Journal of Theoretical & Computational Science
Open Access
ISSN: 2376-130X
ISSN: 2376-130X
Research Article - (2017) Volume 4, Issue 2
In this research work, Influence of Fe and Ni dopant on structural Ba0.6Sr0.4TiO3 nanocrystalline was studied. The pure and (Fe and Ni)-doped Ba0.6Sr0.4TiO3 (Ba1-xSrxTiO3, where (x=0.4), Ba1-xSrxTi1-yFeyO3, where (x=0.4, y=0.1) and Ba1-xSrxTi1-yNiyO3, where (x=0.4, y=0.1)) in powder, abbreviated as (BST), (BSTF) and (BSTN), respectively were prepared by a modified Sol-gel technique. In this process, stoichiometric proportions of barium acetate and strontium acetate were dissolved in acetic acid and titanium (IV) isopropoxide was added to form BST gel. The as-formed gels were dried at 200°C and then calcined at 850°C for crystallization. In order to improved crystallization, surface morphology and optimized grain size used Fe+3 and Ni2+ ions. Samples were characterized by Infrared spectroscopy method (FT-IR), UV-Visible spectroscopy, X-Ray Diffraction technique (XRD), Field Emission Scanning Electron Microscope (FESEM) and Energy Dispersive X-Ray Spectroscopy (EDS). The Nano-scale presence and the formation of the cubic perovskite phase as well as the crystallinity were detected using the mentioned techniques. The results showed that, the adding Fe and Ni to BST structure lead to decrease of the average size of nanoparticles and change the optical properties of the BST. The nanocrystallite sizes obtained were nearly 38, 37 and 34 nm, for BST, BSTF and BSTN powders calcined at 850°C, respectively.
<Keywords: Sol-gel; Ba0.06Sr0.4TiO3; Nanocrystalline; Iron doping; Nickel doping
Barium-Strontium Titanate (BST) is an important ceramic oxide with the perovskite structure and stoichiometric formula BaxSr1−xTiO3 . Recently, barium strontium titanate (BST) have been widely investigated because of its high dielectric constant, nonlinear variation of dielectric constant with the electric field, ferroelectricity, pyroelectric properties and so on. The desired properties make BST a promising candidate material for tunable microwave dielectric devices such as multilayer and voltage-tunable capacitors, dynamic random access memories (DRAM), microwave phase shifters, tunable filters, oscillators, uncooled infrared sensors and etc. [1-3]. Among of all compositions of BST, the Ba0.6Sr0.4TiO3 compoud because of its good combination of low TC, high dielectric constant, relatively low loss tangent and better tunability is a particular importance stoichiometry [4,5].
It is well-known that Ferroelectric and dielectric properties of BST ceramics are strongly dependent on the sintering conditions, grain size, porosity, doping amount of various concentrations and structural defects. Some materials that can improve the listed properties have been tried in the doping. Among these, materials can be noted to Some dopants including, Co2+, Mn2+, Mn3+, Fe+2, Fe+3, Co+2, Co+3, Ni2+, Cr+3, Mg2+, Al3+, Ga3+, etc., which can occupy the B sites of the ABO3 perovskite structure [6-8].
Various preparation methods for BST have been investigated such as solid-state reaction [9], sol-gel [7,10,11], hydrothermal [12,13], spray pyrolysis [14], combustion synthesis [15], and chemical coprecipitation methods [16] and etc. Compared with other methods, solgel process because of its numerous advantages in producing bariumstrontium titanate ceramics has received a strong attention among researchers. Among these advantages, low temperature of this process, possibility for obtaining nanostructures, easy control of microstructure and the crystallization rate of the final product, lower pollution and low cost of operation can be noted [17,18].
In this research, we aim to synthesize nanocrystalline pure BST and (Fe and Ni) co-doping into the BST structure via a modified sol-gel process and investigate the structural of BST samples in powder form, by doping with 10 mole% Fe+3 and Ni2+ ions doped BST ceramics. The Fe+3 and Ni2+ ions substitute at Ti4+ site in BST structure and study of influence of them to microstructure. The structure and the morphology of the prepared samples will be evaluated by using FT-IR, XRD and FESEM.
Nano-structure pure barium strontium titanate, Ba1-xSrxTiO3, where (x=0.4) (BST) and doped with Fe+3 and Ni2+ ions, Ba1-xSrxTi1-yFeyO3, where (x=0.4, y=0.1) (BSTF) and Ba1-xSrxTi1-yNiyO3, where (x=0.4 and y=0.1) (BSTN), in powder forms were prepared, by a modified sol–gel method. The samples were synthesized using barium acetate (ACS reagent grade, 99%, Merck, Germany), strontium acetate (ACS reagent grade, 99.9%, Sigma Aldrich, USA) and titanium (IV) isopropoxide (ACS reagent grade, 99.9%, Sigma Aldrich, USA) as precursors for barium, strontium and titanium, respectively. Acetic acid (Glacial, 99-100% EMPLURA, Merck, Germany) was used as solvent and ethanol (99+%, Merck, Germany) was used to stabilize Titanium (IV) isopropoxide. Iron and Nickel nitrates (ACS reagent grade, 99%, Merck, Germany) using as a precursor for Iron and nickel.
Firstly, Barium acetate and strontium acetate were mixed in a mole ratio of Ba:Sr=6:4, and then dissolved in heated glacial acetic acid with constant stirring. Ethanol was added in Titanium (IV) isopropoxide to form a separate solution at room temperature. After cooling to room temperature, the Ba-Sr solution was added to the as prepared Ti solution drop by drop. Refluxing resulted in the formation of a thick white colored gel. The prepared gel was cooled to 5°C and deionized water was added to it and the solution was stirred magnetically for 60 min. Iron and Nickel nitrate were added to the final solution with molar ratio 10 mole %. Densification of the gels were achieved by sintering in the air for 4 hours at 850°C for BST, BSTF and BSTN samples in a muffle furnace.
The phases of the obtained samples were characterized by X-ray diffraction (XRD) (Philips X-ray diffractometer, 40 kV, 40 mA) in a wide range of Bragg’s angle of 10?-85? using Cu Kα (1.5406 Å) radiation with a step size of 0.02 at room temperature. Microstructure was studied with a field emission scanning electron microscope (Mira 3-XMU). The chemical compounds of samples are characterized by Energy Dispersive X-Ray Spectroscopy (EDS) (EDS microanalyzer in FESEM, Mira 3-XMU). The FT-IR spectra of the calcined powders were recorded with an FT-IR spectrometer (Bruker Vector 33) in the range of 400-4000 cm–1. Also, The UV-Visible absorption spectra of the powder samples were recorded using a UV-VIS spectrophotometer (photonix Ar 2015, Iran) in the spectral region from 200 to 800 nm at room temperature.
FT-IR analysis
Figure 1 shows the IR spectrum of BST, BSTF and BSTN powders calcined at 850°C for 4 hours. FTIR spectra for BST doped with Fe and Ni elements have the same trend for the pure BST sample. The broad band at 3422 cm–1 is related to O-H stretching modes of absorbed water by KBr pellets that were used for FT-IR spectroscopy [19]. The peaks corresponding to barium carbonate were evident at 1630, 1427, 850 and 580 cm–1 [20]. The absorption band at 1427 cm–1 can be interpreted as C=O vibration due to extremely small unavoidable traces of carbonate. It can be seen that as the adding of Fe and Ni to BST structure, the amount of carbonates formed increases. Hence, the result shows that BST nanopowder obtained at 850°C are more pure than BSTF and BSTN at the same temperature. Furthermore, the absorption band at 611 cm–1 is assigned to specific vibrations of Ti–O bonds for pure BST [21]. Also, a shoulder around 954 cm–1 and a dip at 1063 cm-1 appears to be getting more prominent in Fe and Ni doped BST samples corresponding to the formation of new bonds due to dopant Fe and Ni. As can be seen, there are no new peaks appearing for the dopants (Fe3+ and Ni2+) and the only change appeared in the band for Ti-O stretching vibration at 611 cm-1 for pure BST, in which it was shifted to smaller wavenumber cm-1 for BSTF and BSTN, which confirm that Fe3+ and Ni2+ ions completely substitute the Ti4+ ions in the B site of the perovskite structure ABO3 [22,23].
Phase analysis of nano powders
X-ray diffraction was performed to characterize the crystallinity and phase of the nanopowders. Figure 2 shows the XRD patterns of pure BST, BSTF and BSTN calcined in air at 850°C for 4 hours. The XRD patterns indicate that all the samples are crystalline and exhibit cubic perovskite structure with (110) as a major peak, (PDF Card No. 00-034-0411).
As was observed in previous studies, increasing the calcination temperature from 750 to 850°C leads to decompose of impurity phases of barium and strontium carbonate in pure BST [24,25]. But according to the XRD pattern shown in Figure 2, by adding impurity of iron and nickel to BST structure, an amount of impurity phase of barium carbonate remains in the composition calcined at 850°C. As can be seen, in BSTF and BSTN, XRD pattern, a weak line occurs at 24.2 which corresponds to the residual carbonates phase such as BaCO3, SrCO3 and (Ba,Sr)CO3 [25]. This can be confirmed by FT-IR analysis of nano powders calcined at 850°C. However, in all samples, the predominant phase is the cubic perovskite phase of Ba0.6Sr0.4TiO3. The XRD pattern of BSTF and BSTN samples conform to the cubic phase of BST and shows that there are no type of predominant impure phase and no change in the BST structure despite Fe and Ni ions in BST lattice. Due to this it can be concluded that Fe+3 and Ni+2 ions have been substituted at Ti4+ site in BST perovskite structure, appropriately as confirmed by the FT-IR obtain data.
The crystallite size (d) is determined from the Scherrer’s equation:
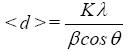 (1)
(1)
where K is the Scherer constant, in the present case K=0.9, λ is the wavelength and β is the full width (in radians) of the peak at half maximum (FWHM). In order to determine the lattice constant, the equation for a cubic crystal was used:
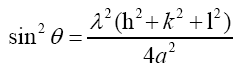 (2)
(2)
where α is the lattice spacing of a cubic crystal and h, k, l are the Miller indices of the Bragg plane.
Tables 1-3 shows details of the calculated X-ray spectrum for the BST, BSTF and BSTN powders calcined at 850°C. The average crystallite sizes of the samples, calculated using Scherrer’s formula for the first four predominant peaks. The results show that when Fe and Ni add to BST structure, the average crystallite size has decreased. As was observed in previous studies [24], increasing the calcination temperature from 750 to 850°C leads to an increase in particle size of BST sample but, by doping Fe and Ni ions to BST despite increasing of the temperature, the crystallite size decrease, which this effect is more obvious for nickel ions.
| h k l | 2θ [deg] | dhkl [Å] | FWHM [deg] | Crystal size [nm] | Lattice parameter [Å] |
|---|---|---|---|---|---|
| 1 0 0 | 22.279 | 3.9869 | 0.1665 | 48.61 | |
| 1 1 0 | 31.826 | 2.8093 | 0.2193 | 37.65 | |
| 1 1 1 | 39.312 | 2.2899 | 0.253 | 33.33 | |
| 2 0 0 | 45.632 | 1.9864 | 0.2748 | 31.35 | |
| Average | 37.74 | ||||
| 3 1 0 | 75.609 | 1.2566 | 3.972 | ||
| 3 1 1 | 80.281 | 1.1948 | 3.961 | ||
| 2 2 2 | 84.378 | 1.1469 | 3.972 | ||
| Average | 3.969 |
Table 1: Details of the calculated X-ray spectrum for BST powder calcined at 850°C.
| h k l | 2θ [deg] | dhkl [Å] | FWHM [deg] | Crystal size [nm] | Lattice parameter [Å] |
|---|---|---|---|---|---|
| 1 0 0 | 22.086 | 4.0199 | 0.1969 | 41.09 | |
| 1 1 0 | 31.555 | 2.8319 | 0.2135 | 38.65 | |
| 1 1 1 | 39.143 | 2.2986 | 0.2288 | 36.84 | |
| 2 0 0 | 45.460 | 1.9928 | 0.2836 | 30.36 | |
| Average | 36.73 | ||||
| 3 1 0 | 75.387 | 1.2568 | 3.9823 | ||
| 3 1 1 | 79.892 | 1.1993 | 3.9775 | ||
| 2 2 2 | 84.117 | 1.1494 | 3.9818 | ||
| Average | 3.9805 |
Table 2: Details of the calculated X-ray spectrum for BSTF powder calcined at 850°C.
| h k l | 2θ [deg] | dhkl [Å] | FWHM [deg] | Crystal size [nm] | Lattice parameter [Å] |
|---|---|---|---|---|---|
| 1 0 0 | 22.122 | 4.0135 | 0.2185 | 37.03 | |
| 1 1 0 | 31.685 | 2.8205 | 0.2253 | 36.64 | |
| 1 1 1 | 39.214 | 2.2946 | 0.3348 | 25.18 | |
| 2 0 0 | 45.562 | 1.9886 | 0.2331 | 36.95 | |
| Average | 33.95 | ||||
| 3 1 0 | 75.483 | 1.2583 | 3.9781 | ||
| 3 1 1 | 79.903 | 1.1991 | 3.9770 | ||
| 2 2 2 | 84.264 | 1.1423 | 3.9761 | ||
| Average | 3.9770 |
Table 3: Details of the calculated X-ray spectrum for BSTN powder calcined at 850°C.
The lattice constant was calculated for the final three peaks for larger angles. This leads to a smaller error in the calculated value of the lattice parameter. The calculated lattice parameter (a) is in good agreement with the result of the X-ray pattern fitting for BST60/40 ceramics (PDF Card No. 00-034-0411) as shown in Table 4. Also, an obvious shift in peaks and an increasing of lattice parameter is observed with doping of Fe and Ni ions in BST that is due to the larger radius of Fe+3 (0.078 nm) and Ni+2 (0.069 nm) ions than Ti+4 (0.0605 nm) ions [26].
| Crystal system | Cubic | Angles (α, β, γ) | 90.00°, 90.00°,90.00° |
| Space group number | 221 | Calculated density | 5.68 |
| Space group | Pm-3m | Volume of cell | 62.33 |
| Lattice parameters | 3.965Å, 3.965Å, 3.965Å |
Table 4: The crystallographic parameters used for the XRD pattern fitting for BST60/40 ceramics.
FESEM and EDS analyses
Figure 3 shows the micro-structure of the nanopowders obtained through field emission scanning electron microscope (FESEM) at high magnifications for pure BST (calcined at 750°C and 850°C) and codoped with Fe (BSTF) and Ni (BSTN) (calcined for 4 hours at 850°C) powder samples in Figures 3a-3d respectively. As was observed in previous studies, increasing the calcination temperature from 750 to 850°C leads to an increase in particle size and increasing of crystallite of BST sample which, the images in Figures 3a and 3b show an increase in grain growth with increasing calcination temperature [24]. Also, Figure 3b shows the particles are nearly cubic in nature and more crystalline. Furthermore, Images in Figures 3c and 3d clearly show that the additive of iron and nickel ions leads to a grain refining and reduce of nanoparticle size and less agglomerated, and they show welldistributed crystallites and dense nanoparticles surfaces at 850°C which, this effect is more obvious for nickel ions. The result is consistent with the results of XRD and FT-IR analyses of the prepared samples.
In order to investigate the composition of the samples, EDS analysis was used. Figure 4 shows the EDS spectra of BST,BSTF and BSTN powders calcined at 850°C. As can be seen, the characteristic X-ray radiation of each element has different energy values with various atomic compositions. In Figure 4a, the presence of Ba, Sr and Ti was detected in the spectra of pure BST, it is clearly seen that the prepared Ba0.06Sr0.4TiO3 ceramic compound is only composed of Ba, Sr, Ti, and O and the pure BST is a dominant phase. Also, Figures 4b and 4c show clearly the presence of Fe and Ni in the BSTF and BSTN compositions, respectively. Table 5 shows Weight % and Atomic % of all elements present in the samples. As can be seen, the addition of iron and nickel in BST structure lead to modifying the stoichiometric ratio of elements of BST compound.
| BST | BSTF | BSTN | |||||
|---|---|---|---|---|---|---|---|
| Element | Line | Weight% | Atomic% | Weight% | Atomic% | Weight% | Atomic% |
| O | Kα | 47.56 | 78.43 | 34.00 | 68.97 | 31.46 | 66.38 |
| Ti | Kα | 29.57 | 16.29 | 31.85 | 21.58 | 29.63 | 20.88 |
| Fe | Kα | ---- | ---- | 1.30 | 0.75 | ---- | ---- |
| Ni | Kα | ---- | ---- | ---- | ---- | 3.74 | 2.15 |
| Sr | Lα | 8.13 | 2.45 | 6.88 | 2.55 | 14.00 | 5.39 |
| Ba | Lα | 14.75 | 2.83 | 25.97 | 6.14 | 21.16 | 5.20 |
| Total | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | |
Table 5: Weight % and Atomic % of all elements present in the BST, BSTF and BSTN samples.
UV-Vis analysis
It is well-known that the grain size of nanoparticles and the amount and type of doping mater are important factors in determining of the electrical and optical properties of the BST [27,28]. In order to obtain the UV spectra of the samples initially, put some of them in methanol, and then place them for 15 minutes in an ultrasonic device to become a uniform disperse solution. Prepared solution was put in the special quartz cells and UV-Vis ray is radiated in the range of 200 to 500 nm. Figure 5 shows the absorption spectra of BST, BSTF and BSTN samples calcined at 850°C. The absorption profiles for all the samples exhibit sharp bands in the UV region. As Figure 5 clearly shows that the adding of Fe and Ni to BST structure lead to displace the edge of the absorption in the UV spectra. The wavelength corresponding to the absorbance edge for BST, BSTF and BSTN nanopowders is 228.32 nm, 231.85 nm and 228.64 nm, respectively. This means that the Fe and Ni dopant can change the optical properties of the BST nanopowders.
The band-gap energy of samples was determined by the Kubelka– Munk model and the Tauc linearization for direct allowed transitions:
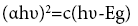 (3)
(3)
where α is the absorption coefficient, hυ is the photon energy and ag is the optical band gap. The optical band gap was determined by extrapolating the linear portion of the plot to α2=0 in the plot of the square of the absorption coefficient versus photon energy [29].
Figure 6 shows the diffusion spectrum (tauc plot) for direct transition of BST, BSTF and BSTN nanopowders calcined at 850°C. The results show that the value of the energy band gap increase with doping Fe in BST structure and decrease with doping Ni, in comparison with pure BST nanopowder. The direct band-gap energy of BST, BSTF and BSTN nanopowders is 4.85, 4.97 and 4.67, respectively. Also, the direct band gap energy of the BST nanopowder was higher than the ones usually found in the literature of the context for BST thin film (around 3.93 eV) [30].
In this paper, the Ba0.06Sr0.4TiO3 (BST), Ba0.6Sr0.4Ti0.9Fe0.1O3 (BSTF) and Ba0.6Sr0.4Ti0.9Ni0.1O3 (BSTN) Nanocrystalline were successfully synthesized by a modified sol-gel technique and studied by XRD, FTIR, UV-Visible, FESEM, EDX techniques. FT-IR spectra and XRD pattern showed present of pure BST structure and un-wanted amount of impure phase of BaCO3 and SrCO3 due to doping Fe and Ni into BST structure. XRD patterns confirmed the cubic structure phase presence of the prepared samples. The average of particle size calculated from the XRD pattern was nearly 38, 36 nm and 34 for BST, BSTF and BSTN for calcination temperatures 850°C, respectively. Also, the XRD pattern showed that the adding Fe and Ni to BST structure lead to an increase of lattice parameter. XRD and FESEM investigation showed that the average grain size of the BST ceramics decreased by adding of Fe and Ni and the microstructure became uniform. EDX analysis confirmed the presence of Ba, Sr, Ti, O, Fe and Ni in the compositions. The effects of Fe and Ni dopant on the optical properties of the BST nanopowders have been studied by UV-Visible spectroscopy. The band-gap energy of BST, BSTF and BSTN nanopowders is 4.85, 4.97 and 4.67, respectively. The result shows that the Fe and Ni dopant can change the optical properties of the BST nanopowders. Additionally, the sol-gel technique applied in this work has can be used to deposit a nano thin film of BST.