Journal of Clinical & Experimental Dermatology Research
Open Access
ISSN: 2155-9554
ISSN: 2155-9554
Review Article - (2020)
Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS) syndrome, also referred to as drug-induced hypersensitivity syndrome, is a distinct, potentially life-threatening adverse reaction. There are 3 sets of criteria that are helping in diagnosis. We present three challenging cases with DRESS syndrome, and discuss the intravenous immunoglobulins in the therapy. IVIG should be given together with corticosteroids. Multidisciplinary approach is the best way in managing patients with DRESS syndrome.
DRESS syndrome; Intravenous immunoglobulins; Eosinophilia
Dress is fairly common drug reaction, although it may be mild and completely resolved without any sequels when the culprit drug withdrawn; but in some cases, it might be life threatening and causing long lasting impairment of vital body systems.
DRESS is abbreviation to Drug Reaction with Eosinophilia and Systemic Symptoms, also known as Drug-Induced Hypersensitivity Syndrome (DIHS). DRESS was first recognized in 1950 by Chaiken, as a sever drug reaction in a patient using anticonvulsant drugs [1], but the term DRESS was introduced by Bocquet et al. [2].
DRESS syndrome is characterized by fever, cutaneous eruption, internal organ involvement, and hematologic abnormalities 1-8 weeks after the administration of the culprit drug [3], with an incidence ranging from 1 in 1, 000 to 1 in 10, 000 exposures in patients taking the drug [4,5].
In 1996, Bocquet et al. [2], proposed its diagnostic criteria for DRESS. In 2006, Shiohara et al. [6], proposed another set of criteria for the diagnosis of Drug-Induced Hypersensitivity Syndrome (DIHS). One year later, in 2007, The European Registry of Severe Cutaneous Adverse Reactions to Drugs and collection of biological samples (RegiSCAR) suggested different diagnostic criteria for DRESS syndrome [7]. These 3 different sets of criteria can make a proper diagnosis and assessment of DRESS syndrome [8].
Bocquet's criteria
Bocquet's criteria require meeting the following 3 features as diagnostic criteria of DRESS [2]:
1) Skin eruption,
2) Blood eosinophilia (>1.5 × 103/μL) or the presence of atypical lymphocytes, and
3) Internal organ involvement, including lymphadenopathies (>2 cm in diameter), hepatitis (liver transaminases values elevated twice the upper normal limit), interstitial nephritis, and interstitial pneumonia or carditis [2].
Japanese group
The criteria established by the Japanese group to diagnose DIHS include the following features [6]:
1) Maculopapular rash developing >3 weeks after starting a limited number of drugs.
2) Prolonged clinical symptoms 2 weeks after discontinuing the causative drug.
3) Fever (>38°C),
4) Elevation of liver enzyme (alanine aminotransferase) ALT (>100 U/L) or involvement of other organs.
5) Leukocytosis (>11 × 103/μL), atypical lymphocytosis (>5%) or eosinophilia (>1.5 × 103/μL).
6) Lymphadenopathy, and
7) Human herpesvirus (HHV)-6 reactivation.
Diagnosis of typical DIHS requires the presence of all 7 criteria, atypical DIHS is diagnosed in patient with 1 to 5 criteria.
Regi SCAR criteria include at least 3 of the following 7 characteristics to establish the diagnosis of DRESS syndrome [7].
1) Skin eruption,
2) Fever (>38°C),
3) Lymphadenopathy at least 2 sites,
4) Involvement of at least 1 internal organ,
5) Lymphocytosis (>4 × 103/μL) or Lymphocytopenia (<1.5 × 103/ μL),
6) Blood eosinophilia (>10% or 700/μL), and
7) Thrombocytopenia (<120 × 103/μL).
Patients were classified into definite, probable, possible or no cases according to the RegiSCAR scoring system [7].
DRESS syndrome is characterized by a long latency (two to eight weeks) between drug exposure and disease onset, a prolonged course with frequent relapses despite the discontinuation of the culprit drug, and frequent association with the reactivation of a latent human herpesvirus infection [2,8]. Skin involvement in DRESS syndrome is a very important sign, it may present with diverse skin rashes [9,10]. Morbilliform eruptions affects 80% of cases (measleslike rash) (Figure 1), otherwise the skin manifestations in DRESS can be as follow:
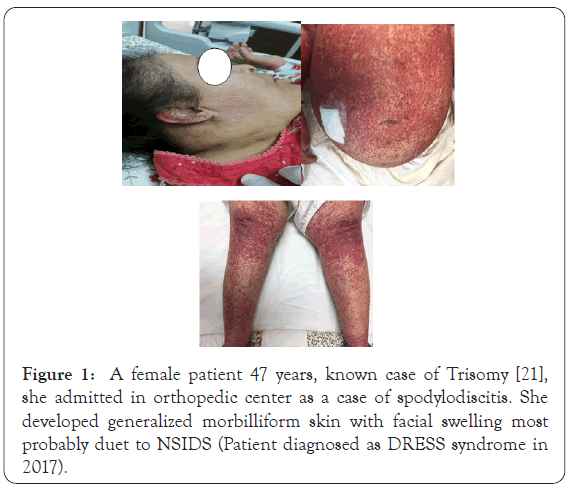
Figure 1: A female patient 47 years, known case of Trisomy [21], she admitted in orthopedic center as a case of spodylodiscitis. She developed generalized morbilliform skin with facial swelling most probably duet to NSIDS (Patient diagnosed as DRESS syndrome in 2017).
1. Exfoliative dermatitis (red peeling skin)
2. Erythroderma occurs in about 10% of cases
3. Facial swelling (30%)
4. Mucosal involvement in 25%
5. Vesicles and bullae
6. Pustules
7. Pupura or target lesions (like Erythema multiforme) and Urticarial lesions
DRESS syndrome symptoms may be severe and involve many different organs. In a retrospective Taiwanese cohort study of 60 patients [11], the following incidences were observed (Tables 1 and 2).
| Organ | Percent of patients with involvement |
|---|---|
| Liver | 80% |
| Kidney | 40% |
| Pulmonary | 33% |
| Cardiac/muscular | 15% |
| Pancreas | 5% |
Table 1: Incidence of organ involvement in DRESS syndrome.
| Abnormality | Percent of patients with abnormality |
|---|---|
| Atypical lymphocyte | 63% |
| Eosinophilia | 52% |
| Lymphocytopenia | 45% |
| Thrombocytopenia | 25% |
| Lymphocytosis | 25% |
Table 2: Incidence of hematologic abnormalities in DRESS syndrome.
The exact mechanism of pathogenesis of DRESS/DIHS is still undetermined but, there are some contributing factors; drugs are the main triggers for DRESS syndrome. DRESS syndrome is considered as an idiosyncratic reaction (type B), the mechanisms involved in this reaction believed to be immune-mediated which caused by immunogenic conjugates formed from the reaction of a reactive metabolite of a drug with cellular proteins. This reaction ultimately leads to immune-mediated toxicity [12]. HHV-6 may be implicated in pathogenesis of DRESS, as its DNA was detected in patients’ serum by polymerase chain reaction [13]. Other additional contributing factors like viral infections and genetic susceptibility may have role in the pathogenesis.
DRESS syndrome as a reaction to some incriminated drugs, is most commonly seen with the use of seven different drug groups [14]:
1. Anticonvulsants, such as the aromatic anticonvulsants (phenytoin, carbamazepine, phenobarbital and primidone), mexiletine, lamotrigine, valproate, ethosuximide, zonisamide.
2. Antidepressants (desipramine, amitriptyline, fluoxetine).
3. Sulfonamides and sulfones (dapsone, sulfasalazine, trimethoprimsulfamethoxazole, salozosulphopyridine).
4. Anti-inflammatory drugs (piroxicam, naproxen, diclofenac, sundilac, phenylbutazone, ibuprofen).
5. Anti-infectives (abacavir, cidofovir, terbinafine, nevirapine, minocycline, linezolid, doxycycline, telaprevir, nitrofurantoin, zalcitabine, spiramycin, metronidazole, piperacillin-tazobactam, ceftriaxone).
6. Angiotensin-converting enzyme inhibitors (captopril, enalapril).
7. Beta-blockers (atenolol, celiprolol). Cases have been reported with allopurinol, gold salts, thalidomide, calcium channel blockers (diltiazem), ranitidine, sorbinil, azathioprine, dobutamine, methimazole, propylthiouracil and efalizumab.
Identification of culprit drugs
Culprit drugs were assessed using Naranjo's scale [15]. Possible or more probable drugs were considered as culprits to induce DRESS syndrome according to this scale. The Naranjo Scale, is a questionnaire designed by Naranjo et al. [15], for determining the likelihood of whether an Adverse Drug Reaction (ADR) is actually due to the drug rather than the result of other factors. Probability is assigned via a score termed definite, probable, possible or doubtful. Values obtained from this algorithm are sometimes used in peer reviews to verify the validity of author's conclusions regarding adverse drug reactions. It is called the Naranjo Scale or Naranjo Score.
DRESS syndrome is characterized by a long latency (two to eight weeks) between drug exposure and disease onset, a prolonged course with frequent relapses despite the discontinuation of the culprit drug, and frequent association with the reactivation of a latent human herpesvirus infection [2,8]. Skin involvement in DRESS syndrome is a very important sign, it may present with diverse skin rashes [9,10]. Morbilliform eruptions affects 80% of cases (measleslike rash) (Figure 1), otherwise the skin manifestations in DRESS can be as follow:
1. Are there previous conclusive reports on this reaction?
Yes (+1) No (0) Do not know or not done (0)
2. Did the adverse events appear after the suspected drug was given?
Yes (+2) No (-1) Do not know or not done (0)
3. Did the adverse reaction improve when the drug was discontinued or a specific antagonist was given?
Yes (+1) No (0) Do not know or not done (0)
4. Did the adverse reaction appear when the drug was readministered?
Yes (+2) No (-1) Do not know or not done (0)
5. Are there alternative causes that could have caused the reaction?
Yes (-1) No (+2) Do not know or not done (0)
6. Did the reaction reappear when a placebo was given?
Yes (-1) No (+1) Do not know or not done (0)
7. Was the drug detected in any body fluid in toxic concentrations?
Yes (+1) No (0) Do not know or not done (0)
8. Was the reaction more severe when the dose was increased, or less severe when the dose was decreased?
Yes (+1) No (0) Do not know or not done (0)
9. Did the patient have a similar reaction to the same or similar drugs in any previous exposure?
Yes (+1) No (0) Do not know or not done (0)
10. Was the adverse event confirmed by any objective evidence?
Yes (+1) No (0) Do not know or not done (0)
Scoring
• ≥ 9=definite ADR
• 5-8=probable ADR
• 1-4=possible ADR
• 0=doubtful ADR
DRESS Syndrome associated with Allopurinol
Allopurinol is often given drug to patients suffering from hyperuricemia, but it carries the highest mortality risk between drugs causing DRESS syndrome [16].
The accumulation of oxypurinol especially with reduced renal clearance leads to a greater risk of developing DRESS syndrome. Multiple studies have shown that advanced age, underlying renal impairment, higher doses, and concomitant use of thiazide diuretics are potential risk factors for developing allopurinolinduced DRESS syndrome [17]. However, febuxostat, a relatively new agent on the market, is a promising surrogate of allopurinol. This agent is the first one marketed in the United States to manage the hyperuricemia of gout since allopurinol was approved in 1964. Febuxostat was approved for medical use in the European Union in 2008 and in the United States in 2009 [18].
A genetic deficiency of detoxifying enzymes leading to an accumulation of drug metabolites, which bind to cell macromolecules causing cell death or inducing secondary immunological phenomena. Eosinophilic activation as well as activation of the inflammatory cascade may be induced by interleukin-5 release from drug-specific T-cells [19]. Genetic associations between Human Leukocyte Antigen (HLA) associations and drug hypersensitivity may occur. These include HLA-B*1502, associated with carbamazepine induced Stevens-Johnson syndrome (SJS) and Toxic Epidermalnecrolysis (TEN) [20]. Furthermore, the association of CBZ-induced drug hypersensitivity reactions seems to be phenotype-specific [21].
A possible virus-drug interaction associated with viral reactivation may also exist. This phenomenon has been previously observed for herpes viruses and Epstein-Barr virus [22]. The sequential reactivations of several herpes viruses (HHV-6, HHV-7, EBV, and cytomegalovirus) can be detected coincident with the clinical symptoms of drug hypersensitivity reactions 6.The pattern of the herpes virus re-activation was noted to be similar to that observed in Graft-Versus-Host Disease (GVHD), thus suggesting that DRESS may resemble GVHD in the sense that antiviral T-cells can cross-react with the drugs and do not only arise from the oligoclonal expansion of drug-specific T-cells [9], that viral reactivation may provide a "danger signal" (danger sign) that stimulates massive clonal expansion of both CD8+ and CD4+ non-specific T cells and causes the complete development of the syndrome [23].
DRESS syndrome severity
DRESS syndrome severity was assessed using the duration of clinical manifestations (clinical symptoms and laboratory abnormalities after treatment), requirement for systemic steroid, or fatality [24]. So there are many factors that may affect DRESS severity and prognosis which was analyzed by Kim et al. [25,26].
Factors contributing to DRESS syndrome severity
Non-fatal cases: Different factors which may be contributing the clinical severity of DRESS syndrome. A study had analyzed 48 patients who satisfied Bocquet's criteria. The duration of clinical symptoms was correlated with WBC count, lymphocyte count, and eosinophil count, but not with hemoglobin, platelets, ESR, CRP, AST, ALT, total bilirubin, creatinine, LDH, or ferritin, in non-fatal cases. The duration of medication before symptoms was not related to the duration of clinical symptoms. The duration of clinical symptoms did not differ according to the culprit drug. Patients given systemic steroids showed higher blood lymphocyte counts compared to those not given steroids. There were no differences in the values of WBC, eosinophil, hemoglobin, platelet, ESR, CRP, AST, ALT, total bilirubin, creatinine, LDH, ferritin, or the duration of medication before symptoms between steroid-used and steroid-naïve patients [25].
Fatal cases: Fatal cases showed higher serum creatinine and ferritin, compared to non-fatal cases. There were no differences in the values of WBC, lymphocyte, eosinophil, hemoglobin, platelet, ESR, CRP, AST, ALT, total bilirubin, LDH, or the duration of medication before symptoms between fatal and non-fatal patients. Chen et al., reported that thrombocytopenia, a history of chronic renal insufficiency, multi-organ involvement, and pancytopenia are frequently found in fatal cases.
In other study tachycardia, tachypnea, coagulopathy, gastrointestinal bleeding, systemic inflammatory response syndrome, and leukocytosis are prognostic factors for death [27].
The histopathological features of DRESS syndrome are generally non-specific. There is no single unique finding that can be used to differentiate DRESS syndrome from other drug eruptions or inflammatory skin disorders. Histopathological patterns in skin lesions of patients with DRESS syndrome.
a) Spongiosis with focal subcorneal pustules;
b) Erythema multiforme-like interface dermatitis with basal vacuolar change and multiple scattered apoptotic keratinocytes in the epidermis;
c) Lichenoid dermatitis and prominent infiltrations of cells in the upper dermis and basal vacuolar change;
d) Vascular damage and perivascular infiltration with prominent endothelial cells, red blood cell extravasation, and some extent of vessel wall damage, resembling a feature of lymphocytic vasculitis;
e) Leukocytoclastic vasculitis: frank fibrinoid necrosis, leukocytoclasia, and red blood cell extravasation; and
f) Superficial perivascular dermatitis [28].
High power of previous slide shows basket-waves hyperkeratosis of stratum corneum pointing to acute process, with eosinophilic spongiosis, superficial and mid perivascular mixed inflammatory infiltrates rich in eosinophils, and there is necrotic keratinocytes and interface erythema multiforme-like interface dermatitis (Figures 2 and 3).
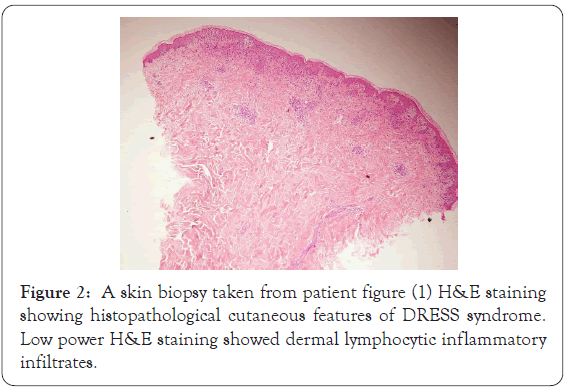
Figure 2: A skin biopsy taken from patient figure (1) H&E staining showing histopathological cutaneous features of DRESS syndrome. Low power H&E staining showed dermal lymphocytic inflammatory infiltrates.
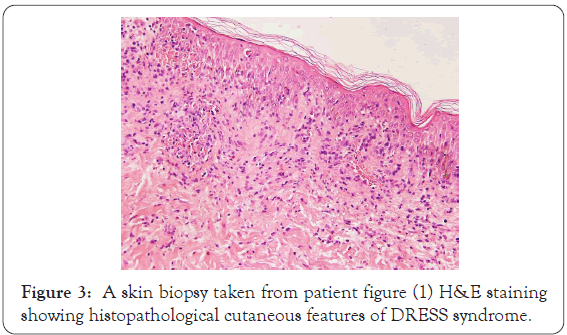
Figure 3: A skin biopsy taken from patient figure (1) H&E staining showing histopathological cutaneous features of DRESS syndrome.
Epicutanous patch test for dress
Patch testing is generally a safe procedure, including in severe adverse drug reactions, like DRESS syndrome. Even when a drug is tested at higher concentrations (20% pet.), the risk of developing a severe reaction is unlikely to occur [29]. Patch test can be performed 6 weeks to 6 months after of the adverse drug reaction, and at least 1 month after discontinuation of systemic corticosteroids, according to the proposed guidelines for performing patch tests in cutaneous adverse drug reactions [30].
Complementary tests during follow-up of patients with dress/dihs protocol
DRESS has an evolutionary behavior syndrome, and so hematological tests should be performed. The initial tests are mandatory to discover if there is visceral involvement, as proposed by Descamps et al., at admission: Complete blood count, ALT, AST, total bilirubin, GGT, alkaline phosphatase, sodium, potassium, creatinine and creatinine clearance, 24 h urine protein and urinary eosinophil count, CPK, LDH, ferritin, triglycerides, calcium and PTH, blood glucose, TAP and TTPA, lipase, protein electrophoresis, c-reactive protein, quantitative PCR for HHV-6, 7, EBV and CMV, blood culture, anti-nuclear factor. Follow up (two times/week): Complete blood count, ALT, AST, creatinine, LDH, other laboratory tests according to changes found on admission tests. Evaluative follow up: quantitative PCR for HHV-6, 7, EBV and CMV, complete blood count, ALT, AST, alkalinephosphatase, creatinine, LDH, ferritin and triglycerides [31,32].
The gold stone in treatment of DRESS Syndrome is immediate withdraw of the culprit drug. In case of patient is on many drugs and the culprit drug is not obvious, dermatologist should discontinue the most suspected one according to his their clinical judgment. Based on experts of the group of drug reactions of the French Society of Dermatology, the published results of a consensus of on the therapeutic management of DRESS/DIHS as follow [32]:
• Absence of signs of severity: topical corticosteroids (potent or very potent), emollients, H1-antihistamines
• Presence of signs of severity (transaminases >5 times normal, renal involvement, pneumonia, hemophagocytosis, cardiac, etc.): corticosteroids equivalent to 1 mg/kg per day of prednisone, multidisciplinary evaluation;
• Presence of signs of severity with confirmation of a major viral reactivation: Combining steroids and antiviral (Ganciclovir) and/ or IVIG.
History
Male patient aged 67 year, diagnosed as multiple myeloma one month ago, he was admitted in Kuwait Cancer Control Center, Al Sabah Hospital (KCCC) and started on chemotherapy in addition to thalidomide, alluprinol, antiviral and methylprednisolone.
Two weeks later he developed fever and skin rash. On examination, the skin showed erythematous macular rash (morbiliform) on trunk and purpuric rash on upper and lower extremities (Figure 4) this case had seen on 20th Sepmtber 2015 according to a request to our center.
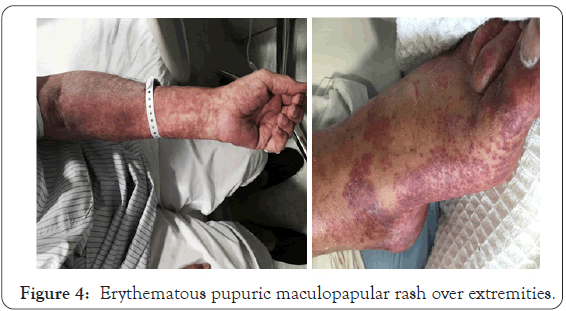
Figure 4: Erythematous pupuric maculopapular rash over extremities.
Investigations
Full blood tests were done for the patient and showed:
C.B.C showed leukocytosis with eosinophilia (W.B.C no. is 20.62 thousand, eosinophil is 11.3) while neutrophil was low (33.9) otherwise normal C.B.C. Liver enzymes was high A.L.T (281 mmol) and L.D.H elevated to 481. Renal function was normal (urea and creatnine). Our D.Ds were, D.H.S (DRESS syndrome), vascuilitis and viral exanthema; further investigations requested which were blood culture virology screening (Hepatitis B and C, C.M.V, E.B.V and H.H.V) [6,7]. Skin biopsy was done. Our recommendations were to stop alluprinol and thaldomide, a consultation to immunologist to do a drug sensitivity test. We changed methylprednisolone with oral prednisolone 60 mg (according to patient weight).
Although systemic steroid treatment started and discontinue of possible incraminted drugs patient skin rash did not improved as the macular lesions became confluent erythema associated with pitting edema (Figure 5).One week later the patient, skin condition fairly improved, the fever subsided, leukocytosis improved (W.B.C=16.2 thousands), eosinophilia became less (eosinophil=9.5). In the day 14th, skin rash progress to involve wide areas of skin. Virology screening was negative for hepatitis, C.M.V, E.B.V and not available for H.H.V [6,7], A.L.T became higher than before=326 mmol, L.D.H also became higher=541 mmol. Blood urea increased to 7.0 with normal creatinine, low albumin, total protein, Na and Ca. Skin biopsy was consistent with allergic drug reaction. I.V.I.G was recommended after consultation of oncologist given in a dose 400 mg/kg/day for 5 consecutive days (total 2 gm/kg body weight). Within a week of I.V.I.G infusion, the patient condition improved, both skin lesions and blood parameters as ALT decreased to 69 mmol. D.H became 231 mmol, G.G.T 87 mmol and W.B.C return to normal count (5.42) and eosinophil decreased to 5 and platelet count increased to 104 thousands instead of 94 thousands. The prednisolone was gradually tapered by 5 mg weekly, in the fourth week the skin exfoliation started associated with fading of skin erythema with continuous improving of blood tests (Figure 6).
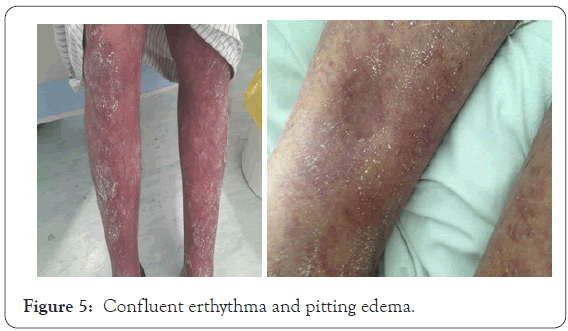
Figure 5: Confluent erthythma and pitting edema.
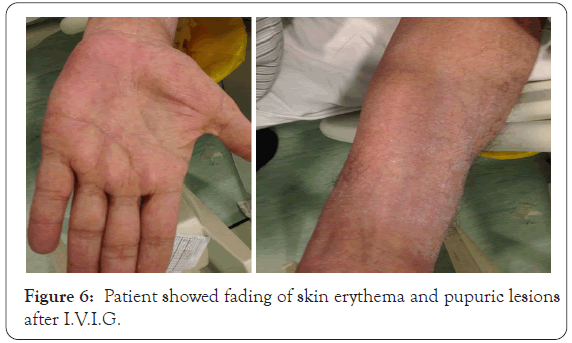
Figure 6: Patient showed fading of skin erythema and pupuric lesions after I.V.I.G.
The challenges in this case
The possible offending drugs in this condition are more than one as, the patient was on thalidomide and allopurinol and both of them can be the culprit drug for DRESS syndrome [17], in addition to chemotherapeutic drugs. Allopurinol-induced DRESS (A-DRESS) syndrome carries the highest mortality risk as it causes renal functional impairment, and accumulation of oxypurinol metabolite [16]. The I.V.I.G infusion in this patient carries more adverse reactions more than other cases as multiple myeloma patient has an increased viscosity (increased serum viscosity) so our decision taken by a multidisciplinary team included his treating oncologist as the patient did not improved on systemic steroid only although he was on proper dose according to his body weight and his condition worsen with time even after discontinue the possible culprit drugs and there was internal orange involvement, so I.V.I.G infusion was mandatory [33].
History
This case was seen on 5th November 2015 from Chest hospital for an 11 years old child, born with congenital complicated heart abnormality (Double cardiac outlet) complained of skin rash. He underwent multiple cardiac surgeries (5 cardiac surgeries done in U.S.A, last surgery since one month). He admitted to C.D.H in Kuwait for post-operative care and follow up of cardiac condition.
Dermatological history
A dermatological consultation sent to our center as he complained of fever and skin rash 5 days ago, on examination: there was erythematous papular skin rash all over the body with facial erythema, provisional sever drug reaction (DRESS syndrome) was postulated as the patient was on a big list of drugs. Current medication the patient on: Tombocor 100 mg, Aldacton 25 mg, Warferin 1 mg tablet, Zestril tablet, Fusix tablet, Digoxin and Vacomycin, Imipenem, Urisofolk.
Investigations
C.B.C showed leukocytosis (white blood count=21.10 thousands) neutrophil=57.4% and eosinophil=5.9%, hemoglobin of 121 gm and platelet count of 79 thousands. Liver enzymes NL, urea=20.30, creatinine=123.9 and very high total and direct bilirubin=582.7 and 404.04 respectively, the blood sugar was high 15.71 mmol. Laboratory tests for nasal and groin MRSA was negative, fungal antigen for Aspergillus by ELISA was negative, atypical mycobacterium negative by Z.N stain. Virology screening for E.B.V DNA and C.M.V DNA were not detected while I.g.G for both E.B.V, C.M.V were detected and I.g.M for them were not detected. A skin biopsy done and it was concide with drug reaction. Ultrasound revealed hepatosplenomegaly. Treatment plan was to discontinue the possible incriminated drugs although by history no newly introduced drugs were introduced, so antibiotics were changed to another group after discussion with the treating cardiologist. Systemic steroid inform of prednisolone 15 mg, and I.V.I.G in dose 400 mg/kg per day for 5 days totally 2 gm/kg was started, two cycles of I.V.I.G were given. Patient gradually improved and showed full improvement after more than 8 weeks (Figures 7 and 8).
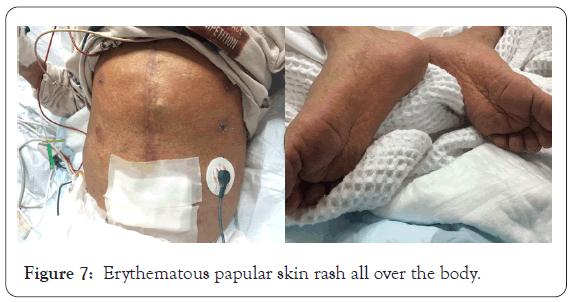
Figure 7: Erythematous papular skin rash all over the body.
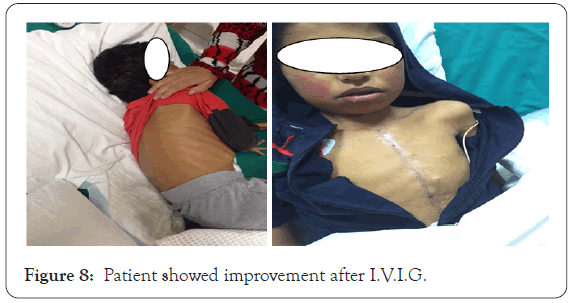
Figure 8: Patient showed improvement after I.V.I.G.
The challenges in this case
The patient has very critical heart problem, on multi-medications for long time (since birth), so we should very careful about medications should be discontinued and correlate the appearance of skin rash with any new started drugs. Antibiotics here were newly introduced here due to the fever, so we recommended changing to other group. Thorough history for this patient revealed that the warfarin tablet color changed according to his mother as the patient was taking (Figure 9) 75 mg daily which has orange color. However, he was before in different dose which have green or yellow color, by research found that here is a reported case allergy as manifested by hives due to changed warfarin tablet dose and so the color changed [34]. Here although I.V.I.G is proper therapy as patient did not improved on systemic steroid but I.V.I.G is a load on the heart as it causes hypervolemia and also increased blood viscosity [35], so the decision was by a multidisciplinary team included dermatologist, pediatric cardiologist and I.C.U physician also it given at very slow rate with supervision of body fluid and electrolytes.
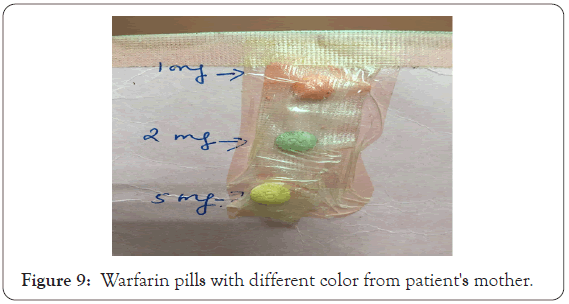
Figure 9: Warfarin pills with different color from patient's mother.
History
A request on 17th August 2015 for a female patient aged 48 years known case of pulmonary T.B who was admitted in P.R.C in Alsabah hospital for treatment. A triple drugs (Isoniazide, Rifampcin and Ethumboto) in addition to Ciprobay and Allopurinol. A consultation was sent to our center as, she complained of skin redness. On examination patient has erythroderma and exfoliative dermatitis of whole body with enlarged lymph node. Patient generally looks ill feverish (39 °C) and shivering. Blood tests showed, C.B.C showed leukocytosis as white blood cells count was 16.8 thousand with esinophalia 11.4%. High liver enzymes ALT was 581 IU/L, AST was 824 IU/L, GGT 752 IU/L, also ALK PHOS increased to 170 IU/L, LDH was highly raised to 1351 IU/L. Serum amylase slightly increased to 106 IU/L. Askin biopsy was taken and it consistent with drug reaction. According to RegiSCAR this case goes with DRESS criteria (Figure 10) [7].
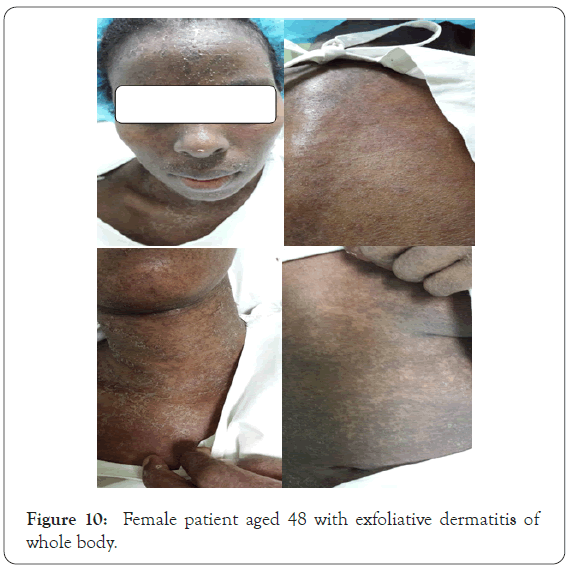
Figure 10: Female patient aged 48 with exfoliative dermatitis of whole body.
The challenges in this case
This patient has active pulmonary T.B and she got DRESS syndrome severe drug reaction. The most important step in treatment of DRESS is to discontinue the most culprit drug, here allopurinol was discontinued, it is usually given with anti-tuberculous drugs for prophylaxis against hyperuricemia [36], more than that, first‐line antituberculosis drugs may be one of the culprit drugs in DRESS syndrome; rifampicin and isoniazid are most frequently involved [37,38]. It is mandatory here to stop both allopurinol and the three anti-tuberculous drugs and reintroduce anti-tuberculous drugs one by one with after complete control of skin rash, this plan firstly was discussed with the treating physicians. The other challenging step was the treatment as systemic steroid should started while patient has active T.B and the anti-tuberculous drugs were temporarily discontinued so this decision was discussed with his treating physicians and also I.V.I.G should combined the prednisolone as an immune-synergetic in addition to control DRESS syndrome.
Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS) syndrome is a potentially life-threatening ADR, it may be result in hemophagocytosis with bone marrow failure, encephalitis, severe hepatitis, renal failure, and respiratory failure. This syndrome has a 10% mortality rate, most commonly from fulminant hepatitis with hepatic necrosis [39]. The most important challenge in DRESS syndrome is early diagnosis and immediate withdrawal of the offending drug, this is mandatory in management of the condition and leading to reduction in morbidity and mortality [40]. Systemic corticosteroids are the gold standard treatment for DRESS, as they interfere with the immune inflammatory sequence of DRESS syndrome [41]. Intravenous Immunoglobulin (IVIG) was given as adjuvant with systemic steroid in DRESS syndrome, especially in cases who do not respond to systemic steroids alone or as monotherapy where steroid was contraindicated [42]. In another studies IV.I. G therapy was successfully in many cases of DRESS syndrome [43,44], another study demonstrated failure of superiority of IVIG over oral corticosteroids to control DRESS syndrome [45]. In contrary our feedback was high efficacy and additional clinical improvement of combination of I.V.I.G with systemic steroids specially in challenging DRESS syndrome cases we managed, this supported and explained by Keno et al., high dose high-dose IVIG therapy for DIHS/DRESS has two immunological effects. First, it compensates for the decreased immunoglobulin concentration and immunological defects for protection of HHV- 6 infection. Batches containing high titers of antibodies against the viruses might be particularly effective in preventing HHV-6 reactivation [46]. Secondly, high-dose IVIG has a substantial antiinflammatory effect that regulates the immune response, as seen in the treatment of autoimmune disorders [47,48]. So these actions of IVIGs can balance the actions of systemic corticosteroid as immunosuppressive drug which makes patients more vulnerable for infection, and by this combination we guard against infection and control immune response in DRESS syndrome.
Drug Reaction with Eosinophilia and Systemic Symptom (DRESS) is a potentially life-threatening syndrome including severe cutaneous eruption, fever, hypereosinophilia, and internal organ involvement. More than 50 drugs can induce DRESS syndrome, in addition to viral infection specially herpesvirus infections; which was postulated as trigger or synergetic factor in pathogenesis of DRESS syndrome. The main treatments of DRESS are withdrawal of culprit drug and corticosteroids. Unfortunately, this is not enough in some cases, and this is critical situation IVIG is a mandatory additional adjuvant therapy. IVIG should be given in association with steroids at a dose of 2 g/kg over five days. These treatments will be conducted through multidisciplinary evaluation; which achieves better success in outcome of DRESS syndrome.
Citation: Almasry IM, Al Sabah H, Lazarevic V, Albathali AA, Alnaki A, AlSumait A, et al. (2020) Successful Management of Some Challenging Cases of DRESS Syndrome with Intravenous Immunoglobulins, Case-Based Review Article. J Clin Exp Dermatol Res. 11:541.
Received: 12-Nov-2020 Accepted: 26-Nov-2020 Published: 03-Dec-2020
Copyright: © 2020 Almasry IM, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.