-rich plasma (PRP) rather than saline alone. Our population was small, and placebo effects are possible; but the effects of treatment resulted in improvements in FSFI greater than previously reported after treatment with any of the currently FDA-approved drugs for female sexual dysfunction. The extended safety profile of BoNT for women of reproductive age at the dosages used, the known regenerative properties of BoNT and PRP, its positive effects on circulation and the autonomic nervous system, and the lack of any FDA-approved therapies that directly affect the female genitalia to improve sexual function warrant offering this innovative treatment to women suffering sexual dysfunction unresponsive to other means.
Botulinum Toxin, Botulinum Toxin Type A, BoNT, Incobotulinumtoxina, Anorgasmia, Sexual Dysfunction, Treatment, Clitoris, Injection, Satisfaction, Sexual Distress, Orgasm, Anorgasmia, Autonomic Nervous System, Parasympathetic Nervous System, Sympathetic Nervous System, Ganglion, Placebo, Saline.
FSAD: Female Sexual Arousal Disorder
FSIAD: Female Sexual Interest/Arousal Disorder
HSDD: Hypoactive Sexual Desire Disorder
PGAD: Persistent Genital Arousal Disorder
FSFI: Female Sexual Function Index
PD5I: Phosphodiesterase-5 Inhibitor
PRP: Platelet-Rich Plasma
Sexual dysfunction in both men and women can be a serious problem that worsens family relations and psychological well-being.
Moreover, sexual dysfunction in women is the only physical problem not diagnosed unless there is accompanying secondary psychological distress [1]. For contrast, consider that even though men with erectile dysfunction or women with hypertension are diagnosed with those disorders, even if not psychologically distressed by such disorders, a woman who is not psychologically distressed does not meet the criterion for sexual dysfunction even though she may suffer symptoms of dyspareunia, anorgasmia, decreased arousal, or decreased satisfaction. Therefore, women neither wanting to start a family nor desiring a sexual relationship may not be distressed, and so are not counted as having sexual dysfunction even if they suffer all of the otherwise diagnostic symptoms.
This unique limit in the definition of female sexual dysfunction partly accounts for the fact that the incidence and prevalence of sexual dysfunction are greater in older men and younger womenolder women who do not complain are not counted [2].
Even with this limiting definition of sexual dysfunction in women, in some reports, sexual dysfunction occurs in 40% of women [3].
Limited treatment options for women
When comparing men and women, there is a disparity between not only diagnostic criteria for sexual dysfunction but also in treatment options. Considering and correcting this disparity is facilitated by reviewing the history of the treatment of men: in the 1980s, it was thought that, of men suffering from erectile dysfunction (ED), 85% suffered because of psychogenic etiologies; therefore, urologists were encouraged to become skillful in sexual counseling [4]. Then, with the discovery of the effects of phosphodiesterase-5 inhibitors (PDE5Is), most men thought to have ED of a psychiatric etiology were found to have a neurovascular cause-a physical etiology. This new understanding that sexual dysfunction in men most often originates in the genitalia, not the brain, triggered a proliferation of FDA-approved drugs and devices shown to improve sex in menover twenty FDA-approved drugs.
Women, in contrast, currently have only two FDA-approved oral medications with on-label indications to improve sex: flibanserin and bremelanotide. Both drugs are approved only for the treatment of hypoactive sexual desire disorder in premenopausal women. Both have a mechanism of action that is psychoactive, with no direct effect on the female genitalia.
Even in the endocrine arena, there is only one FDA-approved drug for sex—prasterone, which is approved only for dyspareunia. Women still do not even enjoy an FDA-approved form of testosterone; dosages for brands approved for men are modified and used off-label to treat the millions of women who benefit from this proven therapy for female sexual dysfunction [5].
Topical lubricants and anesthetics can be used for dyspareunia and arousal. However, these options mostly treat symptoms, not underlying etiologies. Surgical therapies help some types of dyspareunia; surgery to treat phimosis of the clitoral hood can improve sensation; colporrhaphy and other surgeries can improve sensation, and labiaplasty can improve self-image. Most women, however, suffer from sexual dysfunction with etiologies lacking curative surgery. Studies show that properly injected platelet-rich plasma (PRP) can improve sexual function in women [6-8]. But PRP is an autologous blood isolate, not a drug, and is not governed by the FDA.
So, the number of therapies to help women with sexual dysfunction compared with men demonstrates a disparity in both the scope of etiology-directed treatments and the number of FDA-approved medications.
Disparity of FDA Evaluation
The difference in the number of available options of FDAapproved drugs for women suffering sexual dysfunction occurs partly because of the significant difference between men and women in the approval process: a new drug for men need only improve erection firmness or the angle of deformity (physical and objective measurements); but for women to gain FDA approval a new drug must improve satisfaction (a psychological, and subjective measurement)—a more difficult domain to measure and to achieve. For example, if a man takes a PDE5I and his penis grows firmer, that qualifies for approval of the drug by the FDA for the treatment of male sexual dysfunction. If a woman takes a new drug, however, that cures her anorgasmia and improves her libido, but her husband suffers premature ejaculation, she might enjoy increased pleasure, orgasm, and libido but become less satisfied, then (should other women experience the same effect) that drug would be denied approval.
This difference in the approval process disincentivizes pharmaceutical companies to invest the money needed for the research to bring new drugs to market to help women with sex.
That men currently have 20-plus drugs and devices that directly improve their sex by affecting the genitalia indicates no unacceptable disparity between the sexes only if one assumes that the vagina is a passive receptacle and that there are no physical components of the female genitalia that might cause sexual dysfunction. However, the embryology and histology of the genitalia of both men and women display corpus cavernous, glans, prostate (periurethral glands), corpus spongiosum, autonomic nervous system, and similar innervation and vascularization [9].
Two desired traits for the next drug for women’s sex
For all the above reasons, there is a need for new FDA-approved drug therapies for women that work by directly affecting the genitalia to improve sexual function, especially sexual satisfaction. Easier approval might happen if a drug with an already known safety profile in women of childbearing age, already approved for other indications, could be repurposed and approved for a new indication-to improve arousal, orgasm, lubrication, and sexual satisfaction of women.
Previous use of BoNT is limited to attenuation, not enhancement
Thus far, because of its ability to block nerve conduction, botulinum toxin (BoNT) has been studied in females suffering from sexual dysfunction, but only to help with problems that might be improved with less muscle contraction or less sensation. For example, BoNT was shown to help with vaginismus and pelvic floor pain by relaxing muscles by blocking nerve signaling at the motor end plate [10, 11]. Botulinum toxin has also been successfully used to treat detrusor muscle instability (bladder spasms, overactive bladder) and neurogenic balder by the same mechanism: blocking nerve transmission, causing the relaxation of smooth muscle, and relieving pain and other symptoms by directly injecting the smooth muscle of the bladder wall [12].
Regarding sensation, BoNT was studied for the treatment of persistent genital arousal disorder (PGAD) [13] and to help with vulvodynia [14-16]; for both indications, benefits were postulated because of the potential to interfere with nerve conduction and lessen sensation, orgasm, and pain [17].
In summary, thus far, studies of BoNT have been done to lessen muscle contraction by direct injection of the muscle and to lessen sensation (including pain, arousal, and orgasm) by injection near the offending nerves. There are no previous reports of the use of BoNT for directly increasing sensation, desire, arousal, orgasm, or sexual satisfaction.
Previous BoNT injection sites do not include the clitoris
BoNT is injected directly into the muscle to relax muscles and treat vaginismus, pelvic floor pain, and bladder spasms. For persistent genital arousal disorder, BoNT is injected near (not into) the clitoris [18]. To relieve the vulvodynia, BoNT is injected directly into the area of pain [19]. Because of this previous history of the use of botulinum toxin solely for either decreasing the signal of the nerve to contract the muscle or to decrease arousal and sensation, it may seem counterintuitive to inject BoNT directly into the clitoris. Even though BoNT has been widely available to gynecologists (kept in the office for two decades for bladder spasms), there are no published reports of injections of BoNT directly into the clitoris to increase sensation, arousal, orgasm, and satisfaction in women. Even though injection of platelet-rich plasma (PRP) directly into the clitoris to improve sexual function has been described [20], even though the FDA has approved drugs for injection directly into the penis, there are no studies describing the injection of any drug whatsoever directly into the clitoris. More specifically, there are no studies of injecting BoNT directly into the clitoris for any reason.
Therefore, the following ideas are neither expected nor previously explored.
BoNT changes autonomic tone and increases arterial blood flow
To explore reasons for injecting BoNT directly into the clitoris to increase arousal, orgasm, and satisfaction, consider the history of the use of BoNT for the treatment of migraines. Botulinum toxin has been FDA-approved for treating migraines for over a decade. Though the initial strategy for using BoNT to treat migraines might have been aimed toward the relaxation of muscles, the modern view of migraine is that the disorder is initiated by vasodilatation of the circulation of the meninges (not by muscle contraction) [21]. Current neurology literature states that botulinum toxin, when injected into the facial muscles (procerus and the corrugators), is taken up by the afferent neurons and transported via retrograde axonal transport, is delivered to the trigeminal ganglion and the caudate nucleus [22], probably at a rate of about half a centimeter per day.
The trigeminal ganglion and the caudate nucleus are shared by the afferent nerves of the meninges and by the autonomic nervous system. When BoNT arrives at these central locations, both sympathetic nerve transmission and pain neurotransmitters are blocked. The result is a decrease in sympathetic tone, a relative increase in parasympathetic tone, and a decrease in pain sensation; therefore, an attenuation of migraine pain [22].
In summary, the procerus and the corrugators of the facial musculature act as ports through which the more centrally located caudate nucleus and the trigeminal ganglion can be infiltrated with and affected by BoNT, therefore attenuating the sympathetic nervous system, increasing relative parasympathetic tone and decreasing the transmission of pain from the meninges [22, 23].
Furthermore, double-blind placebo-controlled studies have shown that injecting BoNT into the corpus cavernosum of the penis significantly improves erectile dysfunction (ED) even in men with difficult-to-treat etiologies (such as spinal cord injury and longstanding diabetes) [24, 25]. Even men who had failed full-dose therapy with PDE5Is for ED saw improvements in sexual function after the injection of BoNT into the corpus cavernosa [26, 27].
Part of the mechanism of action of improvement in ED from BoNT injection of the corpus cavernosum is thought to be the relaxation of the smooth muscle of the arterioles with an increase in arterial blood flow; PDE5Is also work by relaxation of the smooth muscle controlling arterial blood flow into the penis, resulting in a firmer erection. So, at least in this way, BoNT and PDE5Is affect the same mechanism of action.
Additionally, an increase in the parasympathetic nervous system and a decrease in the sympathetic nervous system tone cause vasodilatation of the arterial blood flow into the penis with an increase in erection firmness. A second mechanism of action of BoNT injected in the corpus cavernosum has been postulated to be (like that seen with the treatment of migraine) that BoNT migrates along the afferent nerves of the sympathetic nervous system, blocking sympathetic tone and increasing relative parasympathetic tone-causing erection. This increase in parasympathetic tone is not part of the mechanism of action of PDE5Is.
Because parasympathetic tone governs erection, this action of BoNT results in both a harder erection and a larger flaccid penis since the parasympathetic tone would be more active even in the baseline, unaroused state.
Previous studies in females have also shown that women taking a PDE5I, as with men, can see an increased blood flow within the corpus cavernosa of the clitoris with a resultant improvement in sexual function [28]. However, unlike with the corpus cavernosum of the penis, the injection of BoNT into the corpus cavernosum of the clitoris has not yet been reported.
Since vasodilatation from PD5Is in women affects blood flow in the same way as PD5Is in men, then there might be an improvement in sexual function in women by injecting BoNT, as is seen in men. Also, if botulinum neurotoxin increases parasympathetic tone in women (as in men), then it could act in a way not afforded by PDE5Is by not only increasing blood flow to the clitoris but also affecting libido through the vasodilatory effects of the parasympathetic system.
Even more, the parasympathetic nervous system of the pelvis connects to the mid-hypothalamus, which is responsible for the cerebral perception of arousal [29]. So, the ability of BoNT to affect the autonomic nervous system might also stimulate the hypothalamus and, therefore, increase arousal.
The hypothalamus also connects to the pituitary gland and affects hormone production. By its effects on the parasympathetic nervous system and the hypothalamus, BoNT could change immediate emotions and the production of pituitary hormones. Such hormonal changes might not be confined to those typically associated with menopause (FSH, LH, and estradiol) but might also extend to dopamine and serotonin balance, which affects orgasmic function.
A recent study demonstrated the vasodilatory effects of BoNT by regulating arterial smooth muscle contraction via the eNOS/sGC/ cGMP pathway, which leads to an increase in endothelial nitric oxide [30]. This is likely another mechanism that contributes to the increased tumescence seen with BoNT injections for erectile dysfunction in males and should have similar effects in the cavernosal tissue of the female clitoris.
Despite these possible beneficial effects, injecting the clitoris with BoNT to increase blood flow, increase parasympathetic tone, and stimulate the mid-hypothalamus (causing increased arousal and a change in hormone production) has not been reported.
If successful, such injections would give results opposite to what has been explored or anticipated previously. All previous uses of BoNT in the female genitalia intended attenuation (not enhancement) by either decreasing arousal (by decreasing sensation), decreasing muscle tone (by decreasing the signal at the motor end plate), or decreasing pain (by decreasing sensation or muscle tone).
BoNT triggers neurogenesis, neovascularization, and collagenases
Moreover, botulinum neurotoxin has been shown in multiple studies (since the 1950s) to propagate neurogenesis and neovascularization and, by these effects, to help heal surgical, traumatic, and chronic wounds [31-34].
Research has demonstrated that, with aging, women see a decrease in the number of nerves within the clitoris, a decrease in blood flow, and a decrease in the number of muscle fibers within the urinary sphincter [35, 36]. Trauma, even from just riding a bicycle [37], can damage nerves, resulting in decreased sensation from the clitoris with resulting sexual dysfunction. If the injection of the corpus cavernosum of the clitoris with BoNT had the same effect that has been shown in studies of wounds, then the increase in the number of nerves and the number of blood vessels afforded by the injection could improve sensation and sexual function (the opposite effect of what has been explored thus far).
A previously published study demonstrated that injection of platelet-rich plasma (also known to cause neurogenesis [38-40] and neovascularization [41-43]) into the clitoris improved sexual function. [20] BoNT might do the same.
Summary of possible effects of BoNT on female sexual function
In summary, men have been helped with ED by injecting the corpus cavernosum with BoNT; women also have two corpus cavernosa that contain innervation and vascularization like men.
If BoNT injected into the clitoris acted centrally, affecting the autonomic nervous system and the hypothalamus, as has been described in the treatment of migraine and the treatment of erectile dysfunction, then such an effect could improve female sexual function by increased parasympathetic tone, increased arousal, and a change in pituitary hormone production. If BoNT exerts a local effect, there could be an increase in arterial circulation with engorgement of the clitoris, also to improve sexual function. There may also be a triggering of neovascularization and neurogenesis that could improve the sexual response in women.
So, BoNT injected in the clitoris might improve libido and orgasm, as well as improve satisfaction. This would be a new and unique treatment with an unexpected breakthrough in the treatment of women's sexual function.
Considering the precedent of BoNT in clinical practice, as well as its proven safety with vaginismus and bladder spasm, women who presented with complaints of anorgasmia, difficulty with arousal, or other symptoms related to sexual dysfunction were offered BoNT injections into the clitoris and were observed for their responses to treatment.
This report describes women who wished to see an improvement in their sexual function in libido, arousal, orgasm, and satisfaction but for whom hormonal strategies were either not wanted or hormones were already at optimal levels; thus, changes in hormonal therapies were not needed. These were also women who chose not to risk the side effects that might be seen with flibanserin and bremelanotide, which could be argued to be more troubling than those seen with BoNT at the doses we used for treatment. These women were treated with the understanding that they could also choose not to receive treatment at all, but they wanted to experience treatments already available to men.
In addition, because of the history of the use of PRP in wound care and the treatment of women for both urinary incontinence and sexual dysfunction, some women chose to have the BoNT reconstituted with autologous PRP (rather than with saline).
This pilot study measures the response of women with varying degrees and types of sexual dysfunction who received the intervention: the injection of the body of the clitoris with BoNT (either alone or combined with PRP).
Thirteen females, ages 32-64, presenting with complaints of sexual dysfunction (desire, arousal, orgasm, or satisfaction), sought improvement by the injection of BoNT into the clitoris. The patients were seen in clinical private practice and were not paid to receive the procedure or complete the pre- or post-treatment surveys. All patients were fully informed of the innovative therapeutic, offlabel, and experimental nature of the clitoral BoNT injection and consented to the procedure.
Exclusion criterion: participants were without thrombocytopenia, myasthenia gravis, ongoing infection, pregnancy, inappropriate affect, high-dose corticosteroids, or clitoral phimosis.
The materials and equipment included the following:
1. 5-cc syringes
2. 3-cc syringes
3. 30-gauge, BD brand needles
4. 18-gauge needles
5. Bacteriostatic saline
6. Regen® PRP tubes
7. The matching Regen® PRP centrifuge
8. Calcium chloride (CaCl) 10%
9. 30% lidocaine ointment
10. Luer-Lok™ connectors
11. IncobotulinumtoxinA (Xeomin®)
A. Anesthesia
First, the clitoral hood was retracted, and lidocaine ointment was applied to the body of the clitoris; inadvertent application to the glans is inevitable, but the distal body of the clitoris must be covered [6]. Injection was delayed for 20 minutes after the application of the lidocaine ointment for complete or nearcomplete anesthesia.
B. Preparation of BoNT
Using an 18-gauge needle on a 1-cc syringe with a Luer-Lok, one cc of bacteriostatic saline was added to a 100-unit vial of BoNT (Xeomin®) [7]. Then, 0.5 cc of the reconstituted BoNT (50 units) was drawn back into the syringe and the needle recapped. These 50 units were then set aside until needed.
C. Preparation of PRP
Only for the seven women who chose BoNT combined with PRP, ten cc of blood was drawn from the arm and centrifuged using a Regen® kit, producing around five cc of PRP supernatant (amount is hematocrit dependent). The Regen tube was inverted five times (to resuspend platelets that might have adhered to the gel), then using an 18-gauge needle, two ccs of PRP were drawn from the Regen tube into a 3-cc syringe.
Next, a third syringe (1-cc with a Luer-Lok connected to an 18-gauge needle) was used to withdraw 0.1 cc of 10% CaCl—also to be set aside.
D. Two methods of Injection of the clitoris
Twenty minutes after the ointment was applied, the clitoris was injected with 50 units of BoNT, either (1) without or (2) with PRP.
1. Injection without PRP
For the six women in the saline-only group (no PRP), two ccs of saline were drawn into a new 3-cc syringe. Then, 0.5 ccs of BoNT (from step A) was attached using a Luer-Lok connector; the 0.5 ccs of BoNT was transferred into the 3-cc syringe-resulting in 50 units of BoNT in 2.5 ccs of saline (0.5 + 2.0) held by a 3-cc syringe.
Then, the Luer-Lok and the now-empty 1-cc syringe were replaced by a ½ inch, 30-gauge needle.
Then, the clitoral hood was retracted to expose the body of the clitoris, which was cleansed with gauze soaked with hypochlorous solution [8].
[7] A 1-cc was used since a larger syringe would result in a less accurate measure of this critical solution where every drop matters.
The injection
The prepared 2.5 ccs of saline carrying 50 units of BoNT were injected from the 3-cc syringe after inserting the 30-gauge needle into the body of the clitoris at the 2’OClock position approximately 3mm proximal to the glans [9].
Only one side of the clitoris was injected.
During injection, only the bevel of the needle was inserted into the tissue of the clitoris (no deeper), and the fluid was meticulously and slowly injected such that it was completely absorbed into the clitoris. Little to no edema was observed—neither in the area surrounding the clitoris nor in the clitoris, indicating that the material hydro dissected deep along the corpus cavernosum and not laterally into the surrounding tissue.
2. Injection of BoNT combined with PRP
If PRP was added (seven of the women), the 18-gauge needle was removed from the 3-cc syringe containing two ccs of PRP (from step C), and a Luer-Lok connector was used to facilitate the addition of the 50 units of BoNT (from step B).
Then, 0.1 cc of 10% CaCl (also from step C) was added to the mix through the same connector, which was replaced with a 1/2-inch 30-gauge needle.
Within 3 minutes of combining the three (BoNT, PRP, and CaCl), the woman’s clitoris was injected with the mix exactly as was done for those women injected without PRP (D1) [10].
E. Instructions for after-injection
The woman was encouraged to, on arrival home, cleanse her clitoris and surrounding area of the remaining lidocaine ointment and proceed with her usual daily activities, including sex [11]. If she received the BoNT combined with PRP, she was also instructed to avoid non-steroidal anti-inflammatory drugs for one week.
This study falls in the category of Medical Practice and Innovative Therapy, which describes an activity that is designed solely to benefit the individual patient(s) and does not require IRB review (University of Virginia Institutional Review Board Health Science for Health Science Research).
The standardized Female Sexual Function Index (FSFI) questionnaire was used to measure arousal, desire, pain, orgasm, satisfaction, and lubrication. Outcomes measured the patients’ sexual responses using self-administered FSFI surveys before and 4-12 weeks after receiving the intervention.
A total of 13 women, ranging from 32 to 64 years old, with an average age of 50, presented to a private clinic seeking improvement in sexual function. Their responses to the FSFI questionnaire on the day of treatment and 4 to 12 weeks afterward populate the following table (* marks those who received PRP) Table 1:
| Pt. # | Desire | Arousal | Lubrication | Orgasm | Satisfaction | Pain | Overall Pre- BoNT | Overall Post-BoNT | Overall Delta |
|---|---|---|---|---|---|---|---|---|---|
| 1 Pre | 4.8 | 4.2 | 3.9 | 4.8 | 4 | 4.8 | 26.5 | ||
| Post | 6 | 6 | 4.8 | 6 | 6 | 6 | 34.8 | ||
| Delta | 1.2 | 1.8 | 0.9 | 1.2 | 2 | 1.2 | 8.3 | ||
| 2 Pre | 4.8 | 4.2 | 3.6 | 4.4 | 3.6 | 6 | 26.6 | ||
| Post | 5.4 | 5.4 | 5.7 | 5.6 | 4.8 | 6 | 32.9 | ||
| Delta | 0.6 | 1.2 | 2.1 | 1.2 | 1.2 | 0 | 6.3 | ||
| 3 Pre | 4.8 | 4.2 | 3 | 2.4 | 6 | 6 | 26.4 | ||
| Post | 6 | 6 | 6 | 5.6 | 6 | 6 | 35.6 | ||
| Delta | 1.2 | 1.8 | 3 | 3.2 | 0 | 0 | 9.2 | ||
| 4 Pre | 3.6 | 3.9 | 6 | 5.2 | 4 | 6 | 28.7 | ||
| Post | 6 | 6 | 6 | 6 | 6 | 6 | 36 | ||
| Delta | 2.4 | 2.1 | 0 | 0.8 | 2 | 0 | 7.3 | ||
| 5 Pre | 2.4 | 3 | 4.8 | 2.4 | 2.4 | 6 | 21 | ||
| Post | 6 | 6 | 6 | 5.6 | 5.2 | 6 | 34.8 | ||
| Delta | 3.6 | 3 | 1.2 | 3.2 | 2.8 | 0 | 13.8 | ||
| 6 Pre | 4.8 | 5.4 | 3.9 | 4.8 | 3.6 | 6 | 28.5 | ||
| Post | 5.4 | 6 | 6 | 4.8 | 4.8 | 5.2 | 32.2 | ||
| Delta | 0.6 | 0.6 | 2.1 | 0 | 1.2 | 0.8 | 3.7 | ||
| 7 Pre | 4.8 | 3.9 | 3.9 | 4.8 | 4.8 | 4.8 | 27 | ||
| Post | 6 | 6 | 6 | 6 | 6 | 5.2 | 35.2 | ||
| Delta | 1.2 | 2.1 | 2.1 | 1.2 | 1.2 | 0.4 | 8.2 | ||
| 8* Pre | 2.4 | 3 | 1.2 | 1.6 | 3.6 | 3.6 | 15.4 | ||
| Post | 6 | 6 | 5.1 | 5.2 | 6 | 5.6 | 33.9 | ||
| Delta | 3.6 | 3 | 3.9 | 3.6 | 2.4 | 2 | 18.5 | ||
| 9* Pre | 5.4 | 3.9 | 1.5 | 1.2 | 4.8 | 4.4 | 21.2 | ||
| Post | 5.4 | 5.4 | 5.7 | 4 | 5.6 | 6 | 32.1 | ||
| Delta | 0 | 1.5 | 4.2 | 2.8 | 0.8 | 1.6 | 10.9 | ||
| 10* Pre | 0 | 0 | 0 | 0.8 | 0 | 0 | 0.8 | ||
| Post | 1.2 | 0 | 0 | 0 | 0.8 | 0 | 2 | ||
| Delta | 1.2 | 0 | 0 | -0.8 | 0.8 | 0 | 1.2 | ||
| 11* Pre | 0 | 0 | 0 | 0 | 0.8 | 0 | 0.8 | ||
| Post | 3.6 | 3.6 | 5.4 | 5.2 | 2.4 | 0 | 20.2 | ||
| Delta | 3.6 | 3.6 | 5.4 | 5.2 | 1.6 | 0 | 19.4 | ||
| 12* Pre | 5.4 | 3 | 1.2 | 4.4 | 4 | 1.2 | 19.2 | ||
| Post | 4.8 | 5.7 | 6 | 6 | 6 | 6 | 34.5 | ||
| Delta | 0.6 | 2.7 | 4.8 | 1.6 | 2 | 4.8 | 15.3 | ||
| 13* Pre | 4.2 | 4.5 | 3 | 2.4 | 3.2 | 5.2 | 22.5 | ||
| Post | 4.8 | 6 | 5.4 | 6 | 5.2 | 5.6 | 33 | ||
| Delta | 0.6 | 1.5 | 2.4 | 3.6 | 2 | 0.4 | 10.5 |
Table 1: Responses to the FSFI Questionnaire
Ten were postmenopausal; nine of the postmenopausal patients were on hormone replacement therapy for at least six months before undergoing treatment, and one (patient #10) was not receiving hormone replacement.
With an FSFI score of <26.55 pre-treatment, nine of the 13 women satisfied a diagnosis of Female Sexual Dysfunction.
Since the pre-injection and post-injection scores are observed from the same individual (a repeated measures design), a paired sample t-test was used, assuming that the differences between paired observations are normally distributed Figure 1.
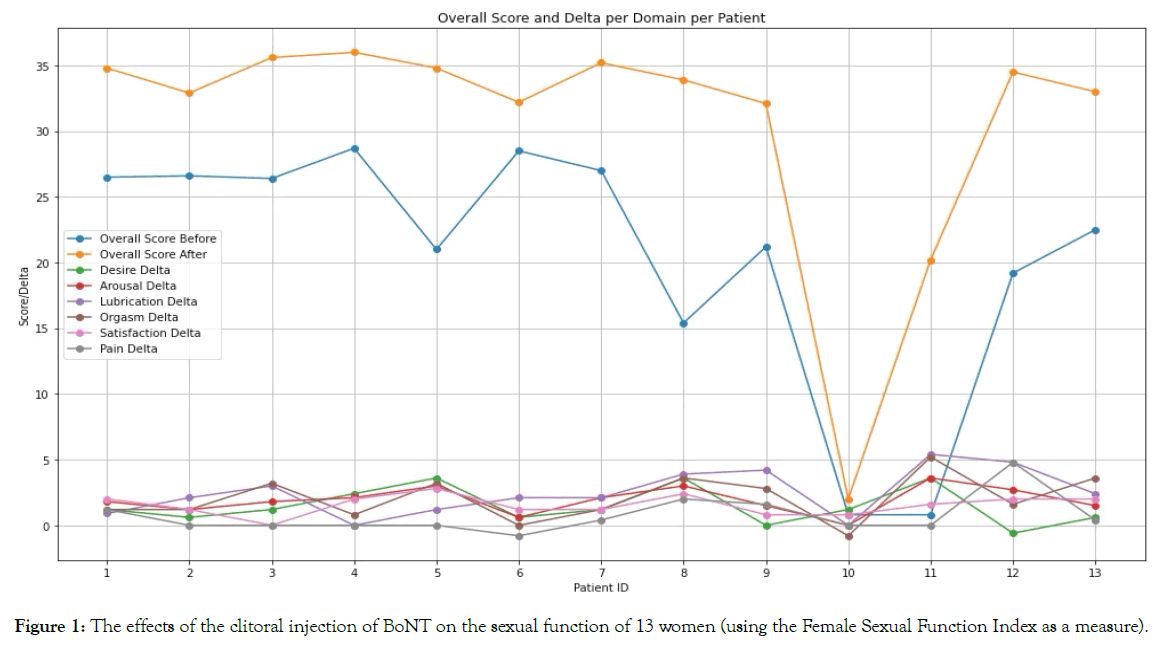
Figure 1. The effects of the clitoral injection of BoNT on the sexual function of 13 women (using the Female Sexual Function Index as a measure).
All but one of the thirteen women significantly improved their scores with treatment; one (patient #10) showed improvement in the overall score, but the change was not statistically significant [12].
The mean of the individual domain scores for all 13 women, for all domains, improved, and the changes were statistically significant (p-values < 0.05), except for the Pain domain, which improved but not significantly (p-value > 0.05).
The mean overall pre-treatment score for all 13 women was 20.35, which increased to 30.56 after treatment, with an average improvement across all participants of 10.2 (p<0.05). A Wilcoxon signed-rank test, comparing the overall scores before and after treatment, showed a W-statistic of 0.0 and a P-value of 0.002 Figure 2.
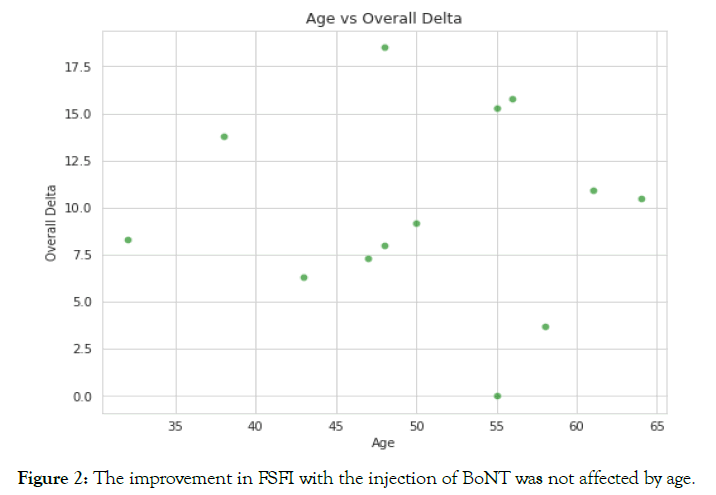
Figure 2. The improvement in FSFI with the injection of BoNT was not affected by age.
Of the 12 with significant improvement in the overall score, 9 (75%) changed from a diagnosis of “female sexual dysfunction” to a diagnosis of “no dysfunction” (FSFI > 26.55).
In summary, results suggest that BoNT injected into the clitoris may improve a woman’s FSFI score on average by 10.2 points, significantly affecting sexual function.
Effects of age on the treatment
There was no correlation between the overall improvement of FSFI and the age of the women treated; an equally positive response was seen across all participating ages.
The effects of the addition of PRP to the BoNT
Six women chose BoNT without PRP, and seven chose the addition of PRP; two-tailed paired sample t-test was used to determine if there was a difference in the effect of treatment between these two groups Figure 3.
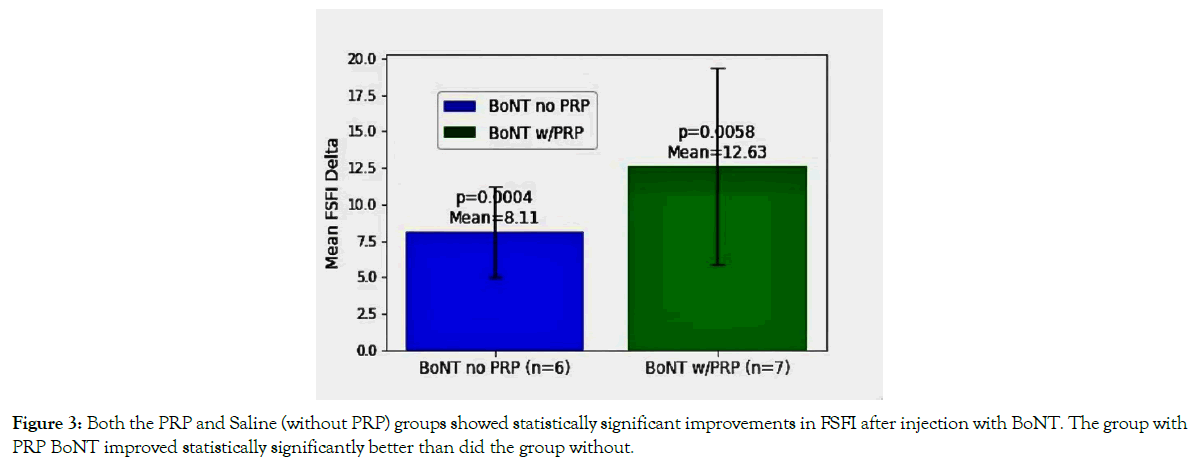
Figure 3. Both the PRP and Saline (without PRP) groups showed statistically significant improvements in FSFI after injection with BoNT. The group with PRP BoNT improved statistically significantly better than did the group without.
For those women injected with BoNT without PRP, the mean increase in overall FSFI was 8.11 (p = 0.0004). For those women who received BoNT with PRP, the overall mean increase in FSFI was 12.63 (p = 0.0058). The observed differences between the two groups, as indicated by a t-statistic of 3.889 and a p-value of 0.006), suggest a statistically significant improvement in FSFI scores for the women treated with BoNT and PRP compared to those treated without PRP.
In summary, both groups (PRP & without PRP) treated with BoNT demonstrated a significant improvement in overall FSFI compared with baseline; the PRP group demonstrated a more pronounced improvement, and this enhanced improvement of 50% was also statistically significant.
Using a histogram of the overall improvement in FSFI for all 13 women and a Shapiro-Wilks test to compare pre- and post-treatment FSFI scores for all six domains, overall before and after scores (and overall mean improvement) confirmed a normal distribution of the data and the validity of a paired sample t-test analysis Figure 4.
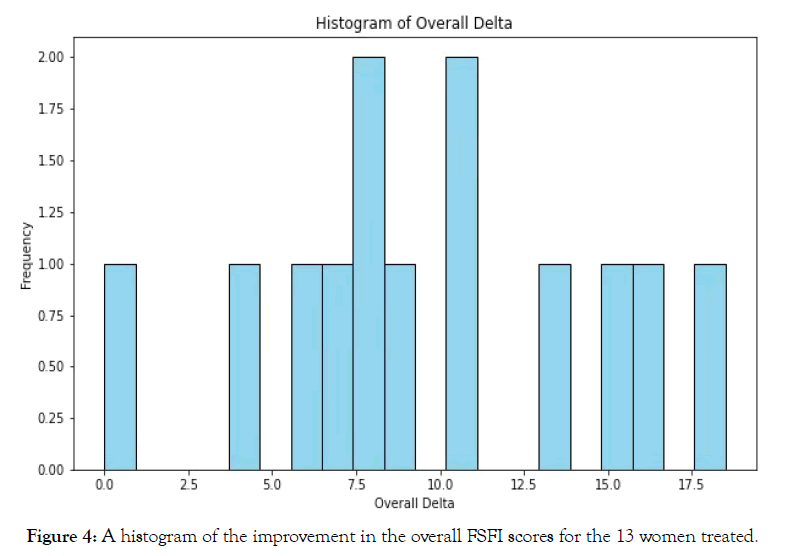
Figure 4. A histogram of the improvement in the overall FSFI scores for the 13 women treated.
The Box-Cox Method was applied and also supported normality of distribution.
Because improvement in the Satisfaction domain is required for the FDA approval of new drugs (or new drug indications) for the treatment of female sexual dysfunction, a correlation analysis was performed between each domain and the Satisfaction domain. The correlation coefficients were as follows: Desire (0.47), Arousal (0.56), Lubrication (-0.06), Orgasm (0.23), Pain (0.28). Therefore, after treatment, the overall increase in Satisfaction correlated most with improvements in Desire and Arousal and least with Lubrication.
We assessed the accuracy of the size of the effect of treatment by calculating the Cohen's d value for the groups treated with BoNT combined with PRP and those treated with BoNT alone. The results are 2.31 for the BoNT plus PRP group and 1.58 for the BoNT alone group. Both Cohen’s d values are greater than 0.8 Figure 5, suggesting a large treatment effect in both groups, with an even stronger effect in the combined PRP and BoNT group. When comparing the groups, Cohen’s d value is 0.856, also indicating a robust treatment enhancement by the addition of PRP to the treatment (with >0.8 indicating a large effect).
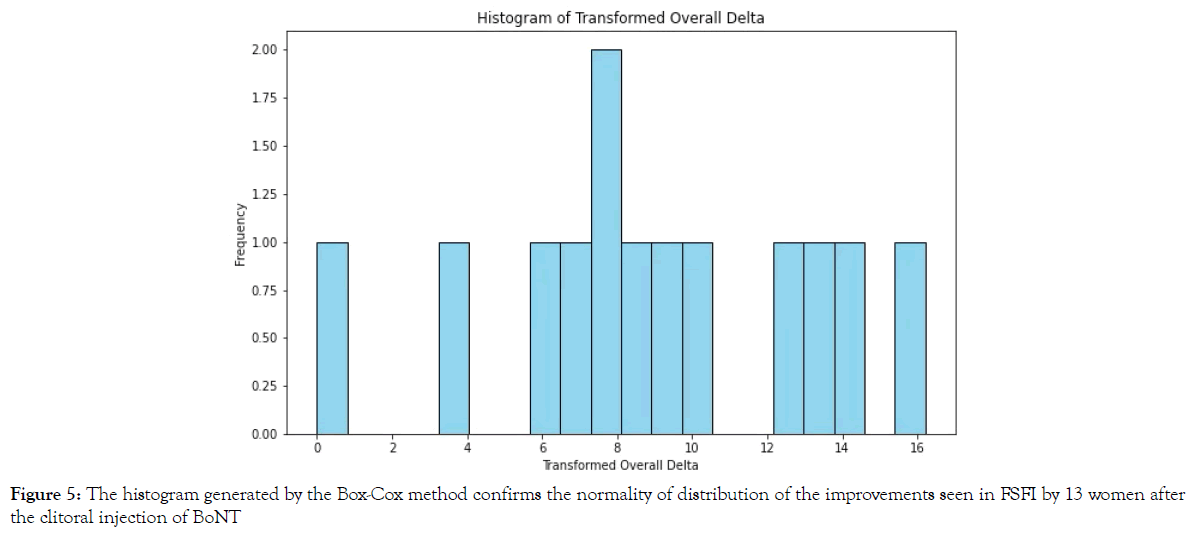
Figure 5. The histogram generated by the Box-Cox method confirms the normality of distribution of the improvements seen in FSFI by 13 women after the clitoral injection of BoNT
Side Effects
Two women voluntarily reported, at one week, an overall subjective decrease in sexual function; but at four weeks post-procedure, both women subjectively reported improved function, and FSFI demonstrated improvements over baseline. This temporary decrease in sexual function seen in two of the thirteen women is not reflected in the numbers since FSFI was not scored sooner than four weeks.
Four of the women treated suffered hyper sexuality for about a week, with arousal and the urge for orgasmic relief sometimes interrupting daily activities.
Eight of the women reported increased engorgement of the labia; one woman canceled a scheduled surgical procedure planned to augment the labia majora because she perceived that the clitoral injection of BoNT triggered sufficient increased blood flow to the labia to accomplish her desired enhancement.
Five of the thirteen women voluntarily reported a side effect of a subjective improvement in urge incontinence.
This is a pilot observation of women who chose to improve sexual function with a clitoral injection of BoNT.
Possible criticisms include that this was not a prospective, doubleblind, placebo-controlled study. One difficulty in executing a placebo-controlled double-blind study for a procedure that involves hydro dissection of tissue to measure regenerative effects is the following: saline injection, in this situation, is not a placebo; in the study of a pharmaceutical agent administered intravenously or intramuscularly (IM) for a somatic effect, saline is a true placebo because there is no tissue disruption by the injection (or the disruption locally with an IM injection is irrelevant to full-body pharmaceutical effects); but with infiltrating tissue to propagate local collagenases, neovascularization, and the remodeling of scar tissue (in short, for improving tissue health), the infiltration or hydro dissection itself disrupts tissue structure and triggers regenerative changes—as has been described in multiple studies in both the dermatology and orthopedic literature [44-46].
Saline infiltration alone triggers a beneficial effect on scarring, leishmaniasis, and the healing of joint and nerve damage [47, 48]. Saline injection of the clitoris is not a placebo. Therefore, a placebo-controlled, double-blind prospective study of the injection of BoNT into the clitoris presents logistic difficulties, and studies that use the injection of saline (or any other liquid) as a placebo could give an erroneous attenuation of the effect of the treatment because the supposed “placebo” could affect benefits (decreasing the statistical significance of benefits of BoNT over baseline).
For future studies, it could be helpful to compare five groups:
(1) A shallow needle puncture of the clitoris
(2) A saline injection
(3) BoNT reconstituted with saline,
(4) BoNT reconstituted with PRP (prepared as in this study, including activation with CaCl. It seems logistically impossible to blind such a study to the injector), and
(5) Injection with PRP alone.
Comparisons with Other Treatments
A further criticism is that current literature suggests the placebo effect with pharmacological interventions for female sexual dysfunction is substantially higher than seen in other conditions; benefits seen in the present study with the clitoral injection of BoNT may be secondary to that placebo effect.
For perspective, Weinberg et al. compared the placebo effect and treatment effect in eight randomized placebo-controlled trials using FSFI as a measure, with 3,959 women included in the metaanalysis; seven different pharmacologic treatments for female sexual dysfunction were included: flibanserin, bremelanotide, bupropion, BoNT for vulvar vestibular pain syndrome, intravaginal prasterone (DHEA), intranasal oxytocin, and ospemifene [49]. The placebo effect was compared with each respective study's treatment effect to determine mean differences; women receiving placebo showed an average 3.62 increase in the FSFI total score, while the treatment arm showed an average increase of 5.35. The weighted effect summary indicates an average treatment increase over a placebo of only 1.73. This translates to 67% of the score improvement attributable to the placebo effect, with only a 1.73-point average increase in FSFI attributable to the pharmacologic intervention Figure 6.
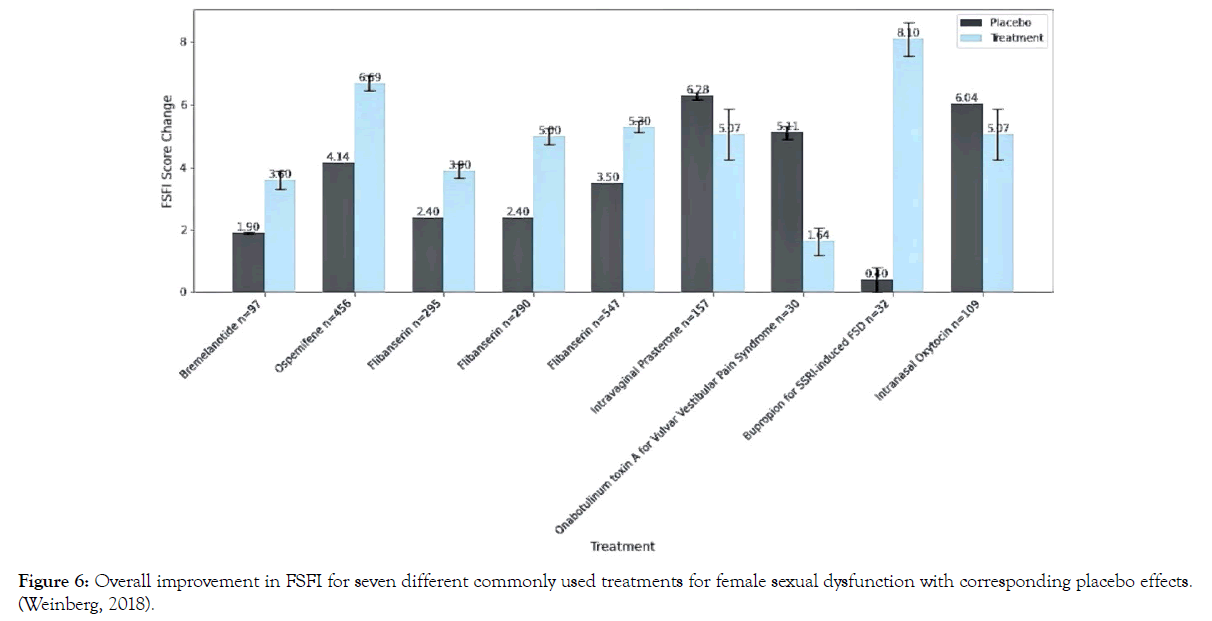
Figure 6. Overall improvement in FSFI for seven different commonly used treatments for female sexual dysfunction with corresponding placebo effects. (Weinberg, 2018).
In review, the present study found a mean difference in FSFI scores between pre-treatment and post-treatment of 10.2 (p<0.05) when combining both groups (with and without PRP); considered separately, injecting BoNT alone resulted in a mean improvement of 8.11 (p<0.05); when PRP was added, the mean improvement over baseline increased to 12.63 (p<0.05). Folding the results of the present study into Weinberg’s metanalysis, comparing the mean increase in FSFI of the clitoral injection of BoNT to other pharmacologic interventions and the placebo effects seen in those treatments shows that if all the effects of our treatment were due to a placebo, the placebo effect from BoNT injected into the clitoris would be greater than not only the placebo effects seen in previous studies but also greater than the reported beneficial effects of all seven of the treatments considered in the metanalysis, including the current drugs approved by the FDA [49]. Moreover, if our numbers were reproduced, clitoral injections of BoNT could be remeasured using either of the two current FDA-approved drugs (flibanserin and bremelanotide) as a placebo, and BoNT injections of the clitoris would still show statistical benefit, and such benefit would be greater than demonstrated by either of those two drugs demonstrated when compared with a true placebo Figure 7.
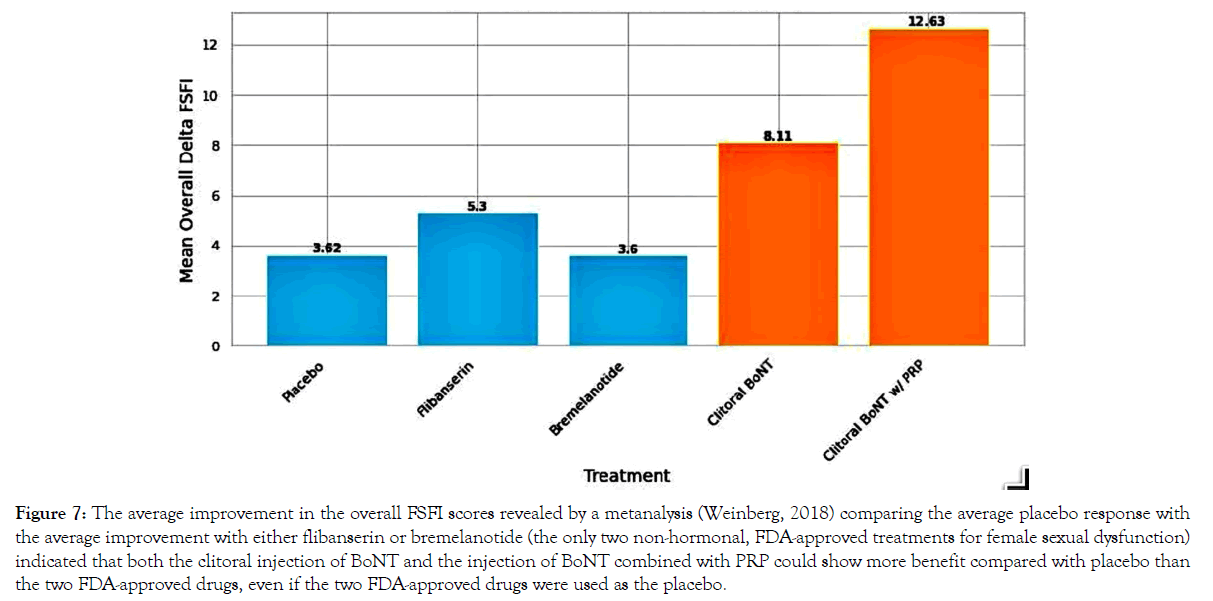
Figure 7. The average improvement in the overall FSFI scores revealed by a metanalysis (Weinberg, 2018) comparing the average placebo response with the average improvement with either flibanserin or bremelanotide (the only two non-hormonal, FDA-approved treatments for female sexual dysfunction) indicated that both the clitoral injection of BoNT and the injection of BoNT combined with PRP could show more benefit compared with placebo than the two FDA-approved drugs, even if the two FDA-approved drugs were used as the placebo.
Note, in Figure 6, that BoNT for vulvar vestibular pain syndrome was less effective than placebo-which would be consistent with the present study looking at enhancing, not decreasing, response.
“Female Satisfaction” to satisfy the FDA
Not only were desire, orgasm, arousal, lubrication, and overall sexual function improved in the present study, but satisfaction also improved (the approval-determining factor for the FDA); therefore, this procedure offers hope for a possible new FDA-approved indication for BoNT for the treatment of sexual dysfunction in women.
Safety
Some advantages of conducting further studies would be the longestablished safety profile for BoNT [50], with millions of doses per year over the past two decades, in women of reproductive age, with few serious sequelae at the dosages used for this treatment [50]. BoNT is considered safe enough to use at doses greater than in the present study for appearance-an important but arguably less serious indication than sexual dysfunction with the implied psychological distress. Still, in addition to the warnings associated with the treatment of sexual dysfunction and injection in general, consent should include all the same warnings used for the consent when BoNT is used for aesthetic purposes, including not be using in the presence of pregnancy, myasthenia gravis, and knowing allergies.
How long does it last?
Six women noticed an attenuation of effects and requested retreatment about six months after the first treatment. Studies of BoNT for erectile dysfunction showed benefits for as long as nine months when higher dosages of 100 units are used [25, 27, 51] implying that the central autonomic effects may extend longer than the 2-3 months [52, 53] usually seen for first-time injections for cosmetic indications, dependent on muscle relaxation (which would only account for arteriolar dilatation as a mechanism of action). The higher dosage might afford the same longer-acting results for women. Even should women need retreatment as frequently as every 12 weeks, this would not be more frequent than what is routinely done with BoNT for aesthetic and migraine indications. Should the regenerative effects (neovascularization and neurogenesis) prove true, and then some components of the effects could be longer lasting and cumulative and theoretically help delay the future development of sexual dysfunction independent of any autonomic effects.
Projected patient cost
Though the time and other expenses of the provider to treat a sexual disorder are more than required for a routine cosmetic BoNT treatment (especially if PRP with the associated preparation time is added), the expense for the actual BoNT material injected for the treatment under consideration is similar to that of routine cosmetic BoNT treatments and less than the cost of the FDAapproved protocol for the treatment of migraine—both of which are trimonthly treatments [54, 55].
The BoNT with PRP combination
The present study suggests that combining BoNT with PRP might provide beneficial synergy, a reasonable conclusion since PRP has been shown over the past two decades to improve tissue health [41, 42, 56-60] and to improve female sexual function [7, 20, 61-66]. However, one study suggested that combining BoNT with PRP could result in decreased effectiveness of BoNT [67]. More studies are needed to investigate the interaction of PRP with BoNT and to investigate other possible carriers with positive synergy.
The ubiquitous and justified criticism of the metanalyses of all PRP studies in other arenas (aesthetics, orthopedics, dentistry, dermatology) laments the multiple ways to prepare PRP and the resulting nebulous definition of what it is. Methods of PRP preparation might, after future studies, be specifically modified for the procedure to be done (avascular joints, diabetic wounds, and the scalp for androgenic alopecia may not respond like the vascular clitoris). More studies are needed to find which of the many combinations of PRP-preparation variables offer optimal outcomes after clitoral injection for improvement in the female sexual response; variables include all of the following and more: blood fractionating methods (centrifuge dimensions and spin times, micropore filters, double-spin, single spin), anticoagulants types and amounts, amounts and types of white blood cells retained, activation agents (platelet-released growth factors and cytokines vary with the activator), and ancillary factors (washing platelets, cooling platelets, aerobic exercise pre-phlebotomy, effects of other medications)—all of which significantly affect outcomes [68-72].
The systems analysis for a personalized approach
As the understanding of female sexual function continues to mature to allow the implementation of the same systems analysis science implemented in other branches of medicine (i.e., respiratory system, cardiovascular system, etc.), other synergies and feedback loops will emerge, and more targeted and personal approaches to treatment will mature which directly address the constraints in the female orgasm system for each woman’s individual spectrum of pathology [73-76]. A growing understanding of a systems-analysis approach to the female sexual response will likely find and embrace more therapies and medications directly addressing the female genitalia (as has been done for the male genitalia).
Effects on urinary continence
As a side effect of the injection of BoNT into the clitoris, some women in the present study reported a significant improvement in the symptoms of urge incontinence. This result would be expected should BoNT migrate centrally and increase parasympathetic tone (as described above) and deserves further study. BoNT has been injected into the urothelium and detrusor muscle of the bladder via cystoscopy for many years to treat overactive and neurogenic bladder. Improvement of sexual function has also been identified in women treated for overactive bladder, regardless of the type of treatment. It is also thought that the reduced coital incontinence and improvement in psychosocial factors do not fully explain the improvement in sexual function in these women [77]. This shared nature is evident when considering the “urethrocoporalcavernosal reflex” [78]. Stimulation of the urethral orifice results in a reflexive vasodilation of the corpora cavernosa and bulbospongiosus erectile tissue and a simultaneous contraction of the ischiocavernosus and bulbospongiosus muscles in both males and females. Other evidence of the relationship between urge incontinence and sexual dysfunction is the often-temporal relationship with the emergence of or worsening of both conditions. Further studies are needed to delineate this relationship.
What about the long-acting BoNTs?
Though long-acting neurotoxins are available, we preferred starting with one of the shorter-acting agents (Xeomin®) with a longer history of safety. Should the decreased function seen for a week (in two of the 13 women treated) become as long-lasting as the effects of the BoNT injected, the shorter-acting agents would be preferred. For an individual woman, should repeated injections with the shorter-acting agents prove beneficial, a longer-acting BoNT is a reasonable consideration, but even in this situation, a longer-acting strategy is riskier and needs more study before implementation in the daily clinic?
But is it FDA-approved?
This treatment of sexual dysfunction in women with BoNT is an off-label, non-FDA approved use of BoNT, but other uses of BoNT are done off-label millions of times per year in the US; for example, for the treatment of bruxism, erectile dysfunction, and depression. Also, specific aesthetic uses are commonly done: “crow’s feet” (orbicularis oculi), down-turned corners of the mouth (depressor angularis oris), smoker’s lines (orbicularis oris), and platysma bands—all of which are done millions of times per year in the US.
Testosterone (arguably with more associated risks than BoNT at the dosages under consideration) is frequently used off-label to help women with sexual dysfunction, as is Oxytocin and bupropion (both of which also primarily affect the brain, not the genitalia).
Moreover, though it is illegal for manufacturers to advertise the off-label use of drugs, 21% to 31% of prescriptions written are for off-label use [79, 80]. The FDA only regulates the marketing and safety of drugs; it does not regulate the practice of medicine [81]. Considering that approximately 25% of prescriptions are written for off-label uses, medicine would be greatly hindered without physicians being free to use their judgment to prescribe off-label. Specifically, a physician may safely and legally prescribe a drug “for purposes other than that for which they were approved,” as long as the use is based on scientific rationale and medical evidence and as long as clinicians “maintain records of the product’s uses and effects” [79, 82].
The frequency of off-label prescriptions is largely explained by a much-discussed problem that hinders the process of drugs gaining on-label approval: when off-label use becomes common, it can disincentivize manufacturers from spending the vast money needed to meet FDA approval, especially with difficult-to-study populations (like children, pregnant women, and women with sexual dysfunction); this situation creates “orphan” populations/ indications where the benefit is known or at least suspected, but the effort to meet FDA approval is beyond what the manufacturer considers reasonable or profitable [83, 84]. So, because of profit considerations, the recognized benefit of a drug can remain an off-label use perpetually-even in the face of mounting supportive research. And, without FDA approval and with resulting legal fears, some physicians will, understandably, hesitate to offer treatmenteven with supporting research in hand.
More research is needed
This is a preliminary study of possible effects on sexual function in women with the clitoral injection of BoNT; much more research is needed to achieve FDA on-label approval. Further studies could include at least the following variables: patient selection, injection techniques (and how they may vary based on symptoms), diluent (including what and how much), best PRP preparation methods, type of BoNT used, and the positive synergy or attenuation of other therapies both pharmacological and psychological.
Based on the parameters of the present study, for a large effect (Cohen's d=0.8), for a significance level of 0.05, and a desired power of 80% for a two-tailed independent samples t-test, the required sample size per group predicted is approximately 25 participants. Yet, even with a smaller sample size (n=6 & n=7) relative to what the power analysis suggests (for an adequately powered study of this type), a large effect size was detected (Cohen’s d=0.856)—suggesting probable positive effects of BoNT injections of the clitoris in future studies of larger size.
Effect of this study on current practice
Wiegel et al. described two groups of women: a study group (n=152) was diagnosed with arousal disorder and the control group (n=244) were women without complaints of sexual dysfunction. The arousal disorder group had a mean FSFI of 20.05 (SD 6.74), and the control group had a mean FSFI of 30.75 (SD 4.80). Wiegel’s study highlights a mean difference of 10.7 points on the FSFI between untreated women with dysfunction and those without [85].
In the current study, our overall average for the 13 subjects fits Wiegel’s report, showing an average overall FSFI score of 20.35 before and 30.56 after treatments.
Since sexual dysfunction is defined by a score of less than 26.6, to have a near-universal effect for the treatment of female sexual dysfunction, a monotherapy would need to improve, on average, FSFI by at least seven; none of the current on-label therapies do so, hence the recommendation to use multimodal therapies for the synergy of effects (with the hope that multiple ineffective therapies might combine for a curative effect). In the present study, BoNT alone raised FSFI by 8.1, giving hope that such therapy could be a successful monotherapy for more women than afforded by the currently available therapies. Moreover, BoNT combined with PRP achieved an improvement of 12.63, which would close the gap between the average FSFI overall of 20.05 seen with women suffering sexual dysfunction and the overall FSFI of 30.75 seen with women without sexual dysfunction.
Current algorithms propagate the same assumptions about women that were made with men in the 1980s, mostly funneling women into therapies that treat the brain and emotions. The current FDAapproved pharmacia for the treatment of female sexual function implies that the female genitalia is a passive tube to receive a penis and deliver a baby with no potential for improved function and pleasure to the woman by the identification and treatment of components of the female orgasm system that lie anatomically within the genitalia. Indeed, there are psychological and social etiologies for female sexual dysfunction that need addressing, but when psychological distress arises from sexual dysfunction caused by a physical etiology, then healing a physical problem seems more desirable than helping one feel better about a problem left unhealed.
Even though further research is needed (as with any new therapy) because the expected harm is minimal, and the current on-label pharmaceutical options for the treatment of sexual dysfunction in women are limited and do not include therapies that affect the genitalia (as are the most common method of pharmaceutical treatment for men), women should be allowed to request and be treated with clitoral injections of BoNT with or without PRP.
Because BoNT injected directly into the clitoris with the described specific method improved sexual function in the domains of orgasm, arousal, and satisfaction, BoNT may be a possible offlabel treatment for female sexual dysfunction. When doing the procedure, BoNT can be used alone, but PRP could also be added and work in synergy with BoNT to improve results. When done, meticulous attention must be paid to the preparation of BoNT and the method of injection to avoid pain and for the best chances of positive results. When PRP is added, the preparation of PRP (including activation and anticoagulant) matters. Because of the difficulty in the FDA approval process for new drugs or new drug indications for female sexual dysfunction, the use of BoNT, even if effective, could remain an orphan indication—never receiving FDA approval. However, because our preliminary study shows statistical improvement in the satisfaction domain of FSFI (which, if confirmed, would trigger FDA approval) and because of the robust improvement seen in overall FSFI to levels usually seen in women without sexual complaints, BoNT could hold promise for a true on-label indication for female sexual dysfunction should one of the manufacturers of BoNT risk the immense expense of further investigation. Because of the 20-year safety profile of BoNT (including for other gynecological indications), because of the strong scientific basis of its possible success (through multiple mechanisms of action that include an improvement in tissue health of the genitalia not seen with the other approved drugs), because it is already being used off-label for the treatment of the genitalia of men by injection into the corpus cavernosum (for ED) and of women (for vaginismus, overactive bladder, and dyspareunia), because (unlike with men) there is currently no FDA approved drug for women, other than topical DHEA, that directly improves sexual function by directly affecting the function of the genitalia (only psychoactive drugs are FDA approved), because the improvement was shown to be sufficient to offer a greater chance of success as monotherapy than anything available, women with ongoing sexual dysfunction unresponsive to other therapies should also be offered clitoral injections of BoNT.
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Citation: Runels C, Runnels A (2024) The Clitoral Injection of IncobotulinumtoxinA for the Improvement of Arousal, Orgasm & Sexual Satisfaction- A Specific Method and the Effects on Women. 13(3):715.
Received: 21-Feb-2024, Manuscript No. 29700; Editor assigned: 24-Feb-2024, Pre QC No. 29700; Reviewed: 09-Mar-2024, QC No. 29700; Revised: 13-Mar-2024, Manuscript No. 29700; Published: 20-Mar-2024 , DOI: 10.35248/2167-0420.24.13.715
Copyright: © 2024 Runels C, Runnels A. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited