Journal of Drug Metabolism & Toxicology
Open Access
ISSN: 2157-7609
ISSN: 2157-7609
Research Article - (2014) Volume 5, Issue 3
14-Aryl/Alkyl-14H-dibenzo[a,j]Xanthenes and Nitriles were synthesized by a green, simple and highly efficient one pot synthetic method. The dehydration of 2-napthol with aldehyde leads to xanthenes and aldoximes to nitriles catalysed by KHSO4-SiO2 in neat and ultrasonication to afford the title compounds in high yields and relatively short reaction times.
Keywords: Ultra-sonication, KHSO4-SiO2, Dibenzo [a,j] Xanthenes, Nitriles
Heterocyclic compounds synthesis always ever green in the field of organic chemistry, due to those compounds having broad spectrum of biological applications [1]. Among them, xanthenes are important class of oxygen heterocycles, those possess diverse pharmacological activities such as antiviral [2], antibacterial [3], anti-inflammatory activities [4] and sensitizers in photodynamic therapy for abolishing the tumour cells [5]. Moreover, these xanthene compounds can be used in laser technology [6] and also used as antagonists for the paralyzing exploit of zoxazolamine [7], pH-sensitive luminous materials for imagining of biomolecules [8] and dyes [9]. Up to now, several protocols have been reported in the literatures for the synthesis of xanthenes (Figure 1), these are mostly attained by cyclocondensation of 2-tetralone with 2-hydroxyaromatic aldehyde [10], trapping of benzynes by phenol [11], cycloacylation of carbamates [12], and phenyl carbonyl coupling reaction of benzaldehyde and acetophenone [13]. Other methods also involve reaction of 2-naphthol with aldehydes [14], aldehyde acetal [15], carbon monoxide [16], formamide [17], and 2-naphthol-1-methanol [18]. However, these methods have some drawbacks such as poor yields, prolonged reaction times, toxic organic solvents, excess reagents, catalysts and harsh reaction conditions. Thus, the development of an alternate milder and clean procedure is highly demanding for the synthesis of benzoxanthenes, which surpasses those limitations.
Nitriles are important class of organic compounds which are widely used as pharmaceuticals, agricultural chemicals, dyes, and material sciences [19]. In spite of extensive application of nitriles, several methods have been reported for the preparation of these interesting compounds. Recent methods which have been successfully applied to synthesis of nitriles, by using both aliphatic and aromatic aldehydes which is catalysed by expensive reagents such as 2,4-dinitrophenyl hydroxylamine [20], hydroxylamine o-sulfonic acid [21], hazardous (selenium dioxide) [22] or corrosive (formic acid) [23]. There are also some reports for the direct conversion of aldehydes to nitriles using different reagents [24-26] such as, hydroxylamine in toluene in the presence of pyridine/selenium dioxide/phosphoric acid/ hydroxylamine hydrochloride/formic acid or MgSO4/p-toluenesulfonic acid [24], N-tosylimines [25], or trimethylsilyl azide [26]. And also synthesis of nitriles from aldoximes is one of the important transformation in organic synthesis [27]. Nevertheless, most of these methods suffer from limitations such as harsh reaction conditions, extensive reaction times, moderate yields, application of hazardous solvents and/or tedious workup procedures. Therefore, finding an efficient and a capable protocol for the preparation of nitriles is of obvious importance.
Now a day’s solid supported reagents have been much explored as stoichiometric reagents and catalysts in organic synthesis [28]. However, their development and applications in organic synthesis are undergoing a incredible renaissance at present, which is certainly being fuelled by the special necessities of combinatorial and green chemistry [28]. KHSO4-SiO2 is class of solid supported reagents which is mild, nonvolatile and non-corrosive recyclable, cost-effectiveness, eco-friendly reagent which has been used for the synthesis of bisindolylmethanes as well as deprotection of alcohols form esters.
The removal of toxic, unstable organic solvents and reduce the cost in organic synthesis is also the most important goal in ‘green chemistry’. Due to the above findings and in continuation of our interest in the development of efficient, economical and new methodologies [29-31], here in we wish to introduce KHSO4-SiO2 as a highly efficient catalyst for the facile and green synthesis of benzaoxaxanthenes and nitriles in ultrasonication and solvent free method. Using this catalyst, the dehydration reactions of benzaoxaxanthenes and nitriles are afforded in high yields and relatively short reaction times.
To the best of our knowledge in the open literature, one-pot synthesis of benzoxanthenes and nitriles catalyzed by KHSO4-SiO2 has not been reported.
All the chemicals were purchased from Sigma-Aldrich and used without further purification. The progress of the reactions was monitored by thin layer chromatography (TLC) using silica gel 60 F254 (pre-coated aluminium sheets) from Merck. 1H NMR and 13C NMR spectra were obtained in CDCl3 on a Varian 300 MHz NMR spectrometer by using TMS as an internal standard. Infrared Spectra (ν max in cm-1) were recorded as KBr pellets on a Perkin -Elmer, FT-IR 100 spectrophotometer. E.S.I. Mass spectra were recorded on API-3000 mass spectrometer. Sonication was performed using Bandelin Sonorex with a frequency of 35 KHz and a nominal power 200W ultrasonic bath for ultrasonic irradiation built in heating 30-80°C thermostatically adjustable. The reaction vessel placed inside the ultrasonic bath containing water.
General procedure for the synthesis of dibenzo [a,j] xanthene (3a-k)
To a mixture of 2-napthol (2 mmol) and benzaldehyde (1 mmol), KHSO4-SiO2 (1 mol%) was added and the resulting mixture was sonicated at 40-45°C. The progress of the reaction was monitored by TLC. After completion of the reaction, the reaction mixture was poured into distilled water and stirred for 5 min. The crude product was collected by filtration under suction, washed with water and recrystallized from hot ethanol to afford pure benzaoxaxanthene derivatives. In order to recover the catalyst totally, the filtrate was dried under vacuum and washed with diethyl ether and reused after drying under vacuum. The physical and spectral data of known compounds (3a-g & 3i-k) were found to be in agreement with the reported data [32] while the characterization data of newly synthesized product (3 h) are given below.
Spectroscopic data for new compounds
14-(2,4-Dimethoxy)-14H-dibenzo[a,j]xanthene (3 h): Yield: 85%; m.p. 180-182ºC; IR (KBr, cm-1): ? 3157, 1412, 1231, 820, 746; 1HNMR (300 MHz, CDCl3, δ /ppm): 8.28-7.14 (m, 14H, Ar-H), 6.42 (s, 1H, Ar-CH), 4.12 (s, 3H, -OCH3), 3.86 (s, 3H, -OCH3); 13C NMR (75MHz, CDCl3, δ/ppm): 157.2, 156.2, 152.4, 146.1, 142.6, 131.3, 128.5, 127.4, 126.2, 125.8, 122.1, 122.1, 117.6, 114.3, 125.1, 50.2, 46.1; MS (EI) m/z /%: 420 (80) (M+2).
General Procedure for the synthesis of nitriles (6a-k)
A mixture of aldaoxime (2 mmol) was added to a catalytic amount of KHSO4-SiO2 and the resulting mixture was sonicated at 40-45°C. The progress of the reaction was monitored by TLC. After completion of the reaction, the reaction mixture was poured into distilled water and stirred for 5 min. The crude product was collected by filtration under suction, washed with water and recrystallized from hot ethanol to afford pure nitriles. In order to recover the catalyst totally, the filtrate was dried under vacuum and washed with diethyl ether and reused after drying under vacuum. All of the reported compounds were identified by their physical and spectral data as follows.
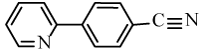
4-(Pyridine-2-yl)benzonitrile (6i): Yield: 86%; m.p. 94-96°C; 1H NMR (300 MHz, CDCl3, δ / ppm): 8.69 (1H, d, J=2.4, Hz, Ar-H ), 8.17 (2H, d, J=8.1 Hz, Ar-H), 7.86-7.72 (4H, m, Ar-H), 7.37 (1H, t, J=5.5 Hz, Ar-H); 13C NMR (75MHz, CDCl3, δ / ppm): 151.1, 149.9, 142.7, 137.2, 133.0, 127.3, 124.5, 121.6, 119.1, 112.3; HRMS (EI) Calcd for C12H8N2+H: 181.0766; Found:181.0762.
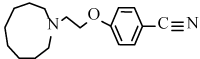
4-[2-(1-azonanyl)ethoxy]benzonitrile (6j): Yield: 92%; m.p. 113- 115 ºC; 1H NMR (300 MHz, CDCl3, δ / ppm): 7.52 (2H, d, J=8.4 Hz, Ar-H), 6.76 (2H, d, J=8.4Hz, Ar-H), 4.29 (2H, t, J=6.3Hz, CH2), 2.93 (2H, t, J=6.3Hz, CH2), 2.89-2.77 (4H, m, N(CH2)2), 1.75-1.61 (12H, m, CH2); 13C NMR (75MHz, CDCl3, δ / ppm): 159.4, 149.1, 128.3, 126.1, 115.6, 66.7, 57.3, 55.6, 28.2, 26.9, 26.0; MS (EI) m/z /% : 273 (25), (M+1) + and 147 (100).
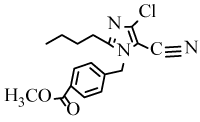
Methyl 4-[(2-butyl-4-chloro-5-cyano-1H-1-imidazolyl)methyl] benzoate (6k): Yield: 93%; m.p. 147-149ºC; 1H NMR (300 MHz, CDCl3, δ / ppm): 8.01 (2H, d, J=8.1 Hz, Ar-H), 7.21 (2H, d, J=8.1 Hz, Ar-H), 5.26 (2H, s, CH2), 3.94 (3H, s, OCH3), 2.63 (2H, t, J=7.5 Hz, CH2), 1.67- 1.58 (2H, m, CH2), 1.37-1.25 (2H, m, CH2), 0.89 (3H, t, J=7.2 Hz, CH3); 13C NMR (75MHz, CDCl3, δ / ppm): 167.1, 160.9, 153.2, 142.1, 139.2, 131.6, 129.7, 126.6, 124.5, 109.3, 57.0, 33.4, 24.7, 22.5, 15.1; MS (EI) m/z /%: 354 (100), (M+Na)+.
Biological activity
The antibacterial and antifungal activities of the test compounds were evaluated by the disc diffusion method20 and their effect was compared with the standard antibiotic Ampicillin and antifungal agent Nystatin. The antibacterial role of all the title compounds (3a–k) was assayed against the growth of Escherichia coli and Pseudomonas aeuroinosa at two different concentrations, 100 μg disc-1 and 250 μg disc-1 (Table 1). The majority of the compounds exhibited moderate activity against both bacteria. Ampicillin was used as a standard reference compound to compare the activities of these compounds. The compounds (3a–k) (Table 1) were screened for their antifungal activities against Aspergillus niger and Fusarium moniliforme along with the standard fungicide Nystatin. It is gratifying to observe that all the compounds (3a–k) exhibited moderate antifungal activity compared with that of the reference compound.
| Compound | Zone of inhibition/mm | |||
|---|---|---|---|---|
| Escherichia coli, Pseudomonas aeuroinosa | Aspergillusniger, Fusarium moniliforme | |||
| 100 μg disc-1 | 100 μg disc-1 | 100 μg disc-1 | 100 μg disc-1 | |
| 3a | 7 | 10 | 9 | 11 |
| 3b | 9 | 9 | 11 | 12 |
| 3c | 10 | 6 | 13 | 13 |
| 3d | 8 | 9 | 12 | 12 |
| 3e | 9 | 8 | 15 | 16 |
| 3f | 8 | 7 | 10 | 15 |
| 3g | 6 | 9 | 8 | 12 |
| 3h | 9 | 8 | 10 | 10 |
| 3i | 7 | 9 | 11 | 12 |
| 3j | 10 | 12 | 14 | 10 |
| 3k | 11 | 13 | 12 | 10 |
| Ampicillin | 20 | 21 | - | - |
| Nystatin | - | - | 25 | 23 |
Table 1: Percentage of inhibition for compounds 3a-3k at concentration of 100 μg disc-1.
In our initial experiment, to optimize the reaction conditions we studied the condensation reaction between benzaldehyde and 2-naphthol (mole rate 1:2) as a simple model catalytic amount of KHSO4-SIO2, under different conditions (Table 2). We found that the best result was obtained when the reaction was carried out under ultrasonication (Table 2, entry 5). In the absence of catalyst, the reaction was carried out in low yield under the same conditions (Table 2, entry 6).
| Entry | Solvent | condition | t | Yielda(%) |
|---|---|---|---|---|
| 1b | CH2Cl2 | Reflux | 6h | 45 |
| 2b | THF | Reflux | 8h | 50 |
| 3 | Neat | 60°C | 4h | 62 |
| 4 | Neat | 80°C | 2h | 70 |
| 5c | Ultrasonication,neat | 200W | 8min | 96 |
| 6c,d | Ultrasonication,neat | 200W | 8min | 25 |
aThe yields refer to the isolated pure products.
bThe reaction was carried out in 5 mL of solvent.
cThe reaction was irradiated by using ultrasonicator laboratory system.
dThe reaction was carried out in the absence of KHSO4-SiO2.
Table 2: The reaction between 2-naphthol and benzaldehyde using KHSO4-SiO2 as a catalyst under different conditions.
With the optimized conditions in hand, to explore the generality of the reaction, we extended our study with different aromatic and aliphatic aldehydes to prepare a series of dibenzo [a,j] xanthenes (3a– k) (Table 3). In all the cases the corresponding benzoxanthenes were obtained in good to excellent yields. However, aromatic aldehydes with electron-withdrawing groups as substrates, the reaction time is shorter than those with electron donating groups. Surprisingly, when piperzaine-1,4-dicarbaldehyde (4) was used, 4-(14H-dibenzo[a,j] xanthene-14-yl) piperazine-1carbaldehyde (3j) was found to be the produced in excellent yield. When we used 4 molar equivalents of 2-napthol, 7-(1-(14H-dibenzo[a,j] xanthene-14-yl) piperidin-4-yl)-7Hdibenzo [ c,h] phenoxazine (3k) was obtained in excellent yield.
| Entry | Product | R | Time (min) | Yield (%) | Melting Point, °C | |
|---|---|---|---|---|---|---|
| Found | Literature | |||||
| 1 | 3a | 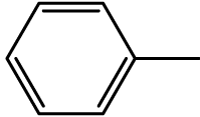 |
8 | 96 | 183-185 | 183-184[33] |
| 2 | 3b | 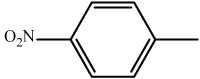 |
15 | 92 | 310-312 | 311-312[33] |
| 3 | 3c | 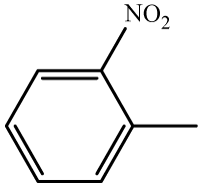 |
22 | 96 | 213-215 | 214-215[33] |
| 4 | 3d | 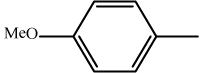 |
18 | 91 | 226-228 | 227-228[33] |
| 5 | 3e | 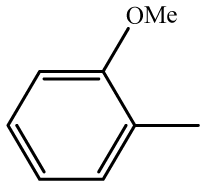 |
24 | 95 | 259-261 | 258-259[33] |
| 6 | 3f | 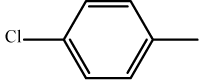 |
14 | 90 | 291-293 | 289-290[33] |
| 7 | 3g | 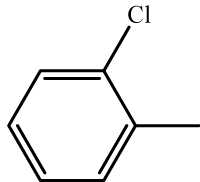 |
20 | 93 | 213-215 | 214-215[33] |
| 8 | 3h | 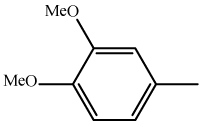 |
30 | 85 | 180-182 | - |
| 9 | 3i | 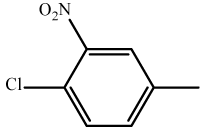 |
20 | 96 | 177-179 | 178 -179[32] |
| 10 | 3j | 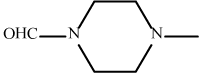 |
28 | 83 | 183-185 | 180-182[32] |
| 11 | 3k | 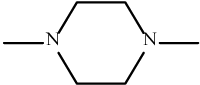 |
30 | 92 | 186-189 | 185 -187[32] |
Table 3: Synthesis of xanthenes catalyzed by KHSO4-SiO2 from 2-naphthols and aldehydes under ultrasonication.
After the successful application of KHSO4-SIO2 as a heterogeneous acidic catalyst in the synthesis of dibenzo [a,j] xanthenes, we decided to use it in the dehydration of aldoximes to nitriles in neat medium under ultrasonication (Figure 2).
In order to optimize the reaction conditions for the synthesis of nitriles as a model reaction, the dehydration of aldaoxime (1mmol) was added to catalytic amount of KHSO4-SIO2 examined using under different conditions. The results are summarized in (Table 4).
| Entry | Solvent | condition | t | Yielda(%) |
|---|---|---|---|---|
| 1b | CH2Cl2 | Reflux | 6h | 39 |
| 2b | THF | Reflux | 8h | 51 |
| 3 | Neat | 60°C | 4h | 64 |
| 4 | Neat | 80°C | 2h | 72 |
| 5c | Ultrasonication, neat | 200W | 40min | 96 |
| 6c,d | Ultrasonication, neat | 200W | 40min | 25 |
aThe yields refer to the isolated pure products.
bThe reaction was carried out in 5 mL of solvent.
cThe reaction was irradiated by using ultrasonicator laboratory system.
dThe reaction was carried out in the absence of KHSO4-SiO2
Table 4: Conversion of aldoximes into nitriles using KHSO4-SiO2 as a catalyst under different conditions.
Efficiency of the catalyst in the synthesis of nitriles with different aromatic and aliphatic aldoximes was made to react (Table 5). As Table 5 indicates, aromatic aldehydes possessing electron-releasing substituents and electron-withdrawing substituents on their aromatic rings afforded the corresponding products in high yields and relatively short reaction times (Table 5, entries 6b-6h). However, when aromatic aldehydes possessing electron-releasing substituents were used, the reaction times were slightly longer in comparison with the others (Table 5, entries 6e, 6f, 6g and 6h). Heteroaromatic aldoximes also efficiently dehydrated and resulted corresponding nitriles with high yields. (Table 5, entries 6i-6k).
| Entry | Substrate | Product | Yield (%) | t(min) | Melting Point, °C | |
|---|---|---|---|---|---|---|
| Found | Literature | |||||
| 6a | 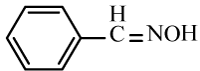 |
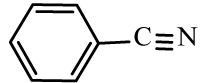 |
90 | 35 | Oil | - |
| 6b | 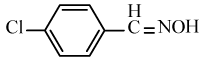 |
 |
95 | 26 | 91-93 | 90-92[34] |
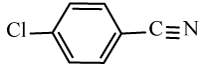
| 6c | 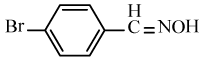 |
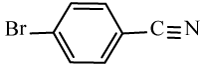 |
98 | 20 | 114-116 | 110-112[34] |
| 6d | 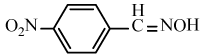 |
 |
97 | 22 | 146-149 | 143-145[34] |
| 6e | 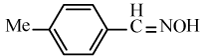 |
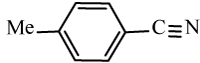 |
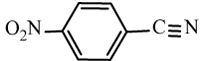
89 | 28 | Oil | - |
| 6f | 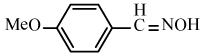 |
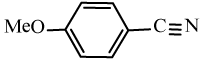 |
96 | 30 | 60-62 | 57-59[34] |
| 6g | 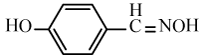 |
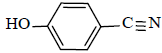 |
88 | 40 | 111-113 | 110-112[34] |
| 6h | 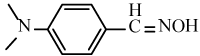 |
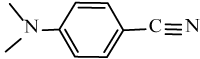 |
90 | 34 | 77-79 | 75-77[35] |
| 6i | 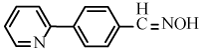 |
 |
86 | 39 | 94-96 | 91-92[36] |
| 6j | 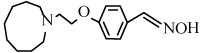 |
 |
92 | 38 | 113-115 | - |
| 6k | 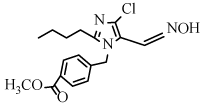 |
 |
93 | 39 | 147-149 | - |
Table 5: Conversion of aldoximes into nitriles using KHSO4-SiO2 as a catalyst under Ultrasonication.
The reusability of the KHSO4-SIO2 was also examined for the preparation of 14-phenyl-14H-dibenzo[a,j]xanthenes (3a) and Nitriles (6a). The catalyst was easily recovered by the addition of ethyl acetate to the reaction mixture; the insoluble KHSO4-SIO2 could be separated by simple filtration, washed twice with ethyl acetate (30 mL), and finally dried by oven at 100°C. The catalyst displayed good reusability after 4 runs (Figure 3).
KHSO4-SIO2 is an inexpensive, non-corrosive, and environmentally benign compound. A convenient and efficient procedure for the preparation of 14-aryl or hetero alkyl-14H-dibenzo[a,j] xanthenes and nitriles in good yields and short reaction times was reported in this work. The notable advantages of this methodology are operational simplicity, generality, availability of reactants, short reaction times and easy work-up.
The authors are thankful to the management of Yogananda Institute of Technology & Sciences, Tirupati, India and also we thank the Malaysian government and Universiti Sains Malaysia for short term grant [304/PKIMIA/6312051] to conduct the following research. Muralikrishna thanks to USM for financial support by way of a fellowship.