Journal of Clinical & Experimental Dermatology Research
Open Access
ISSN: 2155-9554
ISSN: 2155-9554
Research Article - (2014) Volume 5, Issue 2
Neurofibromatosis type 1 (NF1) also known as von Recklinghausen’s disease, is an autosomal dominant disorder characterized by benign tumors called neurofibromas. It is a serious disease, often overlooked. Indeed, people with NF1 have a lower life expectancy, because some of them develop malignant tumors and for many, there is deterioration in their life quality.
In the absence of medical treatment, only surgery is in place for now. However, the problem of neurofibromatosis surgery is that it is potentially very hemorrhagic and neurofibroma skin elasticity seems recurrent after few years.
The aim of this study was to evaluate structure alterations in NF1 skin. At First, mechanical tests that were performed on pathological and control samples have led to the identification of elasticity parameter in traction; then, a comparative histological study was carried out on healthy and pathological skin samples.
Twenty one samples were analysed in uniaxial traction to obtain stiffness coefficient. The mechanical results show that a neurofibromatosis skin is at least four times less elastic than a healthy skin. Histology of the skin samples showed reduced cellularity of pathological samples compared to healthy ones. In addition, the NF1 skin elastin rate is twice lower compared to the healthy one.
This work is part of the understanding of the structure of skin NF1. Although preliminary, this study enables to evaluate a rheological parameter and to correlate it to micro-constituents skin changes.
<Keywords: Neurofibromatosis type 1, Skin elasticity, Mechanical properties
Nf1 is one of the genetic diseases in western countries, affecting one person in 3000 to 3500 births all populations combined [1-4]. It is autosomic dominant. Its severity is related to malignant transformation and impact on quality of life. The development of plexiform neurofibromas is the first cause of deterioration of QoL. Plexiform NF is soft tissue tumors developed ubiquity. There is no medical treatment for these tumors but to be removed surgically. Surgery comes with a high level of complications related to bleeding and poor outcomes related to relapse. Clinically, the skin elasticity is different from a normal skin which could be explanation of poor surgical outcomes. We tested the hypothesis that skin viscoelasticity is different to normal skin by in vitro measures of skin samples.
Hence, a positional cloning strategy was adopted to identify and isolate the Nf1 gene. This strategy means that the observed disease is monogenic or determined by the achievement of a major gene. This method has shown that the gene is located on the long arm of chromosome 17 in the region 17q11.2 [5-7]. The NF1 gene product, neurofibromin is a protein involved in the control of cell differentiation and proliferation, inhibiting the activation pathway of p21 ras [8].
Despite the difficulties inherent in the surgical excision of NF1 tumors, surgical treatment takes an important place in the management of this skin disorders although the skin distensibility quickly reduces the surgical benefit.
Cohesion between the various skin components and its layers led some authors to consider the skin as a homogeneous material [8]. However, the individual layers behaviour of the skin has been studied and most authors agree that the major constituent of the mechanical skin is the dermis [8-11].
One of the first investigators of dermis mechanical behaviour showed the anisotropic behaviour of the skin [12]. Numerous researches have led to the foundations of the skin mechanical behaviour [13-15]. These early studies have shown the nonlinear viscoelastic and anisotropic skin behaviour. This work and the ones succeeded it have implemented various types of mechanical stress to study the skin mechanical behaviour: torsion, indentation, levatometric, ballistometric, suction, traction and tensile testing.
Mechanical tests
Seven patients of both genders were included in our study. Their ages ranged from 17 to 56 years old and the age average was 35 years. Five of these patients were affected by NF1, as defined by NIH clinical criteria, and presented one or more cutaneous neurofibromas with a surgical procedure of removal planned in our department of plastic surgery (Table 1).
| Age | Sex | Localisation of neurofibroma | |
|---|---|---|---|
| Patient 1 NF | 17 years | Female | Left calf |
| Patient 2.NF | 20 years | Female | Left side |
| Patient 3 NF | 17 years | Male | Médial part of left arm |
| Patient 4 NF | 25 years | Female | Right tight and knee |
| Patient 5 NF | 56 years | Male | Butock |
Table 1: NF1 group.
Two healthy control subjects were also included: women of 53 and 60 years old with an indication of brachial dermolipectomy (Table 2). All seven patients were clearly informed of the study and gave their consent. We took skin samples on tumoral skin from each patient and samples on the removed skin from the control group.
| Age | Sex | Localisation of neurofibroma | |
|---|---|---|---|
| Patient C 1 | 53 years | Female | Brachial dermolipectomy |
| Patient C 2 | 60 years | Female | Brachial dermolipectomy |
Table 2: Control group.
In continuum mechanics, relationship stress-strain and strain rate are called behaviour laws [16].
They are from experimental origin and are derived from simple tests on samples performed assuming homogeneity stresses [17].
The simplest experimental model of the stresses (one for which we have chosen to characterize our samples) is the uniaxial stress. It consists of exerting an axial tensile force F in a cylindrical or rectangular sample with a length L0 and S0 section when F is zero. The sample is elongated (l-l0), and strain ε defined as the relative elongation is expressed by the following relationship.
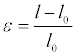
For each value of F, the uniform axial stress is σ.
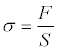
Stiffness coefficient and Poisson ratio characterize a material disregarding its shape and dimensions. Both have a simple interpretation. While the first is specific to a given material and characterizes its extensibility after a tensile test, the second relates the transverse strain and longitudinal deformation of a material subjected to a tensile test. For example, the living tissues are considered incompressible materials and their Poisson ratio is estimated at 0.5.
Stiffness coefficient is a constant that links the stress to the strain. The relationship σ=f (ε) of a living tissue is generally not linear and in this case, this coefficient varies depending on the strain. We define an incremental module to point M, which is the slope of the tangent to the curve at that point. In this case, the material is characterized around a point M by the tangent of the slope, namely:
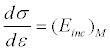
Regarding our tests, the point M corresponds to the breaking point.
For each patient, 10 samples were obtained, for a total of seventy samples. Skin samples were obtained using a calibrated punch (30×4 mm), allowing a geometric standardization samples, then were fixed between the two jaws of the device, and then subjected to tensile tests (Figure 1).
A tensile testing machine MTS Insight (Electromechanical 5kN standard length) is used for the implementation of uniaxial tension test to measure the elongation of a skin sample connected to the force applied thereto at the break.
In addition to geometrical characteristics of the sample, obtained data using the acquisition software representing respectively strain and stretch, we attain the elasticity parameter (Stiffness coefficient), defining thereby a mechanical profile of the material, in this case the skin.
Histological analysis
The second part of the study corresponds to structural analysis of skin samples taken. The purpose of this study is to identify the components of the skin that are responsible for the disorder elasticity.
Two methods of observation of optical microscopy were used:
• Eosin Hematein Saffron staining, for observations of magnification up to ×250. Commonly used in histopathology, it enables at first, the comparison of the general histological organization for the different samples. Haematein (basic dye) stains the nucleic acids in blue-black, eosin (acid dye) turns red more or less intense cytoplasm and some extracellular structures. Saffron stains collagen yellow.
• Orcein staining used on semi-thin sections for observation until a magnification ×300. Weakly acidic, the staining highlights with high selectivity elastin fibers.
In Figure 2, some curve shapes “real stress” versus “stretch” are observed for healthy skin and NF1 skin regardless of age, gender, race or location (Figures 2a and 2b). Average real stress coefficient at break is represented regardless of age, gender, race or location (Figure 2c). We see a clear difference between these two types of skin; the healthy one is more resistant.
Figure 2: Real stress versus stretch curves for a healthy (a) and NF1 (b) skin, regardless of age, gender, race and location, oriented along Langer lines at a 1 Hz frequency during a tensile tests until rupture: healthy patients (light gray symbols) and NF1 patients (dark gray symbols). Average real stress at break regardless of age, gender, race and location (c).*corresponded to p<0.05.
In Figure 3, some curve shapes “real stain at break” versus “stretch” are observed for healthy skin and NF1 skin of the same age, taken from the same location (arm), on people of the same gender and race (Figures 3a and 3b). The difference previously observed in Figure 2, is growing significantly.
Figure 3: Real stress versus stretch curves for a same location (arm skin), oriented along Langer lines at a frequency of 1 Hz during a tensile tests until rupture: (a) healthy patients (light gray symbols) and (b) NF1 patients (dark gray symbols). (c) Average real stress at break according skin type and location. ***corresponded to p<0.001.
Two points emerge from these results:
• The breaking point of NF1 skin is reached for a stress three to four times less than that corresponding to the breaking point of a healthy skin.
• This gap widens even more if we take into account gender, race, age and location.
Average real stress coefficient at break is represented taking into account age, gender, race and location (Figure 3c).
Comparison of average real stress coefficients at break measured in all samples of a healthy skin and skin NF1 shows a control group stress coefficient more than three times higher than that of NF1. When a pairing on the location (arm), gender, age and race is performed, the difference coefficient between the stress coefficients at breakup is to even more important, since the stress of the control group (healthy skin) is eight times greater than that of the skin NF1.
Figure 4 shows stress coefficient and Young modulus, at break for healthy and NF1 samples.
Comparison of stress coefficients at break measured in all samples of a healthy skin and skin NF1 shows a control group stress coefficient more than three times higher than that of NF1. When a pairing on the location (arm), gender, age and race is performed, the difference between the stress coefficients at break is even more important, since the stress coefficient of the control group (healthy skin) is up to eight times than that of the skin NF1.
The same trend is observed for the Young modulus at break in Figure 4, with a larger (up to nine times when considering the location) between healthy skin and NF1 skin, according skin type and regardless of age, gender, race and location and according skin type and location (Figures 4a and 4b).
We have analysed the cellularity of the healthy skin dermis compared to that of neurofibromas using the standard HES staining. There is a decreased overall cellularity in lesions of NF. We notice the very heterogeneous cellularity within the same neurofibroma (Figures 5a-c).
Samples were also stained with Orcein (specific staining) and then compared the healthy skin samples to NF1 samples. Visual quantification of elastin rate shows a decrease of over 50% compared with healthy skin. (Figures 6a, 6a’, 6b and 6b’).
Statistics
Results are presented as mean ± SEM (standard error of the mean). Anova analysis was used to identify differentially expressed mechanical properties between healthy and NF1 skin. Differences were considered significant when P<0.05 (*): ** indicates a significant difference with P<0.01; *** indicates a significant difference with P<0.001.
Tissue mechanical properties are determined by the organization of the extracellular matrix fibers, the cells in the fundamental substance. Agache showed skin non-linear behaviour applying a sharp stress and sustained by a creep test [8]. The resulting curve can be divided into three phases. First purely elastic phase because too fast to be controlled by the tissue viscosity.
In the second phase, viscosity occurs and the last phase corresponds to a linear behaviour and a constant stiffness. He proposed an analog model where Phase I is treated as a spring, Phase II a damper coupled to a spring and Phase III by a damper. When tested in vivo, stretching applied remains below 25% and reversible. Plastic and irreversible deformation could be demonstrated in vitro for larger stretches. When uniaxial traction tests on the ex vivo skin, stress was applied gradually.
The stress/strain obtained curve can be divided into three distinct phases. A first region of low rigidity corresponds to the parallel alignment of collagen fibers in the direction of maximum stretch. In this first phase, the collagen fibers are entangled and elastin is solely responsible for the skin stiffness [10]. The second phase is a transition phase in which the stiffness increases progressively as the collagen fibers align. Once the collagen fibers are sufficiently aligned, they stretch provoke the skin stretching, which is phase 3 [18]. This field is almost linear and corresponds to the collagen fibers elasticity modulus. Beyond this last phase we speak of irreversibility, the tissue is damaged.
The elastic properties of the skin are governed by the geometry and interaction of the collagen and elastin network, while the basic substance contributes to the mechanical behaviour of the skin. The skin has an elastic behaviour at low stretching levels. Beyond, the collagen network is extended and the skin has viscoelastic behaviour [19]. Deformation becomes a function of time and stress. The viscosity of the skin is explained by the fluid displacement, the interaction between the collagen fibers, the ground substance and the dissipative friction fibers [20].
In some parts of the body, the collagen fibers tend to move in the same direction to ensure a “tone” to the skin. Langer lines are power lines that indicate the dominant direction to the surface of the skin. Langer lines were established from cadavers from the oval shape taken by a wound made by a round punch [12]. The electron microscopic examination of tissues confirmed the data shown by Langer. In the retracted skin, collagen bundles appear tortuous, without preferential direction and elastic fibers attach at multiple points. In an uncollapsed skin, the collagen bundles and the finer the elastic fibers are oriented in the line direction and are almost parallel Langer. Thus, the lines of Langer reflect anisotropy of the elastic fibers of the skin. In addition, the tension due to the lines of Langer is an inherent tension of the skin. A clear distinction of the additional stress of age or additional stress induces visceral or muscle contraction. Identification of Langer’s lines should be released on a skin without constraint. Finally, in the implementation of skin tests, it will obviously take into account the characteristics of the lines of Langer studied the skin site.
Many mechanical tests are available to study the skin properties: torsion, indentation, levarometric, ballistometric, suction, and tensile testing. However, depending on the studied mechanical parameter, requested tissue and the way it is tested (in vivo or in vitro), the number of suitable methods decreases.
The NF1 skin viscoelasticity has been previously evaluated, in a non-invasive analysis with cutometer measures, showing an increased distensibility of the NF1 skin compared to healthy skin. Even if they are not reproducible (indeed, Cutometer essentially measures the tissues properties and do not go back to the intrinsic properties of the skin), those results open a new way of research [21].
The present study proposes the first invasive analysis of the mechanical behaviour of the skin in neurofibromatosis type 1 compared to healthy skin, using surgical samples. Therefore, different samples obtained from NF1 and healthy patients were characterized with uniaxial mechanical tests. Also, it was shown that the stiffness coefficient in traction of NF1 skin is up to four times lower than the healthy one. Otherwise, immuno-histological results showed a decreased cellularity and elastin of NF1 skin compared to healthy skin, which confirms stiffness reduction that results from mechanical quantification.
Several authors have attempted to evaluate the elastic properties suitable for the skin [1,22,23]. To do so, they used the cutometer marketed by Courage & Khazaha (Germany), which allows testing with rooms with diameters of 2, 4, 6 or 8 mm. It uses optical imaging to measure the elevation of the skin. A spring located in the head of the system ensures that the pressure remains constant when the system is applied to the skin. Chamber 2 mm is sensitive to mechanical behaviour of stratum corneum but it is not the case for larger diameters. The failure of this device is no pressure measurement is performed in the tank, only the directive gives the initial value of the pressure. A small leak not detected in the vacuum chamber substantially change results and interpretation. In addition, the Cutometer essentially measures the properties of the tissue and does not go back to the intrinsic properties of the skin [21].
The only previous study of skin viscoelasticity in NF 1 patient was an in vivo study, using a cutometer gives the initial value of the pressure. A small leak not detected in the vacuum chamber substantially change results and interpretation. In addition, the Cutometer essentially measures the properties of the tissue and does not go back to the intrinsic properties of the skin 22.Although the results do not tell us about the intrinsic material properties, in this case (skin), they opened a new way of research.
To our knowledge, this is the first time an invasive mechanical study is performed in order to evaluate skin elasticity and stiffness coefficient in NF1 patients. Results obtained in this study are very promising. Indeed, we find that the stress corresponding to the breaking point of a healthy skin is four times higher than that of NF1 skin. The stiffness coefficient of healthy skin is about 5 times higher than that of a skin NF1.
Different clinical types of neurofibromas studied in this work are often entangled in histology (diffuse neurofibroma was found in each histological sample of plexiform neurofibromas), which makes their analysis more complex. What was labeled plexiform neurofibroma, clinically preoperatively is often to wide ranges of diffuse neurofibromas in which is developed plexiform neurofibroma. Substantial problems of skin elasticity found in the biomechanical study could be rather attached to this type of lesions that show a majority in samples, given the microscopic analysis.
Two major points emerge from the histological study: we find that the cellularity, although highly variable from one area to the other of the neurofibromas generally seems very depressed, with low mitotic activity in lesions of NF. The elastin fibers are found at a much lower rate than in healthy skin. This anomaly could be correlated with the loss of skin elasticity for neurofibromatous patients.
Although surgery resection techniques remain one cutaneous manifestations of neurofibromatosis type 1 CO2 vaporization), incomplete or insufficient are often frustrating for both patients and surgeons.
The biomechanical results of this study reflect the clinical feel of skin “hyperextensibility”. They also explain part of the temporary characteristic of the surgical results. However, this is a preliminary study that shows that the rheological parameters of NF1 skin are different from those of a healthy skin. Further studies should be conducted with a larger number of patients and a qualitative analysis of the components of the skin in neurofibromatosis by immunohistochemistry and electron microscopy to expand our understanding of this phenomenon and the reasons that led to it.
It is necessary to quantify more precisely the changes in elastin between healthy skin and NF1 skin. For this reason, we could make a standard immunostaining on histological sections by labeled antielastin. Otherwise, an ultra-structural analysis using scanning electron microscopy and transmission, enabling a qualitative analysis of different components of the skin in question, should be considered. This process will enable the observation of the skin and dermis on biopsy fees fragments in 3 dimensions. The magnification used makes the observation of collagen and elastin fibers in the dermal extracellular matrix possible.
Finally, the measurement of cellularity samples made by semiquantitative method (counting field) will be performed on fresh samples from comparative measurements of the amount of doublestranded DNA spectrophotometry (Picogreen® process).
From a biomechanical point of view, we could consider biaxial tests. These tests are preferable to uniaxial tensile tests for the in vivo skin is biased so multiaxial [24]. Lanir et al. were among the first to achieve biaxial tensile tests on rabbit skin [25,26]. They highlighted the differences in the relaxation of the tissue according to the type of traction used. This better understanding of neurofibromatosis physiopathology could lead us, in the future, to identify and target deficient component of the skin.
For the manuscript entitled “Skin elasticity in Neurofibromatosis type 1: Rheological and histological analysis” to be published in the Journal of Clinical and Experimental Dermatology, the authors Assoul N, Ozil C, Hivelin M, Zidi M, Lantieri L and Bosc R declare no conflict of interest.