Journal of Women's Health Care
Open Access
ISSN: 2167-0420
ISSN: 2167-0420
Research Article - (2019)Volume 8, Issue 5
Objective: Several studies have investigated subjective sleep quality during the menstrual cycle. For example, subjective sleep quality is reportedly worse at the premenstrual phase and during menstruation. However, the influence of sleep quality on menstrual symptoms has not yet been elucidated. The aim of this study was to investigate the association between self-reported sleep quality, psychological distress, and menstrual symptoms.
Methods: Participants were female university students (n=150; mean [SD] age 18.8 years). They completed the Pittsburgh Sleep Quality Index (PSQI), the Menstrual Distress Questionnaire (mMDQ), and Kessler’s Psychological Distress Scale (K6). We also collected demographic data.
Results: Participants were categorized into good sleepers (PSQI total score <5.5, n=74) or poor sleepers (PSQI total score ≥ 5.5, n=76). Poor sleepers experienced significantly more severe menstrual symptoms (p<0.01) and tended to have a less regular or more variable length of their menstrual cycle (p=0.06) than good sleepers. Multiple regression analysis revealed that subjective sleep disturbance (PSQI ≥ 5.5) and psychological distress (K6 ≥ 9) were significantly positively associated with the mMDQ score.
Conclusion: Subjective sleep disturbance and psychological distress in daily life are associated with menstrual problems, including severe menstrual symptoms and menstrual cycle irregularity.
Sleep; Poor sleep; Menstruation; Menstrual cycle; Reproductive health
Menstrual symptoms can have a significant impact on women’s lives. Menses-related health problems, such as menstrual pain, heavy menstrual bleeding, and premenstrual syndrome, are experienced by many women during their fertile years [1]. The prevalence of dysmenorrhea (menstrual pain) ranges from 25% among all women to as high as 90% among adolescents [2]. In a recent study with adolescents, the prevalence rate of severe, moderate, and mild dysmenorrhea were 29%, 43%, and 28%, respectively, and 34% of the sample described their menstrual cycle as irregular [3].
Several studies have investigated subjective sleep quality across the menstrual cycle. For example, subjective sleep quality has been reported to be worse at the premenstrual phase and during menstruation [4]. Romans et al. examined the temporal relationship between subjective sleep quality and the menstrual cycle phase in a community sample of adult women over six months; the authors found that sleep quality was poorest in the premenstruum, but the difference was of little clinical significance and was no longer statistically significant with the inclusion of potential confounding variables, including perceived stress and social support [5]. Women who experience severe premenstrual syndrome have reported significant declines in sleep quality that are associated with their symptoms during the late luteal phase rather than with the early follicular phase of their cycle [6]. Thus, women’s sleep is commonly affected by menstrual cycles and menstrual problems such as menstrual pain.
However, the influence of sleep quality on menstrual symptoms is not yet well understood. Disrupted sleep inhibits luteinizing hormone (LH) secretion, which controls the length and sequence of the menstrual cycle, including ovulation, and ovarian production of both estrogen and progesterone [7], and it has been reported that a shorter sleep duration may alter the menstrual cycle in adolescents [8]. It has been also reported that high levels of distress can delay or impede the LH surge and impair ovarian function by causing dysregulation of the hypothalamic–pituitary–adrenal (HPA) axis. In a prospective cohort study, depression and high perceived stress were associated with an increased probability of anovulation. Therefore, poor sleep quality and/or perceived distress in daily life may cause menstrual problems, such as menstrual pain and menstrual cycle irregularity. This study aimed to identify the association between self-reported sleep quality, psychological distress, and menstrual symptoms.
Participants and ethics
A total of 233 female students in the first grade of the Meiji Pharmaceutical University in Tokyo were approached and invited to participate in the study via posters and information sessions. The survey was uploaded to the learning management system, and participants answered via this platform. Data were collected anonymously. All procedures were conducted in accordance with the guidelines outlined in the Declaration of Helsinki. The Ethical Review Board of the Meiji Pharmaceutical University in Japan approved the study protocol.
Measurements
Questionnaires about sleep quality and sleep habits, menstrual symptoms and menstrual cycle, psychological distress, and demographic variables were included in this survey.
Sleep quality and sleep habits
The Pittsburgh Sleep Quality Index (PSQI) is the most common measure of sleep quality [9,10]. It is a self-reported 18-item survey on subjective sleep quality over the last 4 weeks. The first four items enquire about times (bed time, number of minutes it took for the participant to fall asleep, get up time, and hours of sleep per night). The next 10 items ask how often the participants had trouble sleeping for various reasons (e.g. woke up in the middle of the night, needed to go to the bathroom, was coughing, and bad dreams). Each item is scored on a 4-point scale ranging from “never” to “three times a week or more”. Additional items include a subjective rating of sleep quality (scored on a 4-point scale from from 0=“very good” to 3=“very bad”, the use of sleep medication, and trouble staying awake during the day (scored on a 4-point scale ranging from 0=“never” to 3=“three times a week or more”). The final item measures motivation and enthusiasm to get things done (scored on a 4-point scale ranging from 0=“no problem at all” to 3=“a very big problem”). The total score ranges from 0 to 21, whereby a higher score represents worse sleep quality. The cutoff score for sleep disturbance has been established at 5.5 points. The psychometric properties of both the English and the Japanese version of the PSQI are good [9,10].
Menstrual symptoms and menstrual cycle
The experience of menstrual symptoms within the previous few months was reported. A modified 35-item Japanese version of the Menstrual Distress Questionnaire (mMDQ) was used to assess menstrual pain and symptom severity. The questionnaire includes six subscales, as follows: Pain (6 items), Concentration (8 items), Behavioral Change (5 items), Autonomic Reactions (4 items), Water Retention (4 items), and Negative Affect (8 items) [1]. Items are scored on a 4-point scale ranging from no experience of the symptom to an acute or partially disabling experience of the symptom in the menstrual period compared to the post-menstrual period (which is defined as few days after menstruation). Higher scores indicate more severe symptoms. Cronbach’s alpha for the Pain, Concentration, Behavioral change, Autonomic reactions, Water retention, and Negative affect subscales, and for the total score, were 0.73, 0.81, 0.82, 0.71, 0.61, 0.91, and 0.94, respectively.
The severity of menstrual symptoms was categorized into two groups based on a total score distribution of the mMDQ scores, as follows: “severe” (the top 25th percentile of the score distribution, which was a score of 30-81) and “moderate and below” (the lower 25th percentile of the score distribution, which was a score of 0-29). Participants also reported the last five onset dates of menstrual flow, from which we calculated the length of the previous four menstrual cycles.
Psychological distress
Kessler’s Psychological Distress Scale (K6) consists of 6 items that assess the frequency of psychological distress during the past 30 days [11,12]. The response options range from 0 (none of the time) to 4 (all of the time). Total scores range from 0 to 24, with 9 points or higher indicating a high risk of mood and anxiety disorder. Cronbach’s alpha for the scale was 0.82.
Demographic variables
Sociodemographic and lifestyle characteristics of participants were obtained by a self-reported questionnaire. These variables included age, height, weight, smoking status (yes/no), alcohol consumption (yes/no), duration using displays such as a smartphone, TV, and PC after 8 pm, commuting time from home to the university, and part-time job after 10 pm (yes/no).
Statistical analyses
Correlations between continuous variables were evaluated using Pearson’s correlation coefficients. Participants were categorized into good sleepers (PSQI total score<5.5) or poor sleepers (PSQI total score ≥ 5.5). Thereafter, total and subscale scores of the mMDQ were compared between the two groups using an unpaired t-test. Multiple regression analyses were conducted to estimate the effects of subjective sleep disturbance and psychological distress on menstrual symptoms. The Multicollinearity effect was checked using a variance inflation factor (VIF)<5. Statistical analysis was performed using SPSS version 22 (SPSS Inc., Chicago, IL, USA) and the level of significance was set at p<0.05.
Sample description
Of the 233 students, 150 (64.4%) completed the survey. The mean [SD] age of participants was 18.8 (0.71) years old. The mean body mass index was 20.3 (2.14) kg/m2. None of the participants smoked, and 6.7% reported drinking alcohol. The average duration of using a display after 8 pm was 2.05 hours, and 27.3% of participants had part-time job after 10 pm.
The average PSQI score was 5.5 (2.2) and the number of poor sleepers was 76 (50.7%). The average K6 score was 3.6 (3.9) and the number of participants with a K6 score ≥ 9 was 19 (12.7%).
The average menstrual cycle duration was 32.0 (5.4) days. A total of 60.6% of participants had menstrual cycle irregularity (MCI), which was defined as a cycle fewer than 25 days or more than 39 days during the four previous menstrual cycles. The average mMEQ score was 21.3 (17.0).
The relationship between mMDQ and PSQI / K6 scores
The level of subjective sleep disturbance (r=0.32, p<0.01) and psychological distress (r=0.48, p<0.01) were significantly correlated with the severity of menstrual symptoms (Figure 1).
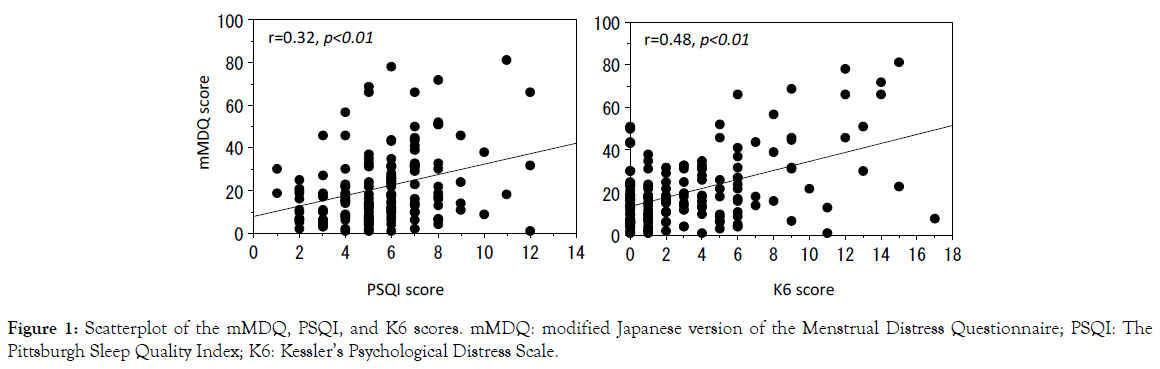
Figure 1. Scatterplot of the mMDQ, PSQI, and K6 scores. mMDQ: modified Japanese version of the Menstrual Distress Questionnaire; PSQI: The Pittsburgh Sleep Quality Index; K6: Kessler’s Psychological Distress Scale.
Menstrual symptoms in those with good and poor subjective sleep quality
The total score of mMDQ was significantly worse in poor sleepers than in good sleepers (t(148)=3.58, p<0.01). There were also significant differences between two groups in Pain, Concentration, Behavioral change, Autonomic reactions, Water retention and Negative affect subscale scores (Table 1). There was no significant between-group difference in menstrual cycle duration (t(148)=0.21, p=0.84), which was 31.9 (5.2) days in good sleepers and 32.1 (5.6) days in poor sleepers. Individual fluctuations in the menstrual cycle and the difference between the maximum and minimum menstrual cycle were larger in poor sleepers than in good sleepers, although these differences did not reach statistical significance (Individual fluctuations: t(148)=1.92, p=0.06; difference between the maximum and minimum: t(148)=1.92, p=0.06).
| Good sleeper (n=74) | Poor sleeper (n=74) | t value | p-value | |||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | |||
| mMDQ total score | 16.4 | 14.2 | 26 | 18.3 | 3.6 | <0.01 |
| Subscale score | ||||||
| Pain | 5 | 3.1 | 6.6 | 4.4 | 2.6 | 0.01 |
| Concentration | 1.8 | 2.6 | 3.8 | 3.9 | 3.8 | <0.01 |
| Behavioural change | 3.6 | 3.5 | 5.6 | 3.7 | 3.4 | <0.01 |
| Autonomic reaction | 0.8 | 1.6 | 1.5 | 2.1 | 2.3 | 0.02 |
| Water retention | 2.2 | 2.2 | 3.4 | 2.5 | 3 | <0.01 |
| Negatice effect | 3 | 4.9 | 5.1 | 5.6 | 2.4 | 0.02 |
| Menstrual cycle (day) | ||||||
| Average menstrual cycle | 31.9 | 5.2 | 32.1 | 5.6 | 0.2 | 0.84 |
| Within person fluctuation | 4.9 | 3.5 | 6.9 | 6.5 | 1.9 | 0.06 |
| Difference between the two extreme values | 11.6 | 7.7 | 16.2 | 15.5 | 1.9 | 0.06 |
Table 1: Differences in menstrual symptoms between good and poor subjective sleep quality groups.
The associated factors with menstrual symptom
Table 2 shows the results of the multiple regression analysis on mMDQ score. We confirmed there to be no multicollinearity, because VIF values ranged from 1.07 to 1.26. Of the variables included in the model, subjective sleep disturbance (PSQI ≥ 5.5) and psychological distress (K6 ≥ 9) showed a significant positive association with mMDQ scores. The other variables in the model, including Body Mass Index (BMI), alcohol consumption, night time job, long commuting time, and menstrual cycle irregularity did not show any significant association with mMDQ score. The adjusted R-squared value was 0.22.
| Independent variables | ß | p value |
|---|---|---|
| Subjective sleep disturbance (PSQI ≥ 5.5) | 0.22 | p<0.01 |
| Psychological distress (K6 ≥ 9) | 0.45 | p=0.02 |
| BMI | - | ns |
| Alcohol consumption (yes) | - | ns |
| Nighttime job (yes) | - | ns |
| Long commuting time (≥ 45 min) | - | ns |
| MCI | - | ns |
| Adjusted R2=0.22 | ||
Table 2: Results of multiple regression analysis of factors contributing to menstrual symptoms.
To the best of our knowledge, this is the first study to examine the relationship between usual sleep quality and menstrual symptoms/ the menstrual cycle. The main finding of this study was that female university students with poor sleep had more severe menstrual symptoms than those with good sleep. The Pain, Concentration, Behavioral change, Autonomic reactions, Water retention, and Negative affect subscale scores were worse in poor sleepers than in good sleeper. In addition, the multiple regression analysis suggested that both subjective sleep disturbance and psychological distress were associated with severe menstrual symptoms.
Several previous studies have suggested that depression, psychotropic medication use, psychological distress, and shift work are associated with menstrual problems. For example, a recent prospective study reported that perceived stress was associated with a greater prevalence of menstrual cycle irregularity and heavier bleed [13]. From the Nurses’ Health Study II with a sample size of over 70,000 women, those who had engaged in more months of rotating shift work in the previous two years showed adverse health outcomes related to irregular menstrual cycle pattern or length [14]. One potential mechanism underlying the link between psychological distress and menstrual problems is the influence of the HPA axis on Gonadotropin releasing hormone (GnRH). Increased reactivity of the HPA axis induced by psychological distress subsequently delays or impedes the LH surge [15,16]. GnRH production, and particularly the LH surge, is also regulated by the central pacemaker of endogenous oscillations, i.e. the suprachiasmatic nucleus. A disruption in the synchronization between the central and peripheral oscillators (including the ovaries) in shift-workers may contribute to reproductive disorders such as menstrual pain, alterations in the length of the menstrual cycle, and menstrual irregularity. This could occur through alterations in the secretion patterns of estradiol and other hormones, or in the sensitivity and response rate of target organs [17].
The present study found that self-reported sleep quality, independently of psychological distress, was associated with menstrual problems in university students who did not engage in shift work. Poor sleepers experienced significantly more severe menstrual symptoms and tended to have a less regular or more variable length of their menstrual cycle than good sleepers. In an experimental study conducted with healthy young women, partial sleep deprivation increased LH and estradiol concentrations [18]. It is therefore plausible that sleep disturbance and poor sleep quality affect reproductive health, including menstruation via hormonal changing. Further studies are needed to examine the pathways, mediators, moderators, and underlying biological and genetic mechanisms between sleep disturbance and menstrual problems.
This study has some limitations. First, the data used in this study were collected by retrospective self-report, which has a potential for recall bias. Objective measures of sleep duration and some sleep problems, such as autography are desirable. In addition, measurements of basal body temperature, and LH surge in the urine would help elucidate the exact menstrual cycle regularity. Second, given that cross-sectional data were used, causality of the observed associations cannot be inferred.
In summary, the current study found that subjective sleep disturbance and psychological distress in daily life are associated with menstrual problems, including severe menstrual symptoms and menstrual cycle irregularity, in female university students. Future research should clarify the relationships between sleep behaviour, sleep quality, and reproductive hormones that regulate the menstrual cycle and ovulation.
This work was supported in part by a Grant-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (JSPS KAKENHI Grant Number 16K07545).
Citation: Komada Y, Ikeda Y, Sato M, Kami A, Masuda C, Shibata S (2019) Subjective Sleep Disturbance and Psychological Distress are Associated with Menstrual Problems. J Women’s Health Care 8:472. doi: 10.35248/2167-0420.19.8.472.
Received: 19-Sep-2019 Accepted: 01-Oct-2019 Published: 07-Oct-2019
Copyright: © 2019 Komada Y, et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original work is properly cited.