Journal of Physical Chemistry & Biophysics
Open Access
ISSN: 2161-0398
ISSN: 2161-0398
Research Article - (2025)Volume 15, Issue 1
At present, the increase of air pollutant gases in the atmosphere and climate changes is one of the most serious and main problems which endanger human life and the environment. Hence, in recent decades, reducing of the sulfur compounds in fossil fuels has been noticeably more considered. The physical and chemical properties of the samples were characterized by sulfur analysis, XRD, SEM, BET, FT-IR and SEM techniques. This research concentrated on an economic, green and practical process to eliminate sulfured compounds from liquid fuels such as gas oil, by using a novel porous nano silica adsorbent. By applying chemical methods and performing modification on the surface of a kind of nano silica adsorbent including deposition of iron, significant adsorption of thiophene and its derivatives were observed. The presence of the iron on the silica absorbent bed cause creating bonding between the sites and the iron atoms and makes considerably removing unsatisfied sulfur molecules. In comparison with other typical desulfurization processes like HDS, this method works on an ambient condition and on the other hand some hard processing conditions and also expensive catalysts are not essential to accomplish a satisfied and effective desulfurization.
Desulfurization; Porous nano silica; Adsorption; Gas oil; Ferric chloride; Piranha solution; Immobilized; Depositing; Lewis-acid
The treatment process of petroleum considerably needs desulfurization of the oil to produce satisfied products. Generally, the removal of sulfur from petroleum is accordingly one of the major and important treatment processes in many refinery companies and based on the sulfur content of crude oil its price will be significantly estimated. The price of crude oil is considerably influenced by its sulfur content. Nowadays, specifications of fuel that govern transportation fuels have dramatically become stringent concerning sulfur content. A large number of petrochemical products are similarly produced to be approximately sulfur-free [1]. To a large extent, the most sulfured compounds in the vacuum gas oil and vacuum residue, are contained the dibenzothiophene family. The chemical nature of the sulfur has a direct bearing on its removal. Desulfurization of compounds that contain aliphatic sulfur, i.e., thiols and sulfides, is easier than desulfurization of compounds that contain aromatic sulfur, i.e., thiophenics.
The desulfurization of petrochemicals and transportation fuel is attracting more attention due to the increased awareness of the adverse effects of burning sulfur containing oils on human health and the environment [2,3]. Actually, sulfur removal of petrol fuels or waste biomass derived fuel oils has become a critical component of the petroleum refining industries [4]. Sulfured compounds in fuel oil (Figure 1), such as Thiophenes (T), mercaptans, Benzothiophenes (BT) and Dibenzothiophenes (DBT), produce Sulfur Oxide (SOx) upon combustion, which are the main sources of acid rain and air pollution. These pollutant compounds also enhance corrosion and deactivation of the catalyst during the desulfurization process of fuel oil in refining industries [5-7]. As a consequence, eliminating such sulfur-containing compounds is significant for producing green fuel oils and to achieve the acceptable international standards of sulfur content (10 ppm-15 ppm) as per the recommendations of the United State Protection Agency (USEPA), given the environmental concerns surrounding sulfur [8,9].
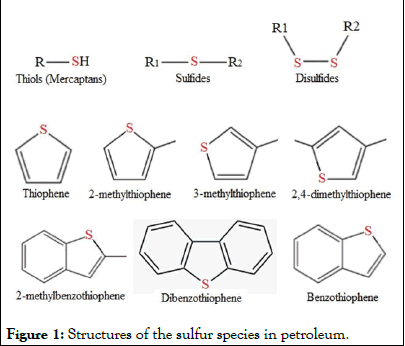
Figure 1: Structures of the sulfur species in petroleum.
Gas oil is one of the most essential energy sources and is derived directly from crude oil by atmospheric distillation units. Furthermore, diesel oil is a complex mixture of various saturated, unsaturated and aromatic hydrocarbons. However, the presence of pollutant contributors such as refractory sulfur and aromatic nitrogen compounds adds to environmental pollution. These compounds are difficult to remove because of their greater molecular weight and higher boiling point. Moreover, these compounds are known to inhibit the hydrodesulfurization process [10].
Generally, techniques for deep desulfurization of liquid hydrocarbon fuels can be classified into catalytic hydrodesulfurization with improved new catalysts, chemical oxidation, adsorption, extractive desulfurization and biodesulfurization by using special bacteria [11-13]
Today, one of the common adsorption techniques are based on the application of activated carbon. Activated carbon is largely noticed due to its adsorption properties, including phenolic compounds, dyes, metals and organic molecules. Hence, that applies to eliminate the sulfured structures [14-16]. On the other hand, natural clays as another adsorption method are totally occurring resources whose extraction does not need complicated equipment and does not create environmentally pollutant substances. Clays are molecularly made of more than two silica tetrahedral sheets with a central octahedral sheet. The main structure of clays is negatively charged and electrically balanced by some of exchangeable cations like Na+, K+, Ca2+. The presence of these ions imparts a high surface polarity that is not chemically friendly to non-polar systems like gasoline and diesel. Unfortunately, the target pollutants, i.e., sulfur compounds, are hydrophobic. For this reason, a modification is required to promote the hydrophobic affinity of natural clay to achieve these non-polar molecules. This process is accomplished by taking advantage of the exchangeable ions that reside on the porous surface of clays. Non-polar quaternary ammonium ions have been utilized previously, to improve the adsorption of phenolic compounds on bentonite [17,18].
Recently, alternative solid acid catalysts and adsorbents are mainly applied in order to replace sulfuric acid because of simple separation conditions from products, environmental friendliness and convenience for recycling [19,20]. In some of the researches, several adsorbent and solid acid catalysts have been investigated such as zeolite, H3PO4/MoO3/SiO2, WO3/SiO2, HPW/MCM-41, H2SO4/SiO2 and ion-exchange resin, which attempt to reduce the prior drawbacks. In the last few decades, Lewis-acid catalysts have been widely utilized in several acid catalyzed reactions because of their low expenses and high efficiency. To promote this treatment system, the study of immobilizing Lewis acid on the support has noticeably been considered, especially, because of its crucial characteristics as a feasible, economical and green method. At present, some of mesoporous materials like SiO2, Al2O3, MCM-41, SBA-15 and montmorillonite have been extensively applied as a carrier because of their proper nano porous structure and noticeable surface area. Between the above supports, silica has some superiority and positive points by comparison with other ones, such as, silica works as a fantastic carrier with many of silicon hydroxyl groups on its surface which could interact well with the metal chlorides and make strongly immobilization between them.
Herein, we would explain making immobilized FeCl3-SiO2 and its application in the desulfurization of the liquid fuels as an efficient and practical adsorbent and catalyst and the achieved results shall be presented completely in this article.
At this research project, the chemical reagents were purchased with high purity from the Merck chemical company. Also, the Infrared (IR) spectra by using (an) instrument of Perkin-Elmer FTIR Spectrum two were recorded. In addition, the surface morphology of SiO2 and FeCl3-SiO2 samples were investigated by SEM Philips (XL-30) instrument. The adsorption and specific surface area parameters in the adsorbent layers were studied by BET Belsorp Mini2. In order to study more accurately the crystalline structure of the adsorbents, the XRD DX27-mini was applied. All sulfur analysis tests were achieved by EDXRF Sulfur Meter (TANAKA) RX-360SH. Furthermore, the oven (Lab.Companion) was utilized for dehydration and drying of the samples.
Selecting the proper Lewis-acid compound
In this section, by considering the importance of the Lewis-acid and its key role in reaction with sulfur, according to the Table 1, ten acid reagents were chosen. After that, three solutions of gas oil with different sulfur content i.e., 168, 1093 and 280 ppm were prepared. Then, 25 (g) of each of the three solutions A, B and C separately, with 2.5 (g) of each ten below acids were mixed and the suspension was stirred for one hour under ambient conditions. In the next step, the filtrate was investigated by the sulfur analysis test. Lastly, regards to the results which obtained from thirty tests, as that would be clear in the Table 1, the best efficiency between those acid compounds belongs to Ferric Chloride (FeCl3) in the all of three solutions.
| Initial solutions | Lewis-acid structures | |||||||||
| CoCl2, 5H2O | PbCl2 | AlCl3 | AgCl | CuCl2 | NiCl2 | MnCl2, 2H2O | ZnCl2 | SnCl2 | FeCl3 | |
| Solution-A (168 ppm) | 160 | 179 | 187 | 186 | 171 | 175 | 180 | 183 | 166 | 157 |
| Solution-B (1093 ppm) | 1104 | 1074 | 1201 | 1081 | 1102 | 1058 | 1099 | 1023 | 1102 | 961 |
| Solution-C (280 ppm) | 296 | 283 | 287 | 299 | 281 | 300 | 267 | 287 | 287 | 208 |
Table 1: The concentration of the sulfur before and after adding the Lewis-acid compounds to the three solutions (A, B, C).
Activation of nano silica beds with Piranha solution
Regarding this part, the silica beds were cut as plates (with dimensions 0.3 cm × 0.5 cm) and then they were put into a glass beaker. After that, some Piranha solution (a mixture of concentrated sulfuric acid and 30% hydrogen peroxide solution by ratio 3:1) poured on them. For better performance and more activation, this step did three times. In fact, by doing several these acts, we could do depositing more number of hydroxyl groups on the silica beds. After activation of porous beds, in a few times, the beds were placed in the boiling distilled water, so this make them to increase from acidic pH to the neutral state. Actually, after adding the Piranha solution, the number of the– OH functional groups substituted on the nano silica bed will be rise, as this reaction would be proved by the FT-IR spectrum in which the peak 1 and 2 intensity in the 3400 (cm-1) region has increased (Figure 2).
Figure 2: FT-IR spectrum of porous beds before and after adding the Piranha solution.
Scanning Election Microscopy (SEM)
The scanning electron microscope technique is one of the wellestablished methods to investigate the surface structure of materials. SEM produces images by probing the sample with a focused beam of electrons, which interacts with atoms on the surface to produce various signals that contain information about the material. SEM techniques allow us to observe the surface of a sample through the interaction of an electron beam. In addition, allow us to perform the analysis of the composition of the surfaces. One of the most representative cases where porosity contributes positive factors to the applicability of a material is porosity offers the advantage of increasing the loading capacity of the vector. The porosity of a material is decisive when evaluating its durability and resistance to adverse conditions. The distribution of pores and their characteristics defines the permeability of the material, that is, its ability to store, thus conditioning its physical and chemical properties. The pores have different properties between them. The most important are its shape and size, its location, its connectivity and its surface-related chemical properties. The pore size of a material can be classified as follows:
Macrospores: They are less than 2 nanometers in size.
Mesoporous: They have a pore size between 2 and 50 nanometers.
Macrospores: They have a size greater than 50 nanometers.
Based on Figure 3 and the size of the pores indicated in the figure at a magnification of 500 nm, the size of the mesoporous has been determined. In order to obtain more accurate parameters and determine other specifications of the surface, such as the surface-to-mass ratio and the average diameter of the pores, the Brunauer-Emmett-Teller (BET) method has been used. Different methods have been considered for measuring surface area and porosity, including microscopic methods and absorption-based methods. In solid samples with non-porous surfaces, the surface area can be approximately measured. However, in samples with a porous structure, there are difficulties in determining the porosity, as well as the total surface area and average pore size. One of the most important methods for measuring the total surface area of porous samples is the BET method, which relies on the adsorption of certain specific molecular species in the gas phase on the surface. BET analysis results of the nano porous bed (Table 2).
Figure 3: SEM image of silica bed structure, SEM (Scanning Electron Microscopy), nano meter of silica bed structure.
| No. | BET plot | ||
| 1 | Vm | 0.085971 | (cm3(STP) g-1) |
| 2 | as,BET | 0.37419 | (m2 g-1) |
| 3 | C | 10.178 | |
| 4 | Total pore volume (p/p0=0.990) | 0.001006 | (cm3 g-1) |
| 5 | Mean pore diameter | 10.757 | (nm) |
Table 2: BET analysis results of the nano porous bed.
Depositing of Lewis-acid (FeCl3) on the nano silica porous bed
In this step, firstly, the silica porous plates which have been activated by Piranha solution in the previous part, placed in the oven for five hours until they would completely dry and dehydrate. Secondly, a specific amount of activated porous nano silica bed 30 (g) was put in an Erlenmeyer flask and then, 15 (g) ferric chloride and 200 (mL) toluene, respectively, were added into the batch and the reflux reaction continued and did for 72 hours. After finishing the reflux reaction which lasted three days, the appearance of the porous bed clearly altered from white to dark brown and consequently, the accomplishing the depositing process of FeCl3 on the bed structures would be confirmed. Finally, the end products were kept in the oven for five hours and dried. Henceforth, the last produced structure of this step would be called subproduct-A
Cellular Glass (GC)
Cellular Glass (GC) or foam glass is a solid material made from expanded glass with a closed cell structure. The majority of the glass is made up of 95 to 98 percent. In the production of cellular glass, powdered glass is mixed with a foaming agent, usually ground calcium carbonate and placed in a furnace at approximately 1000 degrees celsius. The glass powder melts due to the high temperature and the calcium carbonate decomposes at this temperature, producing CO2 gas. The escape of gas from the glass melt creates a cellular glass structure. The cooling stages are then performed, which are done at a slower rate and the cellular glass structure will be more homogeneous. It has very high stability and excellent resistance to chemical solvents and very high compressive strength. The wide working temperature range (-200 to +400 degrees celsius) allows for the use of foam glass in a variety of conditions. Cellular glass foam is free of any environmentally harmful substances. Recycled glass products such as light bulbs, recycled glass bottles, window glass and car glass, as well as computer monitors, are the raw materials for cellular glass foam. It is highly compatible with the environment (Figure 4).
Figure 4: Images of the nano porous silica beds in the three steps including: (A) before activation with Piranha solution, (B) after activation with Piranha solution and depositing of Lewisacid (FeCl3) or subproduct-A, (C) after desulfurization process.
Application of phenol-formaldehyde resin for more desulfurization
At first, some of the porous silica structures, about 30 (g), have been activated by the Piranha solution and passed properly by depositing of ferric chloride, were undergone the reflux system by adding 10 (g) phenol-formaldehyde and also 200 (mL) toluene for 72 hours. After that, they would be dried in the oven for five hours. Hereafter, the produced material of this section would be considered as Subtrans-B. Subsequently, thirty grams of Subtrans-B, 250 (mL) of toluene and 15 (g) FeCl3 were put into a distilling flask and the reflux system was heated for 72 hours. Eventually, the final product which was noticed as subproduct-B, would be obtained after drying in the oven for five hours.
FT-IR spectrum
Layer-by-layer modification of mesoporous silica SiO2 surface using Piranha solution was performed to increase the number of -OH functional groups on the silica support. The comparison of peaks 1 and 2 in the FT-IR spectrum (Figure 5) before and after loading FeCl3 onto the silica support was carried out, as well as the comparison of peaks 2 and 3 after the surface modification and addition of -OH functional groups using phenol formaldehyde resin. Finally, the loading of FeCl3 was performed to increase the surface adsorption capacity of sulfurase in gas oil and the comparison of peaks 4 and 5 in the FT-IR spectrum was conducted. In all cases, the addition of -OH functional groups on the silica support led to an increase in the absorption peak in the region of 3300 cm-1-3500 cm-1 and the loading of FeCl3 onto the silica support resulted in a decrease in the -OH absorption peak. Based on the FT-IR spectrum, the loading process was successful. Therefore, the first layer of FeCl3 on the silica support is used for the reaction of FeCl3 with Si-OH hydroxyl groups and the second layer is for the reaction of the chlorine element of the FeCl3 compound with the hydroxyl group of C-OH of the phenol-formaldehyde resin and will cause a large increase in the Lewis acid groups of FeCl3 on the surface.
Figure 5: FT-IR spectrum of porous beds before and after adding the FeCl3 compound.
Desulfurization of gas oil samples with the synthesized adsorbent structures
Totally, this desulfurization process was done with a continuous circulation system including a pump, for providing a steady current of gas oil and also a column containing porous adsorbent silica beds and some fittings and other accessories. During this study, some the different gas oil samples with various concentrations and qualities were used with different kinds of adsorbent beds. Furthermore, the parameter of the time and its effect on the process efficiency was investigated.
Actually, the existence of sulfur structures, as one of the most disadvantageous petroleum fractions, have created many problems such as corrosion, environmental pollution and huge economic losses. Today, the adsorption method could be considered as an efficient and inexpensive process. On the other hand, selectivity to sulfured compounds in a mixture of thousands of various hydrocarbons is a very crucial and key parameter. Furthermore, some other characteristics such as stability, the capacity of adsorption, regeneration possibility should be noticed to select adequate sorbent material. Also, considering using this nano bed structure by a satisfied porosity characteristic, a considerable increasing occurred in adsorption of sulfured compounds.
According to this study, to remove sulfured compounds, a novel porous nano silica bed was designed and produced which works through chemical adsorption with high efficiency, particularly for desulfurization of gas oil samples. This nano silica bed was prepared after some steps such as activating by Piranha solution and then depositing with ferric chloride. Selecting of Lewis-acid compound as the main substrate for trapping the molecules containing sulfur atom was one of the important parts of the process. Ten proposed Lewis-acid compounds were tested by three solutions with different concentrations and the most satisfied one belonged to ferric chloride. After that, Cobalt (II) chloride (CoCl2, 5H2O) was the second option by the amount 160 ppm. The results which have been demonstrated in Tables 3-5, indicate the quantity and quality analysis of the porous silica beds in all states of their production. By comparison between Tables 3 and 4, clearly, the percentage of iron considerably increased from 0.8 to 3.6 which has proved the acceptable depositing of FeCl3 on the surface of the silica adsorbents. Naturally, time of process could be a key parameter in progressing of reaction. In another part of this study, the time of desulfurization was investigated versus sulfur removal percentage. The data of Table 6 indicated that the elimination of sulfured compounds climbed when the time of process would increase.
| Oxide | %W | Oxide | %W | Oxide | %W | Oxide | %W | Oxide | %W |
| Na2O | 11.3 | MgO | 3.15 | Al2O3 | 1 | SiO2 | 67.2 | P2O5 | 0.09 |
| Cl | 0.26 | K2O | 0.39 | CaO | 13.6 | Fe2O3 | 1.2 | SO3 | ND |
| Element | %W | Element | %W | Element | %W | Element | %W | ||
| Si | 31.4 | P | 0.04 | Fe | 0.8 | S | ND |
Table 3: XRF analysis results based on weight percent of the elements and components of silica beds.
| Oxide | %W | Oxide | %W | Oxide | %W | Oxide | %W | Oxide | %W |
| Na2O | 9.9 | MgO | 2.8 | Al2O3 | 0.92 | SiO2 | 60.51 | P2O5 | 0.06 |
| Cl | 1.8 | K2O | 0.3 | CaO | 9.4 | Fe2O3 | 5.1 | SO3 | 0.01 |
| Element | %W | Element | %W | Element | %W | Element | %W | ||
| Si | 28.3 | P | 0.03 | Fe | 3.6 | S | 0.01 |
Table 4: XRF analysis results based on weight percent of the elements and components of silica beds which deposited with FeCl3.
| Oxide | %W | Oxide | %W | Oxide | %W | Oxide | %W | Oxide | %W |
| Na2O | 10 | MgO | 2.9 | Al2O3 | 0.86 | SiO2 | 57.1 | P2O5 | 0.03 |
| Cl | 3.5 | K2O | 0.26 | CaO | 9.4 | Fe2O3 | 4.1 | SO3 | 0.07 |
| Element | %W | Element | %W | Element | %W | Element | %W | ||
| Si | 26.7 | P | 0.01 | Fe | 2.9 | S | 0.03 |
Table 5: XRF analysis results based on weight percent of the elements and components of silica beds which deposited with FeCl3 after the desulfurization process.
| No. | Time (hour) | Initial sulfur concentration (ppm) | Final sulfur concentration (ppm) | Sulfur remove (%) |
| 1 | 0.5 | 500 | 470 | 6 |
| 2 | 1 | 500 | 401 | 19.8 |
| 3 | 1.5 | 500 | 211 | 57.8 |
| 4 | 2 | 500 | 179 | 64.2 |
| 5 | 2.5 | 500 | 160 | 68 |
Table 6: The relationship between the amount of sulfur removed from the gas oil sample (500 ppm) and the time of desulfurization process by the small grain bed (30 g).
In the next part, the influence of grain size of silica adsorbent (subproduct-B) on desulfurization was surveyed. As that has been shown in the Table 7, the process was done in three conditions for four various gas oil samples. Those were including, the large grain (with diameter 5 mm) with two amounts 30 and 60 (g) and also small grains (with diameter 2.5 mm) with amount 30 (g), which lasted for three hours.
The remarkable results demonstrated when the size of the grain decreased to half, the adsorbent efficiency would double. Inaddition, using of phenol-formaldehyde resin as an additive was helpful and operative to increase more removal sulfur structures.
| No. | Initial sulfur concentration (ppm) | Large grain bed/30 (g) | Large grain bed/60 (g) | Small grain bed/30 (g) | |||
| Final sulfur concentration (ppm) | Sulfur remove (%) | Final sulfur concentration (ppm) | Sulfur remove (%) | Final sulfur concentration (ppm) | Sulfur remove (%) | ||
| 1 | 6380 | 3902 | 38 | 3750 | 41.2 | 3705 | 42 |
| 2 | 1000 | 435 | 60.5 | 348 | 67 | 382 | 63 |
| 3 | 500 | 176 | 68.8 | 168 | 67.5 | 165 | 68.7 |
| 4 | 250 | 62 | 76 | 54 | 78.8 | 55 | 78.8 |
Table 7: The comparison between the desulfurization percentage of four various gas oil samples by the manufactured adsorbent (subproduct-B) with two types large grains (with diameter 5 mm) and small grains (with diameter 2.5 mm) and different amounts of porous beds which took time three hours.
Impact of contact time
Contact time is an important variable in the adsorption process. The efficiency and capacity of adsorption have a direct relationship with contact time. The impact of contact time on the adsorption of sulfur compounds in diesel fuel, with an initial concentration of 500 ppm and a volume of 250 cc, by a small-sized activated porous bed with FeCl3 and formaldehyde phenol as the active agents (30 g), was investigated at time intervals of 0.5-2.5 hours. The effect of contact time on the removal of sulfur is shown in Figure 6. As the figure shows, with increasing contact time, the removal efficiency increases and the maximum removal efficiency occurs at the initial 1.5 hours. Therefore, based on the figure, it can be concluded that the adsorption of sulfur compounds reaches equilibrium after 1.5 hours.
Figure 6: The relationship between the amount of the sulfur removed from the gas oil sample (500 ppm) and the time of desulfurization process by the small grain bed (30 g).
Effect of size and amount on adsorption
The effect of adsorbent size and amount on adsorption intensity under ambient temperature conditions has been investigated in two different states. In the first state, the effect of adsorbent particle size with dimensions of 5 mm and 2.5 mm has been examined Figure 7 and Figure 9. In this state, due to the reduction in particle size, the surface defects and micro-fractures increase in the smaller bed (2.5 mm) compared to the larger bed (5 mm), leading to an increase in adsorption. In the second state, the amount of adsorbent has been increased from 30 grams to 60 grams with the same dimensions of 5mm (Figures 7 and 8). The data in Table 7 shows that no significant change has occurred. Therefore, all dimensions of the adsorbent and the amount of adsorbent bed have been chosen as 2.5 mm and 30 grams, respectively.
Figure 7: The desulfurization percentage of four different gas oil samples by the manufactured adsorbent (Subproduct-B) with 30 (g) of the type large grain (with diameter 5 mm) which took time three hours.
Figure 8: The desulfurization percentage of four different gas oil samples by the manufactured adsorbent (subproduct-B) with 60 (g) of the type large grain (with diameter 5 mm) which took time three hours.
Figure 9: The desulfurization percentage of four different gas oil samples by the manufactured adsorbent (subproduct-B) with 30 (g) of the type small grain (with diameter 2.5 mm) which took time three hours.
The comparison between the desulfurization percentage of four various gas oil samples by the manufactured adsorbent (subproduct-B) with two types large grains (with diameter 5 mm) and small grains (with diameter 2.5 mm) and different amounts of porous beds which took time three hours Figure 10.
Figure 10: The comparison between the desulfurization percentage of four various gas oil samples by the manufactured adsorbent (subproduct-B) with two types large grains (with diameter 5 mm) and small grains (with diameter 2.5 mm) and different amounts of porous beds which took time three hours.
Adsorption isotherms
In this section, in order to investigate more about the sulfur elimination investigation, three adsorption isotherm models including, Langmuir, Freundlich and Dubinin-RadushKevich were applied.
The results regarding Langmuir adsorption isotherm model: Totally, two approaches demonstrate what could be happened during the adsorptive desulfurization process. In the former one, physical adsorption as a reversible reaction, sulfured structures would not chemically be changed by separation and also physisorption works as a multi- layered process by weak van der Waals forces between adsorbate and adsorbent. The amount of energy that is necessary for regeneration should be equivalent to the strength of the adsorption bonding. In the next one, chemical adsorption, comprises a chemical reaction between adsorbent and organosulfur compounds. Chemisorption is approximately a single-layered phenomenon. Usually, sulfur is attached to the sorbent as a sulfide structure. The adsorbent could be regenerated by thermal process or by flushing utilized sorbent with desorbent materials. Concerning the type of the process and also the feedstock nature, sulfur shall be generally removed as H2S, sulfur oxide gases or elemental sulfur which produces in SRU units (Sulfur Recovery Units) of gas refinery companies. Adsorption isotherms are models and equations used to describe the surface properties of adsorbents and provide insights into the process of surface adsorption and empirical data analysis. Isotherms are also an important factor in the design of adsorption systems and describe the relationship between the concentration of the adsorbed substance and the capacity of the adsorbent. In the Langmuir isotherm model, based on single-layer and homogeneous adsorbent material with uniform energy for all adsorption surfaces.
Qe=QmaxbCe/1+bCe (1)
Qe (mg/g): The concentration in the equilibrium state in the solid phase or equilibrium adsorption capacity.
Ce/Qe=1/Qmaxb+1/Qmax *Ce (2)
Ceq (mg/g) or ppm: The concentration in the equilibrium state in the liquid phase or residual.
Qe=(C0-Ce)V/M (3)
Sulfur removal (%)=C0-Ce/C0 *100 (4)
Qt=(C0-Ct)V/M (5)
Qmax (mg/g): The maximum concentration in the solid phase in which the most amount of sulfur has been adsorbed.
According to Equation 2 and achieved results, the (Qmax) is equal 27.77 (mg/g) and also the amounts of (b) and (R2) are obtained respectively 7.45 × 10-4 lit/mg and 0.973 (Figure 11).
Figure 11: The Langmuir adsorption isotherm model graph for the desulfurization of four various gas oil samples by the manufactured adsorbent (Subproduct-B) with large grains (with diameter 5 mm) which took time three hours.
The results based on Freundlich adsorption isotherm model: The adsorption of sulfur compounds in diesel fuel, with an initial concentration (C0) of 6380,1102,525 and 258 ppm and a volume of 250 cc, by samples the manufactured adsorbent (Subproduct-B) with 30 (g) of the large type grain (with diameter 5 mm) which took time three hours. Then each of the initial concentrations (C0) after 3 hours of contact with the activated substrate, were separated and measured for sulfur. The equilibrium concentrations (Ce) of 3902,435,176 and 62 were obtained. Qe were calculated according to equation 3, and then the logarithm of each of the initial concentrations (C0) and the logarithm of Qe were calculated. According to Table 8 and Equation 7, the isothermal adsorption diagram of Freundlich was obtained.
Qe=Kf*C1/n (6)
logQe=logKf+1/nlogCe (7)
| No. | C0 | Ce | logCe | Qe | logQe |
| 1 | 6380 | 3902 | 3.59 | 20.65 | 188.95 |
| 2 | 1102 | 435 | 2.63 | 5.55 | 78.38 |
| 3 | 525 | 176 | 2.24 | 3.24 | 54.32 |
| 4 | 258 | 62 | 1.79 | 1.63 | 38.03 |
Table 8: The results achieved according to Freundlich adsorption isotherm model for desulfurization of four different gas oil samples by the adsorbent (Subproduct-B) with 30 (g) of the type large grain (with diameter 5 mm).
In the Freundlich isotherm model the values of Kf and n constants exist. The non-dimensional index n represents the degree of desirability of the adsorption process (adsorption intensity) and Kf is the adsorption capacity of the adsorbent in terms of L/gr. In this model, values of n less than one indicate weak adsorption and values of 2-1 and 10-2 indicate moderate and strong adsorption, respectively. The values of the n and Kf coefficients are determined as the slope and intercept of the linear plot of logQe versus logCe. Furthermore, the Freundlich isotherm model is based on multilayer and heterogeneous adsorbent material on the adsorbent surface. According to the results of this study and the comparison of the Langmuir and Freundlich isotherm models, the data correspond more to the Freundlich isotherm, which is based on multilayer and heterogeneous adsorbent material. The best choice of the isotherm is based on the coefficient of correlation R2, which is higher for the Freundlich isotherm (R2=0.999) than for the Langmuir isotherm (R2=0.973), indicating multilayer adsorption, heterogeneity of the adsorbent surface and nonuniformity of adsorption sites on the adsorbent surface (Figure 12).
Figure 12: The Freundlich adsorption isotherm model graph for the desulfurization of four various gas oil samples by the manufactured adsorbent (Subproduct-B) with large grains (with diameter 5 mm) which took time three hours.
Based on Equation 7 and the obtained results, the (n) constant is equal to 1.64 that could notice in the range of satisfied and beneficial adsorption (1
lnQe=lnQm-βε2 (8)
ε=RTln(1+1/Ce) (9)
Ea=1/√ 2β (10)
| No. | C0 | Ce | Qe | lnQe | ε | ε2 |
| 1 | 6380 | 3902 | 20.65 | 3.03 | 0.634 | 0.4 |
| 2 | 1102 | 435 | 5.55 | 1.71 | 5.68 | 32.26 |
| 3 | 525 | 176 | 3.24 | 1.17 | 14.03 | 196.8 |
| 4 | 258 | 62 | 1.62 | 0.48 | 39.64 | 1571.3 |
Table 9: The results which got according to Dubinin-RadushKevich adsorption isotherm model for desulfurization of four different gas oil samples by the adsorbent (Subproduct-B) with 30 (g) of the type large grain (with diameter 5 mm).
Figure 13: The Dubinin-Radush Kevich adsorption isotherm model graph for the desulfurization of four various gas oil samples by the manufactured adsorbent (Subproduct-B) with large grains (with diameter 5 mm) which took time three hours.
The Dubinin-Radushkevich isotherm is used to determine the type of adsorption, as information about the adsorption type cannot be obtained using the Langmuir and Freundlich isotherms. Qm is the theoretical saturation capacity, β is the Dubinin-Radushkevich constant related to the average free energy of adsorption in mol2/kj2 and ε is the Polanyi adsorption potential, this is the amount of energy required to remove an adsorbed molecule from the adsorbent surface. This approach has been usually utilized to recognize the chemical and physical adsorption phenomenon with its free energy, where Ea per molecule of adsorbate could be calculated by the Equation 10. In chemical adsorption, the acceptable domain is 20 ≤ Ea ≤ 40. In this study, the amounts of Ea and Qm were respectively computed 21 KJ/mol and 8 mmol/g.
The results of this study demonstrate that the modification of the porous nano silica surface was done with chemical technique, in order to improve adsorption of thiophene and its derivatives from gas oil by iron metal. According to this study, the modified porous nano silica surface by iron, has increased the adsorption capacity several times. The reason of this increase, is the growth in the Lewis acidic sites on the surface of porous silica, accordingly, the acid-base reaction between adsorbent and adsorbed molecules will considerably go up. Additionally, the existence of iron on the surface of the adsorbent bed cause forming the π-bonding between the sulfured compounds and the iron atoms and as a result of that, the more number of molecules shall be able to adsorb on the silica surface. The results of X-ray diffraction analysis reasonably confirm that. The study of the adsorption isotherm models for SiO2 and SiO2/Fe (0.15) explained that the most conformity exists among Freundlich isotherm and the achieved results in related equilibrium reactions. To sum up, according to this research, by modification of commercial silica beds with iron, we could substitute an economic, profitable, efficient and green method, instead of some expensive and complicated processes which require high temperature and pressure such as HDS for removing sulfured structures including, thiophene and its derivatives.
The authors gratefully appreciate the Department of Chemistry and especially the Research Department of the Islamic Azad University of Arak for their kind support.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Ramezani M, Shafiei H, Zolfaghari MR (2025) Study and Design of Novel Process of Adsorption Desulfurization of Liquid Fuels by Appling Modified Nano SiO2 Porous Beds. J Phys Chem Biophys. 15:423.
Received: 16-Mar-2024, Manuscript No. JPCB-24-30211; Editor assigned: 18-Mar-2224, Pre QC No. JPCB-24-30211 (PQ); Reviewed: 01-Apr-2024, QC No. JPCB-24-30211; Revised: 07-Feb-2025, Manuscript No. JPCB-24-30211 (R); Published: 14-Feb-2025 , DOI: 10.35841/2161-0398.25.15.423
Copyright: © 2025 Ramezani M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.