Forest Research: Open Access
Open Access
ISSN: 2168-9776
ISSN: 2168-9776
Research Article - (2025)Volume 14, Issue 3
Concerns about the sustainability of forest ecosystems worldwide, particularly the Indian state of Himachal Pradesh's forests in Solan, have grown due to climate unpredictability. The impact of climatic variability on plant biodiversity in these forests is investigated in this research, with particular attention to changes in species distribution, composition and forest structure. The purpose of this study is to quantify the magnitude of changes brought about by the climate and offer possible mitigating techniques. The timing of the plant life cycle, including the commencement of leafing, flowering and fruiting, has changed as a result of temperature and precipitation pattern changes. Phenological changes may cause a mismatch between the demands of animals and the resources available, which may have an impact on the species' capacity to survive and procreate.
Climate change; Understory vegetation; Biodiversity; Forest; Altitude; Family; Distribution
A significant issue in the Solan region of Himachal Pradesh has been the effect of climatic variability on understory vegetation in various forest types. Known for its varied flora and wildlife, the western Himalayan area includes the Solan district. From subtropical to alpine, the region's diverse climate provides a special home for a variety of species [1]. Climate variability has been shown to impact understory vegetation distribution and composition in the Solan district's various forest types. Being the primary source of food and shelter for species, the understory vegetation is vital to the ecosystem. Moreover, it contributes to the overall health and productivity of the forest ecosystem.
Furthermore, the development and productivity of understory vegetation have been impacted by climatic variability. The development and productivity of understory plants can be impacted by variations in soil moisture and nutrient availability brought about by shifts in temperature and precipitation patterns. Changes in the understory vegetation's productivity can impact the resources available to other creatures in the ecosystem, which can have a domino effect on the ecosystem as a whole. One of the most important environmental issues of our day is climate variability and research on how it affects vegetation is essential. There are many different types of woods in the Solan district of Himachal Pradesh, India. It is crucial for these forests to be conserved to comprehend how climatic variability affects the understory vegetation in these forests.
Study area
The experimental area is situated between 300 meters and 3000 meters above mean sea level in the Himachal Pradesh mid-hill region. The region is located between latitudes 30°50'30" and 30°52'0" N and longitudes 77°8'30" and 77°11'30" E (survey of India Toposheet No. 53F/1).
This region is a transitional zone between humid temperate and subtropical climates. The experimental location experiences significant seasonal and diurnal temperature variations. May and June are typically the warmest months, while December and January are the coldest and have heavy frost throughout the winter. The annual rainfall ranges from 1000 mm to 1400 mm on average, with the majority falling between July and September during the monsoon season and a few pre-monsoon showers. Winter precipitation is frequent but infrequent. Snowfall is an uncommon phenomenon. In winter (January) the lowest temperature is 3°C, while in summer (June) the maximum temperature is 33°C. The Mean Annual Temperature (MAT) is, however, 19°C. The vegetation of the area includes Cedrus deodara, Quercus leucotrichophora, Pinus roxburghii and mixed forest (Table 1) [2].
| Forest type | Location | Latitude | Longitude | Elevation |
| Cedrus deodara | Chail | 30°57'47''.016N | 77°11'42.9612''E | 1750-2250 m |
| Quercus leucotrichophora | Chail | 30°57'47''.016N | 77°11'42.9612''E | 1600-1800 m |
| Pinus roxburghii | Barog | 30°53'26''.196N | 77°10'32.844''E | 1600-1700 m |
| Mixed forest | Parwanoo | 30°50'27.24''N | 76°57'24.12''E | 762-941 m |
Table 1: General details of the study area.
Climatic data
The meteorological observatory at the college of forestry provided the temperature (maximum and lowest) and rainfall data for the period of 2012 to 2022. The average maximum temperature for the period of 2012-2022, according to meteorological data, varied between 24.7°C and 26.5°C. 10.5°C to 12.2°C was the average low temperature recorded. Meteorological data from 2012 to 2022 showed that the average amount of rainfall ranged from 897.1 mm to 1243.4 mm. The resulting data indicated an increasing slant in the maximum and lowest temperatures for the years 2016 and 2022. Although there was a noticeable decline in the average annual rainfall in 2016 and a subsequent progressive increase in rainfall, the maximum annual rainfall of 1243.4 mm was not reached until 2022 (Table 2 and Figures 1 and 2) [3].
| Year | Maximum temperature | Minimum temperature | Rainfall (mm) |
| 2012 | 25.3 | 10.5 | 897.1 |
| 2013 | 25 | 11.5 | 1072.5 |
| 2014 | 24.9 | 10.7 | 1170.7 |
| 2015 | 25.1 | 11.6 | 1008.2 |
| 2016 | 26 | 12.2 | 722.4 |
| 2017 | 25 | 11.9 | 1071.1 |
| 2018 | 24.7 | 11.5 | 1200.2 |
| 2019 | 24.7 | 11.5 | 1053.6 |
| 2020 | 25.9 | 11.1 | 1054 |
| 2021 | 26.5 | 11.4 | 1055 |
| 2022 | 26.5 | 11.7 | 1243.4 |
Table 2: Maximum temperature, maximum temperature and rainfall data (2012-2022).
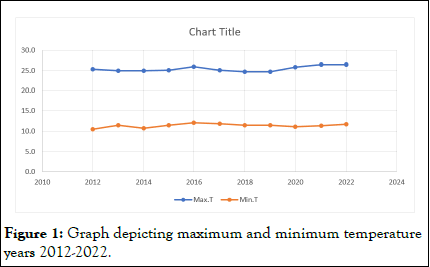
Figure 1: Graph depicting maximum and minimum temperature years 2012-2022.
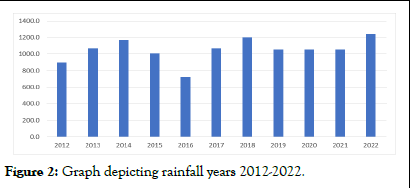
Figure 2: Graph depicting rainfall years 2012-2022.
For carrying out tree enumeration and biomass estimation, three plots of size (0.1 ha × 0.1 ha) were selected with five (5 m × 5 m) and five quadrat sub-plots (1 m × 1 m) were laid out within each tree sample plot the latter two types of subplots to explore shrub and herb related traits, respectively. For the allocation of shrub and herb traits, random sampling method was used (Table 3 and Figure 3).
| Criteria | Size | Replication |
| Plot | 0.1 ha à 0.1 ha | 3 |
| Sub plot 1 (shrubs) | 5 m à 5 m | 5 |
| Sub plot 2 (herbs) | 1 m à 1 m | 5 |
Table 3: Tree enumeration and biomass estimation.

Figure 3: Map of the study area.
For the purposes of the biodiversity research, four primary woods in Solan city-Cedrus deodara, Quercus leucotrichophora, Pinus roxburghii and mixed forest were examined. The Table 4 provides a summary of the distribution of shrub and herb species across four different forest types: Cedrus deodara, Quercus leucotrichophora, Pinus roxburghii and mixed forest. It also includes the total number of species present in each forest type and the overall total representation across all types (Table 4).
| Category | Forest types | ||||
| Cedrus deodara | Quercus leucotrichophora | Pinus roxburghii | Mixed forest | Total representation in forest type | |
| Shrubs | 27 | 37 | 50 | 25 | 139 |
| Herbs | 115 | 132 | 167 | 47 | 461 |
| Total | 142 | 169 | 217 | 72 | 600 |
Table 4: Inventory of shrubs and herbs in different forest types in Solan district of Himachal Pradesh.
Forest type analysis
Cedrus deodara forests: These forests have 27 shrub species and 115 herb species, making a total of 142 species. The presence of a moderate number of both shrubs and herbs suggests that this forest type offers suitable conditions for a diverse understory, though not as rich as in some other forest types [4].
Quercus leucotrichophora forests: With 37 shrub species and 132 herb species, there is a total of 169 species in these oak forests. This indicates a relatively high biodiversity, which could be due to the favourable microclimate and soil conditions provided by the broad-leaved canopy of oak trees.
Pinus roxburghii forests: Exhibiting the highest diversity among the listed forest types, pine forests support 50 shrub species and 167 herb species, totalling 217 species. The high numbers reflect the adaptability of a wide range of species to the conditions found in pine forests, such as light availability and soil acidity.
Mixed forests: These forests contain 25 shrub species and 47 herb species, with a combined total of 72 species. The lower numbers may be due to the complex interactions between tree species in mixed forests, which can create specific ecological niches that are less conducive to a large number of understory species.
The 'total representation in forest type' row shows the sum of shrub and herb species in each forest type, while the 'total' column at the end sums up the total number of shrub and herb species across all forest types, amounting to 600.The data indicates that Pinus roxburghii forests are the most diverse in terms of shrub and herb species, followed by Quercus leucotrichophora forests, Cedrus deodara forests and finally mixed forests (Table 5).
| Sr. no No. | Family | Cedrus deodara | Quercus leucotricophora | Pinus roxburghii | Mixed forest | Total |
| 1 | Acanthaceae | + | + | + | + | 4 |
| 2 | Adiantaceae | - | - | + | - | 1 |
| 3 | Adoxaceae | + | + | + | - | 3 |
| 4 | Agavaceae | - | - | + | + | 2 |
| 5 | Amaranthaceae | + | + | + | + | 4 |
| 6 | Apiaceae | + | + | + | - | 3 |
| 7 | Apocynaceae | + | + | + | + | 4 |
| 8 | Araceae | + | + | + | - | 3 |
| 9 | Araliaceae | + | + | - | - | 2 |
| 10 | Asparagaceae | - | - | + | + | 2 |
| 11 | Aspleniaceae | + | + | + | - | 3 |
| 12 | Asteraceae | + | + | + | + | 4 |
| 13 | Athyriaceae | + | + | - | - | 2 |
| 14 | Balsaminaceae | + | + | + | + | 4 |
| 15 | Begoniaceae | + | - | + | - | 2 |
| 16 | Berberidaceae | + | + | + | - | 3 |
| 17 | Boraginaceae | + | + | + | + | 4 |
| 18 | Brassicaceae | + | + | + | - | 3 |
| 19 | Buxaceae | - | + | + | - | 2 |
| 20 | Cactaceae | - | - | + | + | 2 |
| 21 | Campanulaceae | - | - | + | - | 1 |
| 22 | Cannabaceae | + | + | - | - | 2 |
| 23 | Capparaceae | + | + | - | - | 2 |
| 24 | Caprifoliaceae | + | + | + | + | 4 |
| 25 | Caryophyllaceae | + | + | + | - | 3 |
| 26 | Celastraceae | + | + | + | - | 3 |
| 27 | Clochicaceae | - | + | + | - | 2 |
| 28 | Commelinaceae | + | + | + | - | 3 |
| 29 | Convolvulaceae | - | + | + | + | 3 |
| 30 | Coriariaceae | + | + | + | - | 3 |
| 31 | Crassulaceae | - | - | + | - | 1 |
| 32 | Cucurbitaceae | - | - | + | + | 2 |
| 33 | Cyperaceae | + | + | + | + | 4 |
| 34 | Dennstaedtiaceae | + | + | - | - | 2 |
| 35 | Dioscoreaceae | + | + | + | + | 4 |
| 36 | Dryopteridaceae | + | + | + | - | 3 |
| 37 | Elaeagnaceae | + | + | + | - | 3 |
| 38 | Euphorbiaceae | - | - | + | + | 2 |
| 39 | Fabaceae | + | + | + | + | 4 |
| 40 | Gentianaceae | - | + | + | - | 2 |
| 41 | Geraniaceae | + | + | + | - | 3 |
| 42 | Gesneriaceae | - | - | + | - | 1 |
| 43 | Hydrangeaceae | + | + | + | - | 3 |
| 44 | Hypericaceae | + | + | + | - | 3 |
| 45 | Lamiaceae | + | + | + | + | 4 |
| 46 | Lauraceae | - | - | + | - | 1 |
| 47 | Liliaceae | + | + | + | - | 3 |
| 48 | Lythraceae | - | - | + | + | 2 |
| 49 | Malvaceae | - | - | - | + | 1 |
| 50 | Menispermaceae | - | - | + | + | 2 |
| 51 | Moraceae | - | - | + | - | 1 |
| 52 | Nyctaginaceae | - | - | + | + | 2 |
| 53 | Oleaceae | - | + | - | - | 1 |
| 54 | Ophioglossaceae | + | + | - | - | 2 |
| 55 | Orchidaceae | + | + | - | - | 2 |
| 56 | Orobanchaceae | - | + | - | - | 1 |
| 57 | Oxalidaceae | + | + | + | + | 4 |
| 58 | Paniceae | + | + | - | - | 2 |
| 59 | Papaveraceae | + | + | + | - | 3 |
| 60 | Phyllanthaceae | - | - | + | - | 1 |
| 61 | Plantaginaceae | + | + | + | - | 3 |
| 62 | Poaceae | + | + | + | + | 4 |
| 63 | Polygonaceae | + | + | + | + | 4 |
| 64 | Primulaceae | + | + | - | - | 2 |
| 65 | Pteridaceae | + | + | + | - | 3 |
| 66 | Ranunculaceae | + | + | + | - | 3 |
| 67 | Rhamnaceae | - | - | - | + | 1 |
| 68 | Rosaceae | + | + | + | + | 4 |
| 69 | Rubiaceae | + | + | + | - | 3 |
| 70 | Rutaceae | + | + | + | + | 4 |
| 71 | Saxifragaceae | + | + | - | - | 2 |
| 72 | Scrophulariaceae | + | + | + | - | 3 |
| 73 | Smilacaceae | + | + | + | - | 3 |
| 74 | Solanaceae | + | + | + | + | 4 |
| 75 | Thymelaeaceae | - | + | - | - | 1 |
| 76 | Urticaceae | + | + | + | + | 4 |
| 77 | Verbenaceae | - | - | + | + | 2 |
| 78 | Violaceae | + | + | + | - | 3 |
| 79 | Vitaceae | + | + | + | - | 3 |
| 80 | Woodsiaceae | - | + | + | - | 2 |
| 81 | Zingiberaceae | + | + | - | - | 2 |
| Total | 55 | 62 | 65 | 30 | 212 |
Table 5: Families of plant species in different forest community.
The Table 1 lists 81 plant families and their presence (+) or absence (-) across four different forest types: Cedrus deodara, Quercus leucotricophora, Pinus roxburghii and mixed forest. The last column shows the total number of forests in which each family is present. For example, the Acanthaceae family is present in all four forest types, as indicated by the four pluses (+), resulting in a total count of 4. In contrast, the Adiantaceae family is found only in the Pinus roxburghii forest, marked by a single plus (+) and a total count of 1. The final row sums up the occurrences of all families in each forest type, with 55 in Cedrus deodara, 62 in Quercus leucotricophora, 65 in Pinus roxburghii and 30 in mixed forest, leading to an overall total of 212 presences across all forests.
Cedrus deodara is a type of coniferous tree found in the Himalayas and this column indicates whether each plant family is found within forests dominated by this species. Quercus leucotrichophora refers to a type of oak tree and the column shows the occurrence of plant families in forests where this tree is prevalent. Pinus roxburghii is known as the Chir pine and the corresponding column reveals which plant families are present in these pine forests. Mixed forest suggests a forest with a variety of tree species, not dominated by any single type mentioned above and the column indicates the presence or absence of the plant families in such diverse ecosystems [5].
Row explanation
Each row after the header provides data for a single plant family. For instance, the first row for the Acanthaceae family shows a plus sign in all four forest types, indicating its presence in each one. This results in a total count of 4, meaning the Acanthaceae family is found in all types of forests listed.
Conversely, the Adiantaceae family, shown in the second row, has three minus signs and one plus sign, suggesting it is only found in the Pinus roxburghii forest, giving it a total presence count of 1.
Summary totals
The last row of the table sums up the occurrences of all plant families within each forest type. For example, there are 55 instances of various plant families being present in the Cedrus deodara forest type.
Similarly, the Quercus leucotricophora, Pinus roxburghii and mixed forest types have 62, 65 and 30 instances of plant family presence, respectively.
The grand total (212) is the sum of all the individual totals from the last column, representing the cumulative presence of all plant families across all forest types.
This detailed tabulation allows researchers and ecologists to understand biodiversity in terms of plant family distribution across different forest ecosystems. It can be used to identify patterns of diversity, dominance and rarity among plant families in these habitats.
In Table 5 each forest type presents a unique assemblage of shrub species that can offer insights into the ecological characteristics and biodiversity health of these habitats. The presence (+) or absence (-) of each species within these forest types is documented, providing valuable data on the floristic composition and ecological preferences.
Forest type analysis
Cedrus deodara forests: With 27 shrub species present, this coniferous forest ecosystem supports a moderate level of understorey diversity. The presence of specific shrubs like Arundinaria falcata suggests an adaptation to the cooler, shaded environments typical of Cedrus-dominated landscapes. The absence of certain shrubs may be due to the dense canopy or specific soil conditions that favor coniferous trees over broadleaved shrub species.
Quercus leucotrichophora forests: The presence of 37 shrub species indicates a richer biodiversity, which could be attributed to the broad-leaved nature of oak forests that allows more light penetration and a diverse microhabitat for shrub growth. The variety of shrubs here also points towards a complex ecological web with varied food sources and niches for fauna.
Pinus roxburghii forests: This forest type shows the highest shrub diversity with 50 species, suggesting that pine forests provide a conducive environment for shrub layer development, possibly due to their open canopy structure and acidic soil conditions that many shrubs find favourable.
Mixed forests: Hosting 25 shrub species, these forests might represent transitional zones or ecotones where the diversity is influenced by the intermingling of species from adjacent forest types. The unique shrub species found here, such as Bauhinia vahlii, may indicate a resilience to varied conditions or a specialization to the mixed forest habitat.
Species-specific observations
Species like Lonicera angustifolia and Rubus ellipticus are ubiquitous, found in all four forest types, which could suggest a high adaptability to different environmental conditions or a generalist approach to habitat selection. Certain shrubs have a limited distribution, such as Ampelocissus latifolia and Andrachne cordifolia, which are only found in Pinus roxburghii forests. This restricted presence could be due to specific ecological requirements or competitive interactions within the forest community.
The distribution patterns of shrubs can have significant implications for ecosystem services such as soil stabilization, nutrient cycling and providing habitat for wildlife. Shrub layers contribute to the structural complexity of forests, which is crucial for maintaining biodiversity and resilience against environmental changes Kumar and Sharma.
In conclusion, the detailed analysis of the shrub layer across different forest types provides a snapshot of the ecological dynamics at play. It underscores the importance of considering all vegetation layers when assessing forest health and biodiversity. As an environmental researcher, such data is invaluable for informing sustainable management practices and conservation priorities (Table 6).
Sr. no. |
Shrubs |
Family |
Cedrus deodara |
Quercus leucotrichophora |
Pinus roxburghii |
Mixed forest |
Total |
1 |
Ampelocissus latifolia |
Vitaceae |
- |
- |
+ |
- |
1 |
2 |
Andrachne cordifolia |
Phyllanthaceae |
- |
- |
+ |
- |
1 |
3 |
Arundinaria falcata |
Poaceae |
+ |
+ |
- |
- |
2 |
4 |
Asclepias curassavica |
Apocynaceae |
- |
- |
+ |
- |
1 |
5 |
Asparagus racemosus |
Asparagaceae |
- |
- |
+ |
+ |
2 |
6 |
Bauhinia vahlii |
Fabaceae |
- |
- |
- |
+ |
1 |
7 |
Berberis chitria |
Berberidaceae |
- |
- |
+ |
- |
1 |
8 |
Berberis lycium |
Berberidaceae |
+ |
+ |
- |
- |
2 |
9 |
Boenninghausenia albiflora |
Rutaceae |
- |
+ |
- |
- |
1 |
10 |
Buddleja asiatica |
Scrophulariaceae |
+ |
+ |
+ |
- |
3 |
11 |
Caesalpinia decapetala |
Fabaceae |
- |
- |
- |
+ |
1 |
12 |
Calotropis gigantea |
Apocynaceae |
- |
- |
+ |
+ |
2 |
13 |
Capparis leucophylla |
Capparaceae |
+ |
+ |
- |
- |
2 |
14 |
Carissa opaca |
Apocynaceae |
- |
- |
- |
+ |
1 |
15 |
Cassia floribunda |
Fabaceae |
- |
- |
- |
+ |
1 |
16 |
Cassia mimosoides |
Fabaceae |
- |
- |
+ |
- |
1 |
17 |
Catharanthus roseus |
Apocynaceae |
+ |
+ |
+ |
- |
3 |
18 |
Cissampelos pareira |
Menispermaceae |
- |
- |
- |
+ |
1 |
19 |
Colebrookea oppositifolia |
Lamiaceae |
- |
- |
+ |
+ |
2 |
20 |
Coriaria nepalensis |
Coriariaceae |
+ |
+ |
+ |
- |
3 |
21 |
Cotoneaster bacillaris |
Rosaceae |
+ |
+ |
+ |
- |
3 |
22 |
Craniotome furcata |
Lamiaceae |
- |
- |
+ |
- |
1 |
23 |
Daphne papyracea |
Thymelaeaceae |
- |
+ |
- |
- |
1 |
24 |
Debregeasia saeneb |
Urticaceae |
+ |
+ |
+ |
- |
3 |
25 |
Desmodium elegans |
Fabaceae |
- |
- |
+ |
- |
1 |
26 |
Desmodium multiflorum |
Fabaceae |
+ |
+ |
+ |
- |
3 |
27 |
Deutzia staminea |
Hydrangeaceae |
+ |
+ |
+ |
- |
3 |
28 |
Dumasia villosa |
Fabaceae |
- |
- |
+ |
- |
1 |
29 |
Elaeagnus parvifolia |
Elaeagnaceae |
+ |
+ |
+ |
- |
3 |
30 |
Euphorbia royleana |
Euphorbiaceae |
- |
- |
+ |
+ |
2 |
31 |
Ficus sarmentosa |
Moraceae |
- |
- |
+ |
- |
1 |
32 |
Flemingia fruticulosa |
Fabaceae |
- |
- |
+ |
- |
1 |
33 |
Goldfussia dalhousiana |
Acanthaceae |
+ |
+ |
+ |
- |
3 |
34 |
Himalrandia tetrasperma |
Rubiaceae |
- |
+ |
+ |
- |
2 |
35 |
Hypericum androsaemum |
Hypericaceae |
- |
- |
+ |
- |
1 |
36 |
Hypericum dyeri |
Hypericaceae |
+ |
+ |
+ |
- |
3 |
37 |
Hypericum oblongifolium |
Hypericaceae |
+ |
+ |
+ |
- |
3 |
38 |
Indigofera dosua |
Fabaceae |
+ |
+ |
- |
- |
2 |
39 |
Indigofera heterantha |
Fabaceae |
- |
- |
+ |
- |
1 |
40 |
Isodon rugosus |
Lamiaceae |
- |
- |
+ |
- |
1 |
41 |
Jasminum humile |
Oleaceae |
- |
+ |
- |
- |
1 |
42 |
Justicia adhatoda |
Acanthaceae |
- |
- |
- |
+ |
1 |
43 |
Lantana camara |
Verbenaceae |
- |
- |
+ |
+ |
2 |
44 |
Leptodermis lanceolata |
Rubiaceae |
+ |
+ |
+ |
- |
3 |
45 |
Lespedeza gerardiana |
Fabaceae |
- |
+ |
+ |
- |
2 |
46 |
Lespedeza juncea |
Fabaceae |
- |
- |
+ |
+ |
2 |
47 |
Lonicera angustifolia |
Caprifoliaceae |
+ |
+ |
+ |
+ |
4 |
48 |
Machilus duthiei |
Lauraceae |
- |
- |
+ |
- |
1 |
49 |
Malvastrum coromandelianum |
Malvaceae |
- |
- |
- |
+ |
1 |
50 |
Marsdenia roylei |
Apocynaceae |
- |
+ |
- |
- |
1 |
51 |
Meriandra strobilifera |
Lamiaceae |
- |
- |
+ |
+ |
2 |
52 |
Micromeria biflora |
Lamiaceae |
+ |
+ |
- |
- |
2 |
53 |
Murraya koenigii |
Rutaceae |
- |
- |
- |
+ |
1 |
54 |
Myrsine africana |
Primulaceae |
- |
+ |
- |
- |
1 |
55 |
Parthenocissus himalayana |
Vitaceae |
- |
- |
+ |
- |
1 |
56 |
Parthenocissus semicordata |
Vitaceae |
+ |
+ |
- |
- |
2 |
57 |
Prinsepia utilis |
Rosaceae |
- |
+ |
- |
- |
1 |
58 |
Pueraria tuberosa |
Fabaceae |
- |
- |
- |
+ |
1 |
59 |
Pyracantha crenulata |
Rosaceae |
- |
+ |
- |
- |
1 |
60 |
Randia tetrasperma |
Rubiaceae |
+ |
+ |
+ |
- |
3 |
61 |
Rhynchosia sericea |
Fabaceae |
- |
- |
+ |
+ |
2 |
62 |
Ricinus communis |
Euphorbiaceae |
- |
- |
+ |
+ |
2 |
63 |
Rosa brunonii |
Rosaceae |
- |
+ |
+ |
- |
2 |
64 |
Rubus ellipticus |
Rosaceae |
+ |
+ |
+ |
+ |
4 |
65 |
Sarcococca pruniformis |
Buxaceae |
- |
- |
+ |
- |
1 |
66 |
Sarcococca saligna |
Buxaceae |
- |
+ |
- |
- |
1 |
67 |
Solanum incanum |
Solanaceae |
+ |
- |
+ |
- |
2 |
68 |
Solanum pseudocapsicum |
Solanaceae |
+ |
+ |
+ |
- |
3 |
69 |
Spiraea vaccinifolia |
Rosaceae |
+ |
+ |
- |
- |
2 |
70 |
Thymus mongolicus |
Lamiaceae |
+ |
+ |
- |
- |
2 |
71 |
Tinospora cordifolia |
Menispermaceae |
- |
- |
+ |
+ |
2 |
72 |
Viburnum cylindricum |
Adoxaceae |
+ |
+ |
- |
- |
2 |
73 |
Viburnum mullaha |
Adoxaceae |
- |
- |
+ |
- |
1 |
74 |
Vitex negundo |
Verbenaceae |
- |
- |
+ |
+ |
2 |
75 |
Woodfordia fruticosa |
Lythraceae |
- |
- |
+ |
+ |
2 |
76 |
Zanthoxylum armatum |
Rutaceae |
+ |
+ |
+ |
- |
3 |
77 |
Ziziphus nummularia |
Rhamnaceae |
- |
- |
- |
+ |
1 |
78 |
Ziziphus oxyphylla |
Rhamnaceae |
- |
- |
- |
+ |
1 |
|
Total |
|
27 |
37 |
50 |
25 |
139 |
Table 6: Floristic composition of shrubs species under different forest type in Solan district of Himachal Pradesh.
Table 7 is a comprehensive inventory of herbaceous plant species across four different forest types: Cedrus deodara, Quercus leucotrichophora, Pinus roxburghii and mixed forest. The presence (+) or absence (-) of each species within these forest types is documented, providing valuable data on the floristic composition and ecological preferences of these herbs.
Forest type analysis
Cedrus deodara forests: With 115 herb species present, these forests support a diverse undergrowth. The presence of species like Achyranthes aspera and Ageratum conyzoides, which are widespread across multiple forest types, indicates a hospitable environment for a range of herbaceous plants [6].
Quercus leucotrichophora forests: These forests have an even higher diversity with 132 herb species. This could be due to the rich humus and moisture-retentive properties of the soil typically found under oak canopies, which provide ideal conditions for herb growth.
Pinus roxburghii forests: Exhibiting the highest diversity with 167 species, pine forests seem to offer a unique set of conditions that favour a wide array of herbs. The acidic nature of pine needles as they decompose may influence the soil chemistry and thus the herbaceous layer composition.
Mixed forests: These forests show the presence of 47 herb species, which is considerably lower than the other forest types. This might suggest that the mixed nature of these forests creates specific ecological niches that only certain herbs can adapt to.
Species-specific observations
Some species, such as Ageratum conyzoides and Cyperus rotundus, are ubiquitous across all four forest types, indicating their generalist nature and adaptability to various microhabitats and soil conditions. Other species, like Aerva sanguinolenta and Ajuga integrifolia, have a more restricted distribution, suggesting specific habitat requirements or competitive exclusion in some forest types.
In summary, the detailed herbaceous layer data across different forest types is invaluable for understanding the complex interactions within these ecosystems. It highlights the importance of preserving not just the trees but also the rich diversity of ground-level flora. As an environmental researcher, such information is fundamental for conducting ecological assessments, guiding conservation efforts and ensuring sustainable management of forest resources (Table 7).
| Sr. no. | Herbs | Family | Cedrus deodara | Quercus leuchotricophora | Pinus roxburghii | Mixed forest | Total representation in forest type |
| 1 | Achyranthes aspera | Amaranthaceae | + | + | + | - | 3 |
| 2 | Achyranthes bidentata | Amaranthaceae | + | + | + | - | 3 |
| 3 | Adenocaulon bicolor | Asteraceae | + | + | + | - | 3 |
| 4 | Aerva sanguinolenta | Amaranthaceae | - | - | - | + | 1 |
| 5 | Agave wightii | Agavaceae | - | - | + | + | 2 |
| 6 | Ageratum conyzoides | Asteraceae | + | + | + | + | 4 |
| 7 | Agrimonia pilosa | Rosaceae | - | + | + | - | 2 |
| 8 | Ajuga integrifolia | Lamiaceae | - | - | + | - | 1 |
| 9 | Ajuga parviflora | Lamiaceae | + | + | - | - | 2 |
| 10 | Anagallis arvensis | Primulaceae | + | + | - | - | 2 |
| 11 | Anaphalis busua | Asteraceae | + | + | + | - | 3 |
| 12 | Anaphalis triplinervis | Asteraceae | + | + | + | - | 3 |
| 13 | Anemone vitifolia | Ranunculaceae | + | + | - | - | 2 |
| 14 | Apluda mutica | Poaceae | + | + | + | + | 4 |
| 15 | Argemone mexicana | Papaveraceae | - | - | + | - | 1 |
| 16 | Arisaema intermedium | Araceae | - | - | + | - | 1 |
| 17 | Arisaema tortuosum | Araceae | + | + | + | - | 3 |
| 18 | Artemisia vestita | Araceae | - | - | + | - | 1 |
| 19 | Asplenium adiantum-nigrum | Aspleniaceae | - | - | + | - | 1 |
| 20 | Asplenium anogrammoides | Aspleniaceae | + | + | - | - | 2 |
| 21 | Asplenium trichomanes | Aspleniaceae | - | - | + | - | 1 |
| 22 | Aster thomsonii | Asteraceae | + | + | + | - | 3 |
| 23 | Avena fatua | Poaceae | + | + | + | - | 3 |
| 24 | Barleria caristata | Acanthaceae | - | - | + | + | 2 |
| 25 | Begonia picta | Begoniaceae | - | - | + | - | 1 |
| 26 | Bergenia ciliata | Saxifragaceae | + | + | - | - | 2 |
| 27 | Bidens bipinnata | Asteraceae | + | + | + | + | 4 |
| 28 | Bidens pilosa | Asteraceae | + | + | + | - | 3 |
| 29 | Boehmeria platyphylla | Urticaceae | + | + | + | - | 3 |
| 30 | Botrychium ternatum | Ophioglossaceae | + | + | - | - | 2 |
| 31 | Bupleurum gracillimum | Apiaceae | + | + | + | - | 3 |
| 32 | Campanula benthamii | Campanulaceae | - | - | + | - | 1 |
| 33 | Cannabis sativa | Cannabaceae | + | + | - | - | 2 |
| 34 | Capsella bursa | Brassicaceae | + | + | + | - | 3 |
| 35 | Carduus edelbergii | Asteraceae | - | - | + | + | 2 |
| 36 | Carex nubigena | Cyperaceae | + | + | + | + | 4 |
| 37 | Carpesium abrotanoides | Asteraceae | + | + | + | - | 3 |
| 38 | Cenchrus ciliaris | Poaceae | + | + | + | + | 4 |
| 39 | Cheilanthes dalhousiae | Aspleniaceae | + | + | - | - | 2 |
| 40 | Chenopodium album | Amaranthaceae | - | - | + | - | 1 |
| 41 | Chirita bifolia | Gesneriaceae | - | - | + | - | 1 |
| 42 | Chromolaena odorata | Asteraceae | + | + | + | - | 3 |
| 43 | Chrysopogon fulvus | Poaceae | + | + | + | + | 4 |
| 44 | Cirsium wallichii | Asteraceae | - | - | + | - | 1 |
| 45 | Commelina benghalensis | Commelinaceae | + | + | + | - | 3 |
| 46 | Coniogramme affinis | Pteridaceae | - | + | + | - | 2 |
| 47 | Conyza stricta | Asteraceae | + | + | + | - | 3 |
| 48 | Craniotome furcata | Lamiaceae | + | + | - | - | 2 |
| 49 | Crepis japonica | Asteraceae | - | - | + | - | 1 |
| 50 | Cuscuta pentagona | Convolvulaceae | - | - | + | + | 2 |
| 51 | Cuscuta reflexa | Convolvulaceae | - | - | + | + | 2 |
| 52 | Cyanotis vaga | Commelinaceae | + | + | + | - | 3 |
| 53 | Cyathula tomentosa | Amaranthaceae | + | + | - | - | 2 |
| 54 | Cymbalaria muralis | Plantaginaceae | + | + | + | - | 3 |
| 55 | Cynoglossum wallichii | Boraginaceae | + | + | + | - | 3 |
| 56 | Cyperus niveus | Cyperaceae | - | - | + | + | 2 |
| 57 | Cyperus rotundus | Cyperaceae | + | + | + | + | 4 |
| 58 | Datura stramonium | Solanaceae | + | + | + | + | 4 |
| 59 | Desmodiastrum racemosum | Fabaceae | - | - | + | - | 1 |
| 60 | Desmodium floridanum | Fabaceae | + | + | + | - | 3 |
| 61 | Desmodium hookerianum | Fabaceae | - | - | + | - | 1 |
| 62 | Desmodium microphyllum | Fabaceae | + | + | + | - | 3 |
| 63 | Desmodium triflorum | Fabaceae | - | - | + | - | 1 |
| 64 | Dichanthium annulatum | Poaceae | + | + | + | + | 4 |
| 65 | Dicliptera bupleuroides | Acanthaceae | + | + | + | + | 4 |
| 66 | Dicliptera chinensis | Acanthaceae | + | + | + | + | 4 |
| 67 | Digitalis purpurea | Plantaginaceae | - | - | + | - | 1 |
| 68 | Dioscorea bulbifera | Dioscoreaceae | - | - | + | - | 1 |
| 69 | Dioscorea cordifolia | Dioscoreaceae | + | + | + | + | 4 |
| 70 | Dioscorea kamoonensis | Dioscoreaceae | - | - | + | - | 1 |
| 71 | Diplazium esculentum | Athyriaceae | + | + | - | - | 2 |
| 72 | Diplocyclos palmatus | Cucurbitaceae | - | - | + | + | 2 |
| 73 | Disporum pullum | Clochicaceae | - | + | + | - | 2 |
| 74 | Dryopteris nigropaleacea | Dryopteridaceae | + | + | + | - | 3 |
| 75 | Dryopteris panda | Dryopteridaceae | - | - | + | - | 1 |
| 76 | Duchesnea indica | Rosaceae | + | + | - | - | 2 |
| 77 | Dumasia villosa | Fabaceae | - | + | - | - | 1 |
| 78 | Echinochloa colona | Poaceae | + | + | + | + | 4 |
| 79 | Echinocystis lobata | Poaceae | - | - | + | + | 2 |
| 80 | Epipactis gigantea | Orchidaceae | + | + | - | - | 2 |
| 81 | Erigeron acer | Asteraceae | + | + | + | - | 3 |
| 82 | Erigeron annuus | Asteraceae | - | - | + | - | 1 |
| 83 | Erigeron bellidiodes | Asteraceae | + | + | + | - | 3 |
| 84 | Eriophorum comosum | Cyperaceae | + | + | - | - | 2 |
| 85 | Eupatorium adenophorum | Asteraceae | - | - | + | + | 2 |
| 86 | Euphorbia helioscopia | Asteraceae | - | - | + | + | 2 |
| 87 | Euphorbia pilosa | Asteraceae | - | - | + | + | 2 |
| 88 | Fagopyrum acutatum | Polygonaceae | + | + | - | - | 2 |
| 89 | Fimbristylis rigidula | Cyperaceae | - | - | + | + | 2 |
| 90 | Fragaria nubicola | Rosaceae | + | + | - | - | 2 |
| 91 | Fumaria parviflora | Papaveraceae | + | + | + | - | 3 |
| 92 | Gagea elegans | Liliaceae | - | - | + | - | 1 |
| 93 | Galinsoga parviflora | Asteraceae | + | + | + | - | 3 |
| 94 | Galium acutum | Rubiaceae | + | + | + | - | 3 |
| 95 | Galium aparine | Rubiaceae | - | - | + | - | 1 |
| 96 | Galium asperifolium | Rubiaceae | + | + | + | - | 3 |
| 97 | Galium elegans | Rubiaceae | - | - | + | - | 1 |
| 98 | Geranium himalayense | Geraniaceae | + | + | + | - | 3 |
| 99 | Geranium molle | Geraniaceae | + | + | + | - | 3 |
| 100 | Geranium nepalense | Geraniaceae | + | + | + | - | 3 |
| 101 | Geranium ocellatum | Geraniaceae | + | + | + | - | 3 |
| 102 | Geranium procurrens | Geraniaceae | + | + | + | - | 3 |
| 103 | Geranium wallichianum | Geraniaceae | + | + | + | - | 3 |
| 104 | Girardinia diversifolia | Urticaceae | + | + | + | - | 3 |
| 105 | Gnaphalium hypoleucum | Asteraceae | + | + | + | - | 3 |
| 106 | Gonatanthus sarmentosus | Araceae | + | + | - | - | 2 |
| 107 | Goodyera repens | Orchidaceae | + | + | - | - | 2 |
| 108 | Gypsophila cerastioides | Caryophyllaceae | + | + | - | - | 2 |
| 109 | Habenaria intermedia | Orchidaceae | - | + | - | - | 1 |
| 110 | Hedera nepalensis | Araliaceae | + | + | - | - | 2 |
| 111 | Hedychium spicatum | Zingiberaceae | + | + | - | - | 2 |
| 112 | Heliotropium indicum | Boraginaceae | - | - | - | + | 1 |
| 113 | Heteropogon contortus | Poaceae | + | + | + | + | 4 |
| 114 | Impatiens arguta | Balsaminaceae | + | + | - | - | 2 |
| 115 | Impatiens balsamina | Balsaminaceae | - | - | + | - | 1 |
| 116 | Impatiens laxiflora | Balsaminaceae | - | - | + | - | 1 |
| 117 | Impatiens sulcata | Balsaminaceae | - | - | + | + | 2 |
| 118 | Inula cappa | Asteraceae | - | - | + | - | 1 |
| 119 | Inula cuspidata | Asteraceae | - | + | + | - | 2 |
| 120 | Ipomoea purpurea | Convolvulaceae | + | + | + | + | 4 |
| 121 | Justicia simplex | Acanthaceae | - | - | + | - | 1 |
| 122 | Kalanchoe integra | Crassulaceae | - | - | + | - | 1 |
| 123 | Lamium album | Lamiaceae | - | - | + | + | 2 |
| 124 | Lathyrus aphaca | Fabaceae | + | + | + | + | 4 |
| 125 | Lecanthus peduncularis | Urticaceae | + | + | - | - | 2 |
| 126 | Lepidium sativum | Brassicaceae | + | + | + | - | 3 |
| 127 | Leucas lanata | Lamiaceae | + | + | + | - | 3 |
| 128 | Lindenbergia indica | Orobanchaceae | - | + | - | - | 1 |
| 129 | Malaxis muscifera | Orchidaceae | - | + | - | - | 1 |
| 130 | Mazus surculosus | Scrophulariaceae | + | + | + | - | 3 |
| 131 | Mentha longifolia | Lamiaceae | + | + | + | - | 3 |
| 132 | Mimosa pudica | Fabaceae | - | - | + | - | 1 |
| 133 | Mirabilis jalapa | Nyctaginaceae | - | - | + | + | 2 |
| 134 | Myriactis nepalensis | Asteraceae | + | + | - | - | 2 |
| 135 | Nepeta spicata | Lamiaceae | - | + | - | - | 1 |
| 136 | Nicandra physaloides | Solanaceae | + | + | + | + | 4 |
| 137 | Ocimum sanctum | Lamiaceae | - | - | + | + | 2 |
| 138 | Oldenlandia coccinea | Rubiaceae | - | - | + | - | 1 |
| 139 | Onychium contiguum | Adiantaceae | + | + | + | - | 3 |
| 140 | Oplismenus burmanni | Poaceae | + | + | + | + | 4 |
| 141 | Opuntia dillenii | Cactaceae | - | - | + | + | 2 |
| 142 | Origanum vulgare | Lamiaceae | + | + | + | - | 3 |
| 143 | Orobanche epithymum | Orobanchaceae | - | + | - | - | 1 |
| 144 | Oxalis corniculata | Oxalidaceae | - | + | + | - | 2 |
| 145 | Oxalis corymbosa | Oxalidaceae | + | + | + | + | 4 |
| 146 | Oxalis dehradunensis | Oxalidaceae | - | - | + | - | 1 |
| 147 | Oxyria digyna | Polygonaceae | - | - | + | - | 1 |
| 148 | Parnassia nubicola | Celastraceae | + | + | + | - | 3 |
| 149 | Parthenium hysterophorus | Asteraceae | + | + | + | + | 4 |
| 150 | Pennisetum glaucum | Poaceae | + | + | + | + | 4 |
| 151 | Peristrophe bicalyculata | Acanthaceae | + | + | + | - | 3 |
| 152 | Persicaria capitata | Polygonaceae | + | + | - | - | 2 |
| 153 | Phlomis bracteosa | Lamiaceae | + | + | - | - | 2 |
| 154 | Pilea scripta | Urticaceae | + | + | + | - | 3 |
| 155 | Plantago depressa | Plantaginaceae | + | + | + | - | 3 |
| 156 | Polygonum amplexicaule | Polygonaceae | + | + | - | - | 2 |
| 157 | Polygonum hydropiper | Polygonaceae | - | - | + | - | 1 |
| 158 | Polygonum pubescens | Polygonaceae | - | - | + | - | 1 |
| 159 | Polystichum lobatum | Dryopteridaceae | + | + | + | - | 3 |
| 160 | Polystichum squarrosum | Dryopteridaceae | - | - | + | - | 1 |
| 161 | Primula denticulata | Primulaceae | - | + | - | - | 1 |
| 162 | Pteridium aquilinum | Dennstaedtiaceae | + | + | - | - | 2 |
| 163 | Pteris cretica | Pteridaceae | + | + | + | - | 3 |
| 164 | Pteris dactylina | Pteridaceae | - | - | + | - | 1 |
| 165 | Ranunculus hirtellus | Ranunculaceae | + | + | + | - | 3 |
| 166 | Rubia cordifolia | Rubiaceae | + | + | + | - | 3 |
| 167 | Rumex hastatus | Polygonaceae | - | - | + | + | 2 |
| 168 | Rumex nepalensis | Rubiaceae | + | + | - | - | 2 |
| 169 | Rumex obtusifolius | Polygonaceae | - | - | + | - | 1 |
| 170 | Salvia coccinea | Lamiaceae | - | - | + | - | 1 |
| 171 | Salvia glutinosa | Lamiaceae | - | - | + | - | 1 |
| 172 | Salvia lanata | Lamiaceae | - | + | + | - | 2 |
| 173 | Scutellaria grossa | Lamiaceae | - | - | + | - | 1 |
| 174 | Scutellaria repens | Lamiaceae | + | + | - | - | 2 |
| 175 | Senecio laetus | Asteraceae | - | - | + | - | 1 |
| 176 | Setaria viridis | Poaceae | - | + | + | + | 3 |
| 177 | Siegesbeckia orientalis | Asteraceae | - | - | + | - | 1 |
| 178 | Smilax aspera | Smilacaceae | - | - | + | - | 1 |
| 179 | Smilax glaucophylla | Smilacaceae | + | + | + | - | 3 |
| 180 | Solanum nigrum | Solanaceae | - | - | + | - | 1 |
| 181 | Solanum virginianum | Solanaceae | - | - | + | + | 2 |
| 182 | Sonchus asper | Asteraceae | - | - | + | - | 1 |
| 183 | Sonchus wightianus | Asteraceae | - | - | + | - | 1 |
| 184 | Sopubia trifida | Scrophulariaceae | - | - | + | - | 1 |
| 185 | Stellaria media | Caryophyllaceae | - | - | + | - | 1 |
| 186 | Stellaria monosperma | Caryophyllaceae | + | + | + | - | 3 |
| 187 | Swertia angustifolia | Gentianaceae | - | - | + | - | 1 |
| 188 | Swertia cordata | Gentianaceae | - | - | + | - | 1 |
| 189 | Swertia paniculata | Gentianaceae | - | + | + | - | 2 |
| 190 | Tagetes erecta | Asteraceae | - | - | + | + | 2 |
| 191 | Tagetes minuta | Asteraceae | - | - | + | - | 1 |
| 192 | Taraxacum officinale | Asteraceae | + | + | + | - | 3 |
| 193 | Thalictrum foetidum | Ranunculaceae | + | + | + | - | 3 |
| 194 | Thalictrum foliolosum | Ranunculaceae | - | - | + | - | 1 |
| 195 | Themeda anathera | Poaceae | + | + | + | - | 3 |
| 196 | Theropogon pallidus | Liliaceae | + | + | - | - | 2 |
| 197 | Tridax procumbens | Asteraceae | + | + | + | - | 3 |
| 198 | Trifolium repens | Fabaceae | + | + | + | - | 3 |
| 199 | Urochloa panicoides | Poaceae | + | + | + | + | 4 |
| 200 | Urtica dioica | Urticaceae | + | + | + | + | 4 |
| 201 | Urtica parviflora | Urticaceae | - | - | - | + | 1 |
| 202 | Valeriana jatamansi | Caprifoliaceae | + | + | - | - | 2 |
| 203 | Verbascum thapsus | Scrophulariaceae | - | - | + | - | 1 |
| 204 | Veronica persica | Plantaginaceae | + | + | - | - | 2 |
| 205 | Vicia hirsuta | Fabaceae | + | + | + | - | 3 |
| 206 | Vicia sativa | Fabaceae | - | + | + | - | 2 |
| 207 | Vinca major | Apocynaceae | - | - | + | - | 1 |
| 208 | Vincetoxicum hirundinaria | Apocynaceae | - | - | + | - | 1 |
| 209 | Viola canescens | Violaceae | + | + | + | - | 3 |
| 210 | Woodsia elongata | Woodsiaceae | - | + | + | - | 2 |
| Total | 115 | 132 | 167 | 47 | 461 |
Table 7: Floristic composition of herbs species under different forest communities in Solan district of Himachal Pradesh.
Amidst all terrestrial ecosystems, forests are the vastest, intricate and biologically active systems. According to Elouard et al., modelling the dynamics and operation of forests as well as describing different ecological processes require an understanding of forest structures. Deeper understanding of the shape and organisation of the vegetation in forest communities is achieved via quantitative investigations. Realising the importance of understanding plant groups, variety, population and distribution for the protection and restoration of the environment has led to the realisation that competent management of the Himalayan ecosystem is necessary. It would be extremely difficult to conserve biological resources in their natural environment without an understanding of the distribution and dynamics of these resources. This knowledge can serve as a reasonable foundation for planning and management choices (Figure 4) [7].
Figure 4: Histogram of families of plants and plant species of different forest communities.
Figure 4 illustrates the family abundance of various forest communities. Of the 81 plant families found, the following families were commonly found in all four studied forest types: Acanthaceae, Amaranthaceae, Apocynaceae, Asteraceae, Balsaminaceae, Boraginaceae, Cyperaceae, Dioscoreaceae, Fabaceae, Lamiaceae, Oxalidaceae, Poaceae, Polygonaceae, Rosaceae, Rutaceae, Solanaceae and Urticaceae. Only one or two forest communities had families like Agavaceae, Araliaceae, Asparagaceae, Athyriaceae, Begoniaceae, Campanulaceae, etc. The forest communities with the highest number of families were Pinus roxburghii forest (65), followed by forests with Quercus leucotrichophora (62), Cedrus deodara (55) and mixed forests (30). Hence comparing between the number of plant species and the number of families found in different forest communities [8].
The appropriateness of a particular ecological niche that has supported the germination, development and domination of these species may be attributed to the dominance of a small number of species over their peer group. Numerous researchers, including Pielou, Ram et al., Uniyal et al., Kumar and Ram, Rawat and Chandra and Iqbal et al., have occasionally reported on the dominance of a small number of species in distinct forest types [9].
The biotic interactions between overstory and understory plants and terrain-related abiotic variables can explain the variations in floral spectrum across forest community types; changes in aspect and elevation have a substantial impact on biomass, height and gap frequency. The frequency and intensity of disturbances, such as wind damage and drought mortality, can also be influenced by terrain, which in turn affects the biotic structure and composition. Terrain also has an impact on the local suite of species. It has been widely accepted by researchers that are dependent on a region's time, altitude, slope, latitude, aspect, rainfall and humidity are what control the distribution of species in forests.
Every specie's ability to tolerate and adapt to its surroundings determines whether or not it can exist in a given location. Important ecological traits that are correlated with both anthropogenic influences and the current environment include the organisation of plant communities and their patterns of diversity. Goodall and Perry believe that the range of niches inhabited by these species indicates their extensive biotic range [10].
The research paper "change in dynamics of understory composition with change in altitude in Western Himalayas" delves into the significant impact of climate change on the understory vegetation in the forests of Himachal Pradesh, particularly in the Solan region. The study highlights the importance of understanding the changes in species distribution, composition and forest structure due to climatic variability.
The findings of the research emphasize that climate unpredictability has led to alterations in the timing of plant life cycles, including leafing, flowering and fruiting. These phenological changes may result in mismatches between the needs of animals and the available resources, potentially affecting the survival and reproductive capacity of species in the ecosystem.
Furthermore, the paper underscores the crucial role of understory vegetation in maintaining the overall health and productivity of forest ecosystems. It points out that variations in soil moisture, nutrient availability and productivity of understory plants are influenced by shifts in temperature and precipitation patterns, ultimately impacting the resources available to other organisms in the ecosystem.
The study area, situated in the mid-hill region of Himachal Pradesh, experiences significant seasonal and diurnal temperature variations, with diverse vegetation ranging from subtropical to alpine. The research underscores the necessity of conserving these forests to comprehend the effects of climatic variability on understory vegetation comprehensively.
In conclusion, the research paper highlights the urgency of addressing climate change impacts on forest ecosystems and emphasizes the need for further studies to develop effective mitigation strategies. By understanding the changing dynamics of understory composition in response to climate change, researchers and policymakers can work towards preserving biodiversity and ensuring the resilience of forest ecosystems in the western Himalayas.
[Crossref] [Google Scholar] [PubMed]
Citation: Goyal S, Brahmi MK, Bhardwaj SK, Sharma U, Dogra KS, Jangra MS (2025) Special Dynamics of Understory Vegetation Composition in Shivalik Hills of Western Himalayas. J For Res. 14:570.
Received: 20-May-2024, Manuscript No. JFOR-24-31568; Editor assigned: 23-May-2024, Pre QC No. JFOR-24-31568 (PQ); Reviewed: 06-Jun-2024, QC No. JFOR-24-31568; Revised: 02-Jun-2025, Manuscript No. JFOR-24-31568 (R); Published: 09-Jun-2025 , DOI: 10.35248/2168-9776.25.14.570
Copyright: © 2025 Goyal S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.