Journal of Plant Biochemistry & Physiology
Open Access
ISSN: 2329-9029
ISSN: 2329-9029
Review Article - (2019)Volume 7, Issue 2
Sweet potato (Ipomoea batatas (L.) Lam.) has several phytochemicals including anthocyanin that provide many special health-promoting functions as well as other functional components. Sweet potato is thought to be a healthgiving food owing to the numerous diversities of natural products, especially antioxidants. Anthocyanins are natural hydro-soluble pigments which give the purple, blue and red colouration evident in fruits, leaves, flowers, and storage organs. Anthocyanins are beneficial to human health due to their potent antioxidative properties which protect against several chronic disorders, thus a valuable constituent in the human diet. The pathway for biosynthesis of anthocyanin has been clearly defined with its key regulatory genes identified and segregated in diverse species. Cyanidin or Peonidin 3-sophoroside-5-glucoside with associated acylated derivate are the two primary anthocyanins identified in purple-fleshed sweet potato. Anthocyanins in sweet potato are of great importance to plants, animals, humans and possess scientific benefits as well. This article provides a summary of current findings on the function, structure, and biosynthesis of anthocyanin in sweet potato.
Biosynthesis; Sweet potato (Ipomoea batatas (L.) Lam.); Antioxidant; Anthocyanin; Purple-fleshed
As naturally occurring pigments, anthocyanins are accountable for the bright and distinct colouration of several plant tissues [1]. They are an essential hydro-soluble pigment which is abundant in nature. Anthocyanins belong to the flavonoid family of phytochemicals and their flavylium (2-phenylchromenylium) ions distinguish them from the other flavonoids (Figure 1) [2,3].
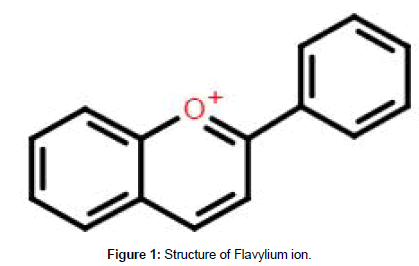
Figure 1. Structure of Flavylium ion.
Anthocyanin originates from the two Greek words Anthos meaning flower and ‘kyanos’ meaning dark blue [4]. Anthocyanins are present in fruits, leaves, vegetables, flowers, stem and storage organs, such as roots and tubers [5-8]. Generally, anthocyanins accrue in leaves, flowers, stems and fruits of Solanaceous vegetables precisely in potato tubers, fruits of pepper and tomato, eggplant peels, as well as the flesh of certain species of potato [9-11].
Research on anthocyanin has grandstanded due to their remarkable antioxidative properties. Anthocyanins possess antioxidant and antiinflammatory properties making them good protective agents. These natural antioxidants scavenge free superoxide radicals, protect biological systems from their damaging effects posed on macromolecules including carbohydrates, proteins, lipids and DNA, impairs the imminence of cancers [12], suppress the risk of arthritis, cardiovascular diseases, diabetes and neurological diseases [13,14]. Moreover, anthocyanin has other beneficial dietary effects on human health [15] such as improvement of visual acuity, antimutagenic properties, antimicrobial potencies and protection of the skin against UV-B radiation. Also, anthocyanins are a natural alternative to synthetic food colours [16]. These natural antioxidants are reported to be present in sweet potato, especially purple-fleshed sweet potato.
Sweet potato (Ipomoea batatas (L.) Lam.) is a dicot perennial plant [17] belonging to the Convolvulaceae (morning glory) family [18]. It is considered the 7th most essential staple food crop in the world with reference to production [19]. Sweet potato is cultivated extensively and consumed in tropical and subtropical regions specifically in Sub-Sahara Africa, East Asia, and the Pacific Islands [20,21] with about 95% of the production mainly from Asia and Africa [22]. Globally, Ipomoea batatas is considered as a major staple crop especially in developing countries [23]. It is an excellent source of energy with various valuable by-products [24], such as carbohydrates, β-carotene, dietary fibre, minerals and other nutrients, the quantities dependent on the variety [25].
Also, sweet potato has several phytochemicals [20] that provide many special health-promoting functions as well as other functional components [26]. Ipomoea batatas is reckoned to be a health-giving food by virtue of its numerous diversities of metabolites, specifically antioxidants [27]. However, there are variations in size, the colour of flesh (white, orange, cream, yellow, and purple) and skin colour [28] among the various sweet potato cultivars globally. The anthocyanin contents in purple-fleshed sweet potato are relatively higher compared to other flesh colours (white, cream, yellow or orange) with little or no anthocyanins [8].
Purple-fleshed sweet potato has gained recognition by dint of their copious physiological properties including antioxidative, antimutagenic, and antihypertensive activities [29]. Such sweet potatoes have similar nutritional benefit as white, cream, yellow and orange-fleshed sweet potatoes but contain several useful pigments, such as anthocyanin [29]. Although summarised information regarding the occurrence of anthocyanin, its functions and structure in sweet potato is limited, this review summarises previous reports on anthocyanins, their structure, functions and biosynthetic pathway in sweet potato.
Classification of anthocyanins
By chemical composition, anthocyanins may be grouped into two major classes; flavonoids and phenolics. However, anthocyanins are a diverse class of flavonoid compounds made of anthocyanidin aglycons bound to one or more sugar moieties [30,31]. Meanwhile, studies have discovered more than twenty (20) anthocyanidin but only six (6) main types; malvidin, cyanidin, peonidin, delphinidin, pelargonidin and petunidin occur in plants (Figure 2) [1,32-34].
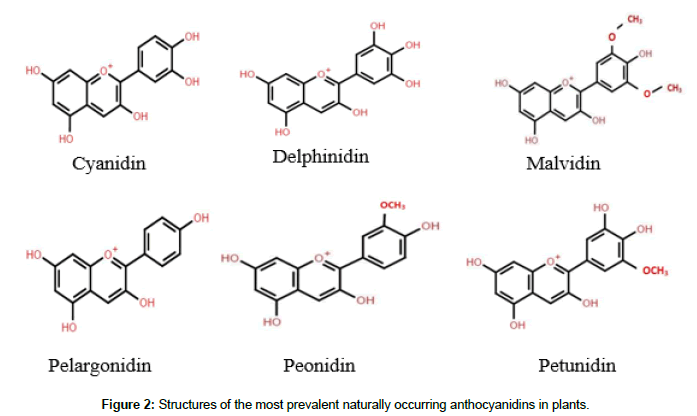
Figure 2. Structures of the most prevalent naturally occurring anthocyanidins in plants.
Virtually, they are versatile natural hydro-soluble plant pigments seen as poly-methoxyl and poly-hydroxyl glycosides obtained from flavylium ions or 2-phenylbenzopyrylium [32]. Flavonoids, the most important group of secondary plant metabolites and bioactive compounds, responsible for the broadest colour range in different plant tissues belong to the class of phenylpropanoid [35]. Even though only O-glycosylation are found in most plant species, other substitution motifs including 3-deoxy, 5 and 7-methoxy and 6-hydroxy anthocyanidins are also found in some plant species [36]. However, some current studies have revealed the occurrence of C-glycosylation in diverse species [37].
Chemical structure of anthocynanins
Chemically, anthocyanins are seen as glycosides of poly-hydroxyl or poly-methoxyl derivate of the flavylium (2-phenylbenzopyrylium) ion consisting of a double benzoyl ring (A and B) which is divided by a heterocyclic ring (C). The basic structures of anthocyanins as shown in Table 1, occur as glycosylated aglycons of their respective anthocyanidin.
| Aglycons | Abbreviation | Pattern of Substitution | Visible color | ||
|---|---|---|---|---|---|
| R1 | R2 | R3 | |||
| Cyanidin | Cy | OH | OH | H | Orange - red |
| Peonidin | Pn | OCH3 | OH | H | Orange - red |
| Pelargonidin | Pg | H | OH | H | Orange |
| Delphinidin | Dp | OH | OH | OH | Purple |
| Malvidin | Mv | OCH3 | OH | OCH3 | Blue - red |
| Petunidin | Pt | O CH3 | OH | OH | Purple |
Table 1: Chemical structure of anthocyanin (Structural identification of anthocyanidin aglycons).
Generally, they occur as 3-glycosides or 3, 5-di-glycosides of galactose, glucose, arabinose or rhamnose attached to a sugar residue [38,39]. The naturally non-methylated anthocyanidin glycosides (cyanidin, pelargonidin, and delphinidin) mostly exist in eighty percent of pigmented leaves, sixty-nine percent of fruits and fifty percent of flowers [40]. Variations in colour and structure amongst anthocyanins depend on the B-ring substitution pattern, the glycosylation pattern, the nature and number of esterification of saccharides, the pH, temperature, and the occurrence of co-pigments [3,41]. Metal ions, light, ascorbic acid, oxygen, and other enzymes lead to colour degradation of anthocyanins, consequently affecting their structure and stability. In retaining the colour and the functionality of anthocyanins, it is essential to increase their stability as they can easily degrade to produce brown or colourless compounds because of their high reactivity rate [42]. Chemical structure of anthocyanins mainly depends on esterification of sugars built on carboxylic acids, p-coumaric acid, aliphatic acids, acetic acid, malonic, oxalic, as well as succinic acid [43].
At low pH, anthocyanins are more stable and highly coloured but the chemical structure and colour changes as pH increases. The flavylium ion species is highly concentrated and gives purple and red hues at pH1– 3 whereas pH4-5 produces the colourless carbinol. Quinoidal blueviolet species and chalcone colourless species dominate at pH 6-7 and pH 7-8 respectively. However, due to the susceptibility of anthocyanins to pH flux, its bioavailability is limited [41]. Comparatively, the glycosylated anthocyanins found in purple-fleshed sweet potatoes are in their aromatic acylated forms and have excellent tolerance for high temperatures and pH [44] (Table 1). Chemical structure of anthocyanin (Structural identification of anthocyanidin aglycons) (Figure 3).
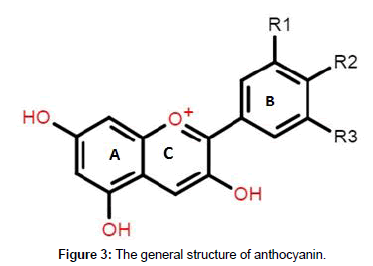
Figure 3. The general structure of anthocyanin.
Functions of anthocyanins
Anthocyanins in sweet potato have several functions which are beneficial to both plants and animals, especially its role in human health. As a water-soluble natural pigment, anthocyanins play significant roles in so many fields. Anthocyanin enhances reproduction in plants due to the bright colours it confers on several fruits and flowers which invite insects for pollination and seed dispersal [45-47]. Again, anthocyanin synthesis is affected under stress conditions [48] or infection by pathogenic organisms [49] and may offer protection for plants against oxidative destruction including those triggered by high irradiation when the capacity for carbon fixation declines.
Anthocyanins also safeguard plants against some biotic and abiotic stresses which may offer them an improved adaptation to climatic changes [50,51]. As photoprotective agents, anthocyanins protect the photosynthetic tissues by absorbing surplus visible and ultraviolet radiation and also act as superoxide radical scavengers [52,53]. Furthermore, anthocyanins accrue in underdeveloped somatic tissues and light-exposed parts of fruits to offer protection against photoinhibition and photo-bleaching under light stress without considerably affecting the process of photosynthesis [54-57]. In addition, coloured anthocyanins have the potency to reduce insect and pathogenic infestations. For example, tomato fruits enriched in anthocyanin were not susceptible to gray mold [58]. Also, large numbers of Helicoverpa armigera died and pupation delayed in Spodoptera litura when fed with anthocyanin-rich leaves relative to those victualled with green leaves [59].
Concerning improving post-harvest performance in vegetables, anthocyanin acts as antioxidants which inhibit the oxidation of lipids and sustain membrane probity to impede cell-aging [60]. Another study revealed that tomato with appreciable amounts of anthocyanins had extended shelf-life and over-ripening was significantly reduced [58,61].
Aside its countless roles of in plants, anthocyanins derived from purple-fleshed sweet potato offer a striking impact on human health owing to their biological functions which work effectively against free radicals, bacterial infections, cancers, inflammations, diabetes [62], prevention of cognitive disorders and neurodegenerative diseases [63]. Reportedly, dietary anthocyanins are related to offering immunity against certain cancers, cardiovascular diseases and many chronic disorders [13,64]. Some studies have accredited the shielding effects of dietetic anthocyanins to their antioxidative capability, but their bioactivity is minimal and may probably stimulate health by repressing specific signalling pathways connected with inflammation and disease occurrence [65].
Therefore, the vast quantities of anthocyanins in the tubers of purple-fleshed sweet potato make it a potential component to develop pharmaceuticals including antioxidative, antineoplastic and antiinflammatory agents [20]. Usually, purple-fleshed sweet potato is transformed into puree (cooked puree, dried, and powdered) and used as a functional ingredient in the food processing industries [66-69]. Purple-fleshed sweet potatoes are used to produce coloured food products with excellent colour potency [69,70]. Meanwhile, crude purple-fleshed sweet potato anthocyanins are reported to inhibit the development of Bacteroides, Prevotella and Clostridium histolyticum [71].
Regulators of anthocyanin biosynthesis
In many plant species, the primary regulatory genes of anthocyanin biosynthesis have extensively been analysed and isolated [32,72]. Though there is post-transcriptional modulation of anthocyanin biosynthesis, the primary level at which anthocyanin biosynthesis is inducted or shut down in plants is controlled by the expression of genes that regulate the biosynthetic process. [73]. Structural and regulatory genes are the two main types of biosynthetic genes.
The structural genes translate the enzymes which catalyze every reaction step while the regulatory genes encrypt transcriptional components that regulate structural gene expression [30,74,75]. In dicot plants, structural genes have two divisions; early (CHI, CHS, FLS, F3′H and F3H,) and late (UFGT, ANS/LDOX and DFR) biosynthetic genes [74]. These structural genes operate under the MYB-bHLHWD40 (MBW) regulatory network made up of the MYB, basic helixloop- helix (bHLH) and WD40 replicate families. For instance, the MYB domain C1 protein which regulates anthocyanin biosynthesis in Zea mays requires a bHLH partner to activate the flavonoid structural genes and the dihydroflavonol reductase (DFR) promoter, although the MYB domain P protein which controls phlobaphene to stimulate the promoter lacks a bHLH partner [76]. These MYB proteins have a central responsibility of regulating the biosynthesis of different secondary metabolites, signal transduction, resistance to diseases as well as growth and developmental fluctuations [74].
The structurally conserved MYB genes comprise 100–160-bp DNAbinding regions which consist of one or more replications. The R2R3 MYB genes which bear two repeats are the predominant group of MYB genes involved in the flavonoid pathway in plants. Thirteen (13) out of the 126 constituents of the R2R3 sub-division have been identified to regulate flavonoid metabolism in Arabidopsis while 14 R2R3 MYB associates were identified to control the biosynthesis of flavonoid in grapes [77]. Previous studies have established that structural genes associated with anthocyanin synthesis were uniformly expressed and their expression levels dependent on the concentration [15,78]. Three genes have been found in Vitis vinifera to code for CHS [79,80] while in Malus pumila, MdF3H, MdANS, MdCHS, pDFR and pUFGluT are responsible for anthocyanin biosynthesis [81].
Moreover, the UFGT transcriptional levels are overly expressed in red fruits than white skinned grapes [79] compared to CHS, UFGT and ANS in citrus [82]. Therefore, to increase the synthesis of anthocyanin solely depends on structural gene expression relative to a particular species [83]. The IbMADS10 gene appeared to control anthocyanin biosynthesis in sweet potato [84]. However, the IbMYB1 was found to regulate the biosynthesis of anthocyanin out of the two MYB genes (IbMYB1 and IbMYB2) identified in the purple sweet potato (Ayamurasaki) cultivar, precisely its tuberous roots as reported by Mano et al. [85].
Biosynthetic pathway of anthocyanin
Anthocyanins are produced through the phenylpropanoid pathway (Figure 4). Phenylalanine ammonia lyase (PAL) deaminate phenylalanine which is produced through the shikimate pathway on the cytoplasmic surface of the rough endoplasmic reticulum (RER) to produce trans-cinnamic acid [86,87]. Cinnamate 4-hydroxylase then transforms trans-cinnamic acid into p-coumaric acid. In some plant species, tyrosine ammonia lyase (TAL), a minor substrate for phenylalanine ammonia lyase, converts tyrosine to p-coumaric acid [88,89]. A combination of p-coumaric acid with co-enzyme A is digested by 4-coumarate-CoA ligase (4CL) to yield p-coumaroyl-CoA [90]. The biosynthesis of polyketides (flavonoids, stilbenes, isoflavonoids, and pyrenes), monolignols (lignin and lignans, salicylic acid and aromatic volatiles) and coumarins occur as the phenylpropanoid pathway diverges.
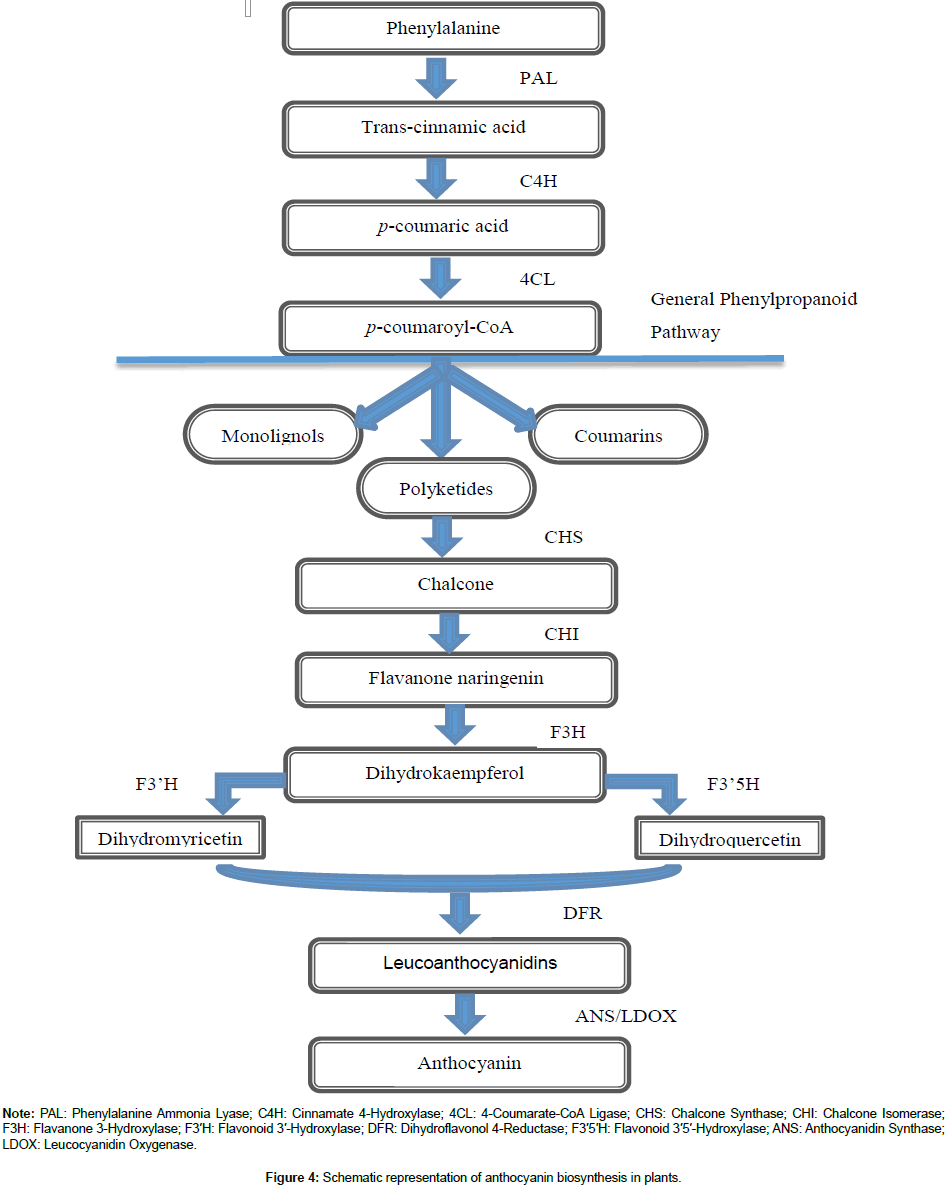
Figure 4. Schematic representation of anthocyanin biosynthesis in plants.
As the flavonoid progresses, chalcone synthase (CHS) converts the condensed p-coumaroyl-CoA combined alongside three molecules of malonyl-CoA to synthesize chalcone [91]. Chalcone isomerase (CHI) converts chalcone to flavanone naringenin which is later digested by flavanone 3-hydroxylase (F3H) to form flavonol dihydrokaempferol (DHK or aromadendrin). DHK is then utilized by flavonoid 3′-hydroxylase (F3′H) or flavonoid 3′5′-hydroxylase (F3′5′H) to synthesize dihydroquercetin (taxifolin) or dihydromyricetin (ampelopsin) respectively. The DHK is again converted by dihydroflavonol 4-reductase (DFR) to form leucoanthocyanidins which are also transformed into coloured anthocyanidins (delphinidin, cyanidin and pelargonidin,) by anthocyanidin synthase (ANS) also termed leucocyanidin oxygenase (LDOX). Methyltransferases (OMT) and acetylates embellish anthocyanidins to produce anthocyanidin- 3-O-glucoside (a chemically constant hydro-soluble pigment) by 3-O-glycosyl transferases (3GT) [92]. The Multi-antimicrobial extrusion (MATE) protein and ATP-binding cassette (ABC) transporters confined in the tonoplast help link anthocyanins with glutathione S-transferase (GST) to effectively segregate towards the vacuole and may also adhere to anthocyanoplasts, a pre-vacuolar section proceeding to the vacuole [93–96]. Moreover, aromatic acylated anthocyanins build-up at high levels inside the vacuole to form AVI (anthocyanin vacuolar inclusions) in some species [97].
Anthocyanin in sweet potato
Sweet potato (Ipomoea batatas (L.) Lam.) is identified to be an exceptionally nutritious vegetable containing high levels of dietary fibre, minerals (such as Ca, Mg, K and Zn), polysaccharides, vitamins (including B1, B2, C and E), phenolic compounds such as anthocyanins, beta-carotene and tocopherol [98–103]. Research has focused on developing new sweet potato varieties with an excellent measure of phytochemicals such as anthocyanins because of its nourishing health benefits. For instance, purple-fleshed sweet potato, accommodates several beneficial and functional pigments such as anthocyanins, compared to other flesh colours (white, cream, yellow or orange) with little or no anthocyanins. In addition to the appreciable measures of anthocyanin, its storage roots contain the same nutritional value as yellow, cream, orange and white -fleshed sweet potatoes.
Traditional red-skinned sweet potato naturally holds high volumes of anthocyanins in its skin, but usually peeled off before eating. Thus, in most Asian countries, red-purple-fleshed sweet potatoes with considerable amounts of phenolics such as anthocyanins have been developed and introduced over the past years [104-109]. Purplefleshed sweet potatoes have gained recognition due to their multiple physiological functions [29] and a variety of beneficial health effects including radical-scavenging [110,111], antimutagenic [112], hepato-protective [113], antihypertensive, chemopreventive [114], antihyperglycemic [115] and antidiabetic activities [116] as well as anti-inflammatory, antimicrobial activity, protection against ultraviolet radiation and reduction of memory loss [117,118]. Anthocyanins influence the biological effects seen in purple-fleshed sweet potato and have the potency to be used as natural food colours against synthetic ones [29].
Comparatively, anthocyanins extracted from purple-fleshed sweet potato exhibits high colour stability than those extracted from plants such as raspberry, red cabbage, strawberry, apple and perilla owing to their unique mono- or di- acylated forms [119,120]. Primarily, 93% of anthocyanins obtained from purple-fleshed sweet potato existing as acylated compounds show improved strength and biological properties [44,121,122]. Nonetheless, only a handful of purple-fleshed genotypes have been established to be commercially profitable [111].
Purple-fleshed sweet potato is typically categorized as either cyanidin-dominated or peonidin-dominated depending on the proportion of cyanidin to peonidin aglycons which is an essential determiner of flesh colour and perhaps specific functional differences. Usually, sweet potatoes with high levels of peonidin have pale red to reddish flesh and are termed red fleshed. On the other hand, flesh colour changes to purple and dark purple as the content of cyanidins increase. Structurally, cyanidin has a stronger antioxidative activity because it accommodates more hydroxyl groups than peonidin [98].
Cyanidin and peonidin 3-sophoroside-5-glucoside acylated with p-hydroxybenzoic, ferulic, p-coumaric and caffeic acids are the main anthocyanins among the 39 anthocyanins discovered in purple-fleshed sweet potato [44,70,98,123]. Amongst the 12 individual anthocyanins detected and evaluated in the new purple-fleshed sweet potato cultivar (P40), eleven (11) of them were acylated while seven (7) were cyanidin derivate. The most prevalent anthocyanin which accounted for about half of the total quantity of anthocyanin in P40 were cyanidin 3-caffeoyl-p-hydroxy benzoyl sophoroside-5-glucoside, peonidin 3-caffeoyl sophoroside-5-glucoside, and cyanidin 3-(600-caffeoyl- 600-feruloyl-sophoroside)-5-glucoside. It was concluded that P40 might be the maiden cyanidin-dominated purple-fleshed sweet potato containing a remarkable measure of anthocyanin [98]. Another study also identified peonidin types as the major anthocyanins identified in the purple-fleshed variety from China. Five anthocyanins of peonidintype monomers exhibited a robust antioxidant property in vitro which might be a potential natural probiotic source and can hinder the proliferation of infectious agents in the intestine such as S. aureus and S. typhimurium. The findings uncovered the probable beneficial effects of incorporating the Chinese variety of purple-fleshed sweet potato in human nutrition. Also, the properties of anthocyanin monomer make it beneficial for the production of pharmaceutical and used as a functional food [57].
Zhu et al. [57] concluded that the major anthocyanins among the twelve types identified and isolated from ten Chinese genotypes were peonidin or cyanidin 3-sophoroside-5-glucoside together with their acylated derivate. However, the major anthocyanin in purple-fleshed cultivar, Tainung 73 were cyanidin and peonidin with their acylated derivatives [124]. In Eshu No. 8, also a purple-fleshed variety, the major anthocyanins identified were cyanidin or peonidin 3-sophoroside-5- glucoside mono or di-acylated with phenolic acid on the 3 position of the sophorose moiety [125].
Sweet potato is a nutrient-filled vegetable with several healthpromoting benefits. It contains considerable amounts of anthocyanin, a natural pigment making sweet potato an excellent antioxidant and anti-inflammatory agent. Anthocyanins are the essential hydro-soluble natural pigments and are composed of anthocyanidin aglycons bound to sugar moieties. The biosynthetic pathway of anthocyanin has been completely elucidated and the regulatory genes together with their respective enzymes have been identified and isolated in numerous species. Cyanidin, malvidin, pelargonidin, delphinidin, petunidin and peonidin are the major anthocyanins in plants. The antioxidant properties of anthocyanin make them good protective agents against neurological and cardiovascular diseases coupled with other beneficial health effects. Alternatively, anthocyanin plays significant roles in plants by producing bright colours which invite insects for pollination and seed dispersal to enhance reproduction, safeguard photosynthetic structures and scavenge free radicals. Anthocyanins may also be adopted as natural substitutes to artificial food dyes. Anthocyanins from sweet potato especially, those with purple-flesh are mainly categorized as peonidin or cyanidin 3-sophoroside-5-glucoside together with their mono or di-acylated derivate while the IbMADS10 gene regulates anthocyanin biosynthesis. Due to the limited information on anthocyanins in sweet potato, it is suggested that more research should be focused on this area to determine the functions, structure and biosynthesis in sweet potato.
This work was supported by National Natural Science Foundation of China -Guangdong Joint Fund, China (U1701234) and Studies on Resistance Resources and Molecular Mechanisms of Sweet potato Weevil in South China (U1701234).
All authors had the responsibility for manuscript preparation, critical discussions and approval of the final manuscript.
The authors declare no conflict of interest.
Citation: Amoanimaa-Dede H, Hongbo Z, Kyereko WT, Yeboah A, Agyenim-Boateng KA (2019) Structure, Functions and Biosynthetic Pathway of Naturally Occurring Anthocyanin in Sweet Potato - A Review. J Plant Biochem Physiol 7: 234. doi: 10.35248/2329-9029.19.7.234
Received: 23-Apr-2019 Accepted: 08-May-2019 Published: 15-May-2019 , DOI: 10.35248/2329-9029.19.7.234
Copyright: © 2019 Amoanimaa-Dede H, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.