Journal of Sleep Disorders & Therapy
Open Access
ISSN: 2167-0277
ISSN: 2167-0277
Research Article - (2022)Volume 11, Issue 1
Objective: Nocturnal Enuresis (NE) frequently occurs in children with Sleep Disordered Breathing (SDB). Many options to address SDB and NE are available to include pharmaceuticals and surgery. This retrospective study examines the relationship between NE and dentofacial anomalies modified or remodeled by intervention with a pre-formed Mono-block Oral Appliance (MOA).
Methods: In a retrospective study using secondary data from a third-party source, 107 questionnaire scores of children ages 5-12 were reviewed. The secondary data score sources included a sleep, breathing and habit questionnaire; kids intake form; pediatric sleep questionnaire; head and neck exam; child home observation sheet and patient profile. The primary endpoints for the study were the number of days between the start date of MOA therapy and the resolve date of bedwetting according to the age of the child. In a linear regression analysis, comparisons were made between the age and bedwetting resolved in days when using an MOA.
Results: A linear regression analysis was conducted to evaluate the prediction of resolving NE with an MOA. The scatterplot indicated an association between NE, therapy with an MOA and the age of the child.
Conclusion: The study shows a strong predictability of resolving NE at an early age with the use of an MOA.
Sleep disordered breathing; Nocturnal enuresis; Obstructive sleep apnea; Bedwetting; Pediatric; Oral appliance
NE: Nocturnal Enuresis; SDB: Sleep Disordered Breathing; FDA: Food and Drug Administration; SNE: Secondary Nocturnal Enuresis; RME: Rapid Maxillary Expansion; CI: Confidence Interval; AI: Airway Intelligence; IRB: Investigational Review Board; HIPPA: Health Information Portability and Protection Act; EMR: Electronic Medical Record
Nocturnal Enuresis (NE) is one of the most frequent pediatric pathologies. The prevalence of Primary Nocturnal Enuresis (PNE) is around 9% in children between 5 and 10 years of age, with approximately 40% having one or more episodes per week [1]. NE episodes have been shown to affect the quality of life in children and their families with an emotional impact causing anxiety, embarrassment, social isolation and problems with self-esteem [1,2]. The emotional impact of bedwetting can affect relationships with family and friends, schoolwork and in turn affect sleep patterns. Nocturnal Enuresis is defined as an involuntary loss of urine during sleep after the age of 5 years occurring over a continuous six-month period, affecting approximately 15% of children up to the age of 15 [3]. The prevalence of NE dramatically decreases to 5% between 6 and 10 years of age and 1% to 2% between the ages of 10 and15 years [3,4]. Of this population, 80% of NE cases lack subtle daytime symptoms related to a mono symptomatic or single principal symptom, of NE with the bedwetting diagnosis classified as primary or secondary NE [3,4]. The etiology of mono symptomatic enuresis is not clearly understood, but is hypothesized as a sleep disorder with low arousability, high arousal threshold and frequent urination or hyperactivity associations [5-7]. Furthermore, the association of psychological disorders and sleep related breathing disorders are common in nearly 80% of children with NE having concurrent sleep apnea present; hence the strong correlation between NE and sleep apnea [4,5,7,8]. NE can occur in all stages of sleep; however, the sleep patterns or hypnograms, appear to differentiate between children with enuresis versus children without enuresis [6,8]. To further associate NE and Sleep Disorder Breathing (SDB), Polysomnography (PSG) detects an arousability threshold in stage N2 of sleep and stage N3, which is reduced in children with enuresis [6,8].
In the last two decades, there has been an increasing recognition where SDB may be a plausible root cause of NE [1-4]. Medical and surgical interventions are options to address nocturnal enuresis [9]. Medicinal interventions, such as anti-diuretic therapy, have been an option that may help reduce the number of wet nights without changing the sleep pattern [5,10]. It has also been previously found that adenoidectomies and tonsillectomies reduce the nocturnal resistance airflow and may alleviate bedwetting in children with SDB and tonsillar hypertrophy [9].
There have been many proposed treatment modalities, such as bladder training, fluid management, night alarms and medications to minimize or resolve NE [4]. Beyond these current modalities, mandibular advancement therapy and adjustment has been suggested for refractory NE [11]. The rationale is, improving nasal airflow and increasing airway patency may reduce the hormones involved in the production of urine during the night [11-13]. There is promising outcome data regarding the management of such nasal and airway resistance cases by implementing an orthodontic device that can increase the maxillary width of an average of 5 mm within 2 to 3 weeks [11,13,14].
Maxillary width is an important consideration when working with children and dentofacial morphology, as the maxillary bone articulates with 10 other craniofacial bones affecting maxillary expansion, further influencing the structures of the temporal mandibular joints and those adjacent to the pharyngeal and nasal passages [11-14]. Beyond craniofacial morphology, the eustachian tube should be evaluated and considered through assessment of any hearing changes or frequent otitis media or middle ear infections [12]. This is relevant, as the eustachian tube is an extension of the nasal passage; hence, the same inflammation properties as nasal tissue related to the impact of nasal flow and conductive hearing loss in addition to TMJ dysfunction [12,13]. With the use of a device that can manipulate the teeth, expand the maxilla to establish nasal breathing and increase of the nasal chamber radius by expanding the nasal floor, it is hypothesized and found in studies that the nasal floor, nasal flow and volume will increase by a power of four under Poiseuille’s Law [13].
Rapid Maxillary Expansion (RME) is an option to increase maxillary width where nasal airflow can be achieved and/or increased by way of orthodontics, which can contribute to the treatment of NE by this approach [15-17]. Non-randomized clinical studies and trials were found to show a relevant association between RME in treating NE children. In a systematic analysis and review, six relevant studies with 80 children and a mean age of 118 months found that the median time to become completely dry was 2.87 months, with a Confidence Interval (CI) of 95%, 2.07-2.93 months [9]. After a year, the rate of becoming completely dry was 31% [11]. Within the studies, the presence of a posterior cross bite as a risk factor and signs of upper respiratory obstruction during sleep significantly decreased and increased the chance of improvement [11,12]. There were no other factors that significantly predicted the outcome after any adjustments in a Cox regression model. Conclusively, the research found that rapid palatal expansion could be considered when other treatment modalities have failed, especially with a cure rate of 31% sounding promising, when compared to the spontaneous cure rate [9,11,14]. In the literature review, studies showed that the high level of evidence from a rigorous randomized controlled trial examining the association of rapid maxillary expansion and NE are lacking. This is a first of kind study examining the use of an oral device and the predictability of resolving NE.
Although the information is not publicly available, the retrospective study protocol did not require a review by an Institutional Review Board (IRB) because the study did not directly involve human subjects, the unit of analyses was questionnaire scores from no identifiable data provided by a third party and it did not meet the definition of human subjects. The data set recorded by the author cannot readily be ascertained directly or through identifiers linked to the subjects, the author did not contact the subjects and the author will not re-identify subjects.
The objective of the retrospective study was to examine data scores and the utility of anatomical repositioning of the teeth and jaw as a predictor for early intervention and treatment solution to resolve NE. Secondary data scores were gathered from a sleep, breathing and habit questionnaire; kids intake form; pediatric sleep questionnaire; head and neck exam; child home observation sheet; and patient profile through data mining of 10 members of a team of technicians who work as an established group of experts managing data in a cloud platform. This team of 10 members performed the data mining based on the inclusion and exclusion criteria with primary endpoints for statistical analysis. Inclusion criteria were children between the ages of 5 and 12, who had a current history of bedwetting, consistent use of the mono-block positioner for treatment and concurrent symptoms of SDB. Exclusion criteria were kids below the age of 5 or older than 12 not seeking treatment with the use of a mono- block positioner, a new patient starting treatment who has no data reported, any patient involved in an Investigational Review Board (IRB) clinical trial with another positioning device or any patients with known genetic conditions affecting the airway, such as Down Syndrome. The primary data endpoint was the number of days the bedwetting resolved based on the treatment start date and the age of the child using the mono-block device.
Data collection
Several documents were used in gathering data scores: the sleep, breathing and habit questionnaire; kids intake form; pediatric sleep questionnaire; head and neck exam; child home observation sheet; and patient profile. The forms used were not universal, validated questionnaires. The sleep, breathing and habit questionnaire is a patient-directed form for children and adolescents consisting of 28 questions asking parents to rate symptoms on a scale of 0 (no occurrence) to 3 (occurs 5 to 7 times a week). Bedwetting is listed as one of the questions. The kid’s intake form is a standardized form traditionally used on the first visit listing the reason for the visit and past medical history. The history of NE and bedwetting is listed on the intake form, as well as the symptoms of breathing disorders while sleeping. The pediatric sleep questionnaire provided information on the sleep habits of the child that included bedwetting. The child home observation sheet is a 2-page document asking the parent to journal or log activities for a more complete picture of health.
The mono-block appliance
The mono-block device (Vivos Guide™ Series, Vivos Therapeutics, Inc.) is an FDA-registered, Class I, preformed BPA- free, polymer tooth positioner and orthodontic appliance for patients as young as 4 years of age and up, generally worn for 8 to 10 hours while sleeping. The monoblock oral appliances are used as guided growth and development devices due to their orofacial myofunctional effects that tend to promote nasal breathing, decrease the symptoms of sleep-disordered breathing and reposition and encourage the tongue to sit more up and forward in the mouth (Figure 1). There were no reports of adverse events among the population group.
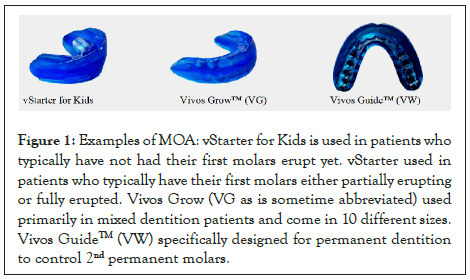
Figure 1: Examples of MOA: vStarter for Kids is used in patients who typically have not had their first molars erupt yet. vStarter used in patients who typically have their first molars either partially erupting or fully erupted. Vivos Grow (VG as is sometime abbreviated) used primarily in mixed dentition patients and come in 10 different sizes. Vivos GuideTM (VW) specifically designed for permanent dentition to control 2nd permanent molars.
Statistical analysis
IBM SPSS Statistics is statistical software used to solve business and research problems by means of ad-hoc analysis, hypothesis testing and predictive analytics. Companies use IBM SPSS statistics to understand data, analyze trends, forecast and plan to validate assumptions and drive accurate conclusions. SPSS software (IBM Corp. Released 2020. IBM SPSS Statistics for Windows, version 28.0. Armonk, NY; IDM Corp.) was used to compute the bivariate linear regression analysis of the predictor variable (age) and the dependent criterion variable (NE resolved in number of days) in this non experimental study design. Four correlation indices were used in the output, with r2 to obtain an index that directly told the predictability of Y from X.
In a retrospective, multicenter, cross-sectional study using secondary data from 55 centers, 548 pre-formed Mono-block Oral Appliance (MOA) device patients were identified. Of the 548 patients, 46% (N=254) had documentation uploaded into the database and 42.1% (N=107) of the patients were identified as bedwetters with SDB symptoms prior to the start of the MOA. Of the 107 identified patients, 37 met the inclusion criteria for the study. The analysis showed that 97.3% of children between the ages of 5 and 12 who were bedwetting and had early intervention with the MOA were more successful in decreasing and resolving with a period after bedwetting. In the study sample, 54% (N=20) had bedwetting resolved within 14 days after starting with Guide™ Series. One valid measurement was absent in the AI system (Table 1).
| Frequency | Percent | Valid percent | Cumulative percent | |
|---|---|---|---|---|
| Resolved | 32 | 84.2 | 86.5 | 86.5 |
| Improved | 4 | 10.5 | 10.8 | 97.3 |
| Not resolved or improved | 1 | 2.6 | 2.7 | 100.0 |
Table 1: Percentage of bedwetting improved or resolved.
Table 2 shows the mean age of MOA therapy was 7.76 years in the successfully responding group. The mean number of days of MOA therapy to resolve and improve bedwetting was 79.72 days or 2.62 months and the mean number of months of guide therapy was 13.86 months. Two outliers were identified at 416 days and 491 days. The outliers impact the variability in measurement and may be excluded, as they differed significantly from the other observations. When considering the two outliers, the mean number of days to resolve was 57.76 days or 1.90 months. Furthermore, the data correlate better than the literature review of 9.83 years and 2.87 months to complete dryness with 14 days. The linear regression analysis indicated with a high probability (p<.001) that the associations of success are largely due to early intervention and the younger age, because the age of bedwetting was correlated with the national average age and gender of the participants. Adjusting the factors and data show that children 8 years and older had a higher increase of failure due to noncompliance and falling out of the therapy regiment. The study sample shows a higher rate of success between the ages of 5 and 7 years.
| N | Minimum | Maximum | Mean | |
|---|---|---|---|---|
| Age | 37 | 5.00 | 12.00 | 7.45 |
| Guide Use in Months | 36 | 1.03 | 34.87 | 13.86 |
| BW Res/Improved in Number of Days | 36 | 0 | 491.00 | 79.72 |
Table 2: Descriptive statistics of the sample.
In the initial literature review, the statistics showed how many children have NE based on age in previous data. In Figure 2, our findings show that of the 107 children reporting NE in the population, 5 to 7 years was much higher at 56.1% (n=60), 8 to 10 years was much higher at 32% (n=34) and at 11 to 12 years was a few percentage points higher at 12% (n=13) compared to the previous data. The results were consistent with previous research showing a prevalence of NE among boys at 71% (n=76) more often than girls at 29% (n=31).
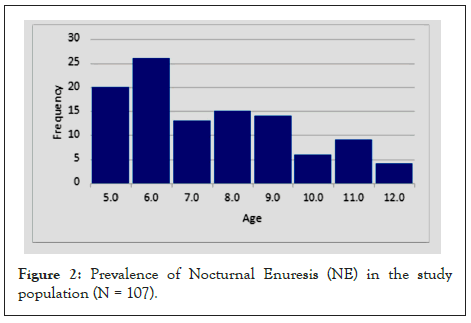
Figure 2: Prevalence of Nocturnal Enuresis (NE) in the study population (N = 107).
The results of the study in Figure 3 show a few similar findings within the sample group using the MOA and NE; children 5 to 7 years was 49% (n=18), 8 to 10 years was 32% (n=12) and 11 to 12 years was a few percentage points higher at 19% (n=7) compared to the previous data in the literature. The results were consistent with previous research showing a prevalence of NE among boys at 70% (n=26) more often than girls at 30% (n=11).
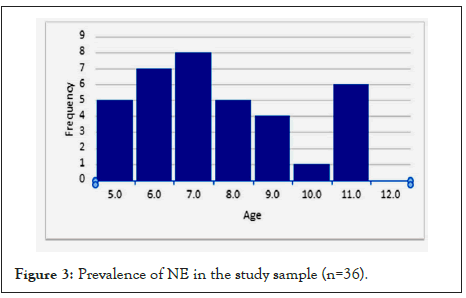
Figure 3: Prevalence of NE in the study sample (n=36).
A bivariate linear regression analysis was conducted to evaluate the prediction of the MOA resolving bedwetting. The scatterplot for the two variables, as shown in Figure 4, indicates that the two variables are linearly related, such that as overall use of the MOA increases, the bedwetting decreases and is resolved. The r2 value of 0.092 indicates that the regression predicts the early intervention of MOA improving and resolving NE. The regression equation for predicting the overall resolve of bedwetting is Bedwetting (NE) resolved=Overall MOA use +4.35.
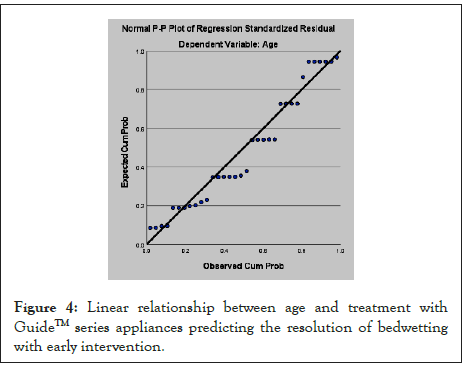
Figure 4: Linear relationship between age and treatment with GuideTM series appliances predicting the resolution of bedwetting with early intervention.
Figure 5 shows the correlation between the age of the child and the effectiveness of using the MOA in resolving bedwetting, 47% (n=34) of the population sample.
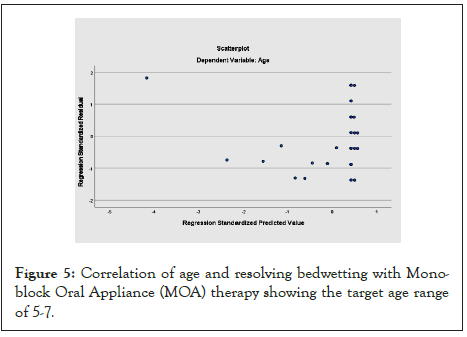
Figure 5: Correlation of age and resolving bedwetting with Mono- block Oral Appliance (MOA) therapy showing the target age range of 5-7.
NE is known to cause a considerable amount of embarrassment and distress to a patient and their family, requiring intervention that is predictable for a successful outcome. The opinion that children will grow out of an enuretic state is true; however, it is unreasonable to assume that NE will resolve on its own without appropriate treatment in the presence of breathing disorders while sleeping. Many modalities of treatment have been available to resolve bedwetting, to include alarms, urocath therapy, behavior modification, pharmaceuticals and surgery to resolve SDB, if there is adenotonsillar hypertrophy. Within the current day literature review, there is limited information, if any, on a successful method for treating bedwetting in children with SDB and limited data on the use of an oral device improving bedwetting in children. In fact, there are no FDA-approved oral devices to help in resolving and reducing bedwetting episodes [18].
The data in the study demonstrate predictability and promising outcomes with early intervention using the MOA to resolve bedwetting. In addition to resolving bedwetting, children can receive additional the positive benefits of the technology. Furthermore, the study allows for further research and clinical trials towards an FDA clearance of the MOA for use in NE in the presence of SDB. Limitations to the study were the retrospective nature of the study and the secondary analysis of the data (access amount of data and consistency of the data collection). There is also bias resulting from use of questionnaires and possible selection bias, as these children were all chosen from dental offices where children with dentofacial issues are likely to present [19-24].
The current spontaneous resolution rate for NE in the literature review is about 15% per year in the general pediatric population; however, the data outcomes from this study suggest that the likelihood of resolution from NE with early MOA intervention is more likely to be 97.3% [24-27]. These findings represent a potentially significant advancement in this novel treatment modality, considering the emotional impact this disorder has on children and their families.
Limitations of this study include the secondary nature of the analyses, the subjectivity of the answers and the retrospective nature of the questionnaire scores, which can have an emotional component at the time the parent completes the form. Nevertheless, despite the study limitations, these findings are compelling.
Current knowledge/study rationale: The current market for treating bedwetting in children because of SDB and apnea is limited to surgical intervention, pharmaceutical intervention and alarm systems. This retrospective study is the first of its kind that examines the use of an oral appliance device as an alternative to the current modalities of treatment and therapy.
Methods: The use of a mono-block tooth positioner allows an alternative early intervention and care to resolve NE. By resolving bedwetting as a symptom of breathing disorders during sleep, the quality of life and sleep habits for children can be improved.
Bedwetting has been recognized as a symptom of SDB treated by many behavioral and pharmaceutical therapies. Tonsillectomy and adenoidectomy surgery is also considered an option for treating NE in children with SDB. In recent years, orthodontics and dentistry have recognized the effects of orthodontic treatments and appliances that can change the craniofacial morphology of children, while showing improvement in treating bedwetting and other SDB symptoms. What is yet to be determined is if this effect is sustained into adulthood. Abolishing NE sooner while treating SDB may result in improvement of self-esteem and potentially quality of life of affected children and their families. Further research is required to determine best potential candidates for such therapy and predictors of success.
The author wishes to thank Manisha Witmans, MD; Vivos’ providers Jonelle Chrichton, DDS; Stacey Kreuz, DDS; Bahar Esmaili, DDS; Eileen Saunders, DDS; Hal Stewart, DDS, FACD; Kelly HeeJung Kim, DDS; Kevin Goles, DDS, DASBA; and Merrily Sandford, DDS. Thank you to Corby Dixon for his valuable contributions in obtaining and collating the data for the study.
Funding
There was no financial support for the study. Dr. Davidson is contracted medical advisor and research consultant for Vivos Therapeutics, Inc.
[CrossRef] [Google scholar] [Pubmed]
[Cross Ref] [Google scholar] [Pubmed]
[CrossRef] [Google scholar] [Pubmed]
[CrossRef] [Google scholar] [Pubmed]
[CrossRef] [Google scholar] [Pubmed]
[CrossRef] [Google scholar] [Pubmed]
[CrossRef] [Google scholar] [Pubmed]
[Google scholar] [Pubmed]
[CrossRef] [Google scholar] [Pubmed]
[CrossRef] [Google scholar] [Pubmed]
[CrossRef] [Google scholar] [Pubmed]
[CrossRef] [Google scholar] [Pubmed]
[CrossRef] [Google scholar] [Pubmed]
[CrossRef] [Google scholar] [Pubmed]
[CrossRef] [Google scholar] [Pubmed]
[CrossRef] [Google scholar] [Pubmed]
[CrossRef] [Google scholar] [Pubmed]
[CrossRef] [Google scholar] [Pubmed]
[CrossRef] [Google scholar] [Pubmed]
[CrossRef] [Google scholar] [Pubmed]
Citation: Davidson KP (2022) The Association of Nocturnal Enuresis and Breathing Disorders in Children with Sleep Disordered Breathing: A Retrospective Review of Pediatric Cases Treated with a Preformed Monoblock Oral Appliance. J Sleep Disord Ther. 11:359.
Received: 05-Jan-2022, Manuscript No. JSDT-22-15413; Editor assigned: 07-Jan-2022, Pre QC No. JSDT-22-15413 (PQ); Reviewed: 21-Jan-2022, QC No. JSDT-22-15413; Revised: 24-Jan-2022, Manuscript No. JSDT-22-15413 (R); Published: 31-Jan-2022 , DOI: 10.35248/ 2167-0277.22.11.359
Copyright: © 2022 Davidson KP. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.