Forest Research: Open Access
Open Access
ISSN: 2168-9776
ISSN: 2168-9776
Research Article - (2020)Volume 9, Issue 2
This study assessed infestation of Proholopterus chilensis in Nothofagus obliqua trees in the Valdivia Province, Chile. P. chilensis is a native xylophagous insect that produces internal galleries in the stem during larval stage, decreasing value recovery in the most profitable log of the tree. The objectives of the study were to evaluate the current infestation of P. chilensis in Valdivia Province, to investigate the relationships among infestation, trees, site and flora attributes, to increase information about P. chilensis infestation patterns, and to make suggestions about silvicultural practices that could reduce damage. Results indicated that the level of infestation was low, compared with previous data. Hierarchical logistic regression models showed that estimated tree height and stand density were significant to explain probability of a tree being attacked by P. chilensis. Site altitude, flora diversity and Importance Value Index of Chusquea quila and Shannon-Weaver index were not significant predictors of infestation.
Xylophagous; Nothofagus forests; Proholopterus chilensis; Infestation patterns
Most of the insects living in the Chilean natural forests are native, and some of them cause economic damage, but in very specific areas; however, they cannot be considered as a pest since they are part of the growth, development, and dynamics of these forests. Moreover, they have contributed to design the forests ecosystems that we have today [1]. Preliminary studies of infestation patterns of Proholopterus chilensis (Col: Cerambycidae) in Nothofagus obliqua (Mirb.) Oerst have shown that tree diameter and environmental humidity have significant effects on infestation [2,3]. However, this knowledge is insufficient to develop silvicultural approaches that reduce the damage, particularly in the Valdivia Province of Chile, where infestation has been historically high.
P. chilensis is a native xylophagous insect with a long life-cycle (2-4 years); however, the economic damage occurs during the larval stage, when larvae remain protected inside the tree and dedicated to feed and grow [4,5]. This insect is associated with living trees, and the galleries are mainly located in the first 4 m from the base of the stem, which concentrates up to 60% of the tree volume [6-9]. Butt logs of infested trees are downgraded from appearance lumber or veneer end-use and, instead, they are used as fuel wood or chips for the wood-composite industry [10-12]. Thus, most of the studies that report the damage of xylophagous insects, such as P. chilensis, are mostly referred to as wood value loss, because infestation rates that have been registered have not affected the biodiversity of the ecosystems where the Chilean Nothofagus forests grow. Thus, Díaz [13] reported that there is no intersection between galleries of different larvae; thus, there would be no competition for the resource with other native xylophagous insects.
N. obliqua is a native tree with a broad distribution in the South- Central part of Chile between 33° and 41° 30’ latitude South in the Andes Mountains, Central Valley, and Coastal Mountains [14]. This species is highly valued due to its wood quality and durability for appearance and structural end-uses. In the Valdivia Province, there are 130,430 ha of Nothofagus forests with N. obliqua, and according to CONAF [15], these forests could produce around 321.4 million m3 of timber; nevertheless, only 63% of this volume would be commercial due to forest health problems [16].
Valdivia is the most frequently infested province by P. chilensis, with Díaz [17] reporting 22.4%, 43.5% and 9.5% of infestation, respectively. Kruuse [6] explained that the high environmental humidity and the quality of wood tissue of Nothofagus trees growing in Valdivia would be optimal for insect proliferation. In contrast, trees growing in areas farther North are not affected by P. chilensis, as those sites present lower humidity and higher temperature that preclude mating, oviposition and larvae survival [17]. In the same way, a study carried out in Valdivia concluded that N. obliqua trees facing South (lower radiation and lower temperatures) had more damage than trees facing North [3]. Besides environmental humidity, infestation depends on tree attributes, flora biodiversity, site, and silviculture.
Suárez and Cabrera [2,3] showed a positive relationship between tree diameter at breast height (dbh) and infestation. Suárez [2] analyzed the influence of flora biodiversity on xylophagous infestation when studying herbivory patterns of P. chilensis and Cheloderus childreni (Col: Oxypeltidae) on N. obliqua and Nothofagus dombeyi, respectively. They concluded that the number of understory species had a negative influence on C. childreni infestation but no influence on P. chilensis. These results could be extended to study the relationships between xylophagous infestation and individual understory species, such as Chusquea quila (Poaceae: Bambuseae), which has been shown to obstruct the establishment and growth of trees, especially Nothofagus sp. [18].
Altitude is another variable that could influence P. chilensis, since altitudinal gradients are correlated to temperature changes that vary the structure and the ecosystem processes in the forest. Temperature decreases by around 0.5°C per 100 m of elevation [14]. Altitude affects habitat quality for wood-boring insects, because their reproductive behavior is stimulated by sun exposure [19]. Baldini and Oltremari [20] reported that stands located under 250 masl displayed more insect attacks in the Araucanía Region (North of our study sites). These authors also pointed out that as the stocking increases the infestation decreases, although there would be an attack preference for stockings lower than 500 stem per ha.
There is no much information about the effect of silviculture on P. chilensis infestation; however, stocking appears to influence insect attack in other species. Amman [21] found a negative correlation between Pinus contorta stand density and mountain pine beetlecaused mortality (Dendroctonus ponderosae). However, in the case of Douglas-fir beetle (Dendroctonus pseudotsugae), high stand density is positively correlated with infestation [22]. These examples show that density management treatments, such as thinning, are expected to play a role in the management of infestation problems. This study was conducted to explore relationships among P. chilensis infestation, trees, site and flora attributes in the Valdivia Province, aiming at increasing information about P. chilensis infestation patterns, as well as suggesting silvicultural approaches that could reduce P. chilensis damage.
Location and selections of study plots
The study established 36 non-permanent 400 m2 square plots between December 2012 and January 2013, covering the eight communes of the Valdivia Province in Los Ríos Region, Chile. The number of plots per commune was as follows: Corral (3 plots), Lanco (3 plots), Los Lagos (7 plots), Máfil (3 plots), Mariquina (4 plots), Paillaco (3 plots), Panguipulli (9 plots) and Valdivia (4 plots) (Figure 1 and Table 1).
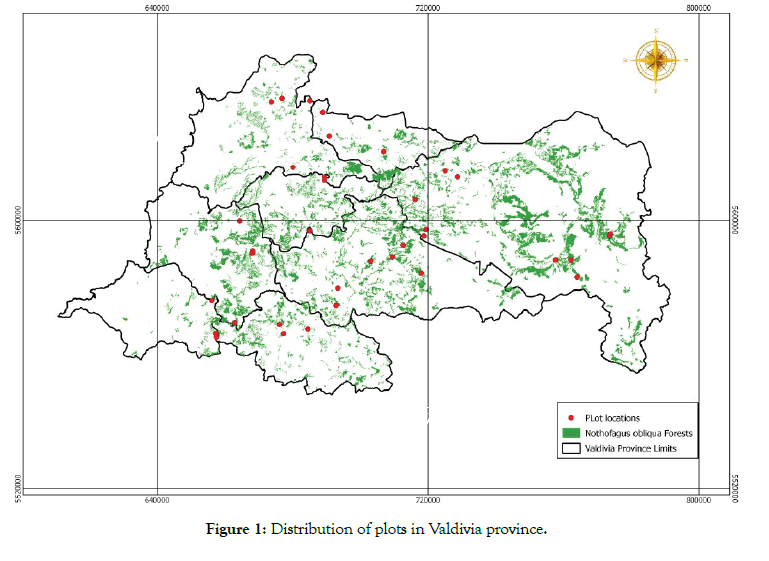
Figure 1: Distribution of plots in Valdivia province.
Table 1: Summary of P. chilensis damage indicators and stand variables per commune, where codes are CO (Corral), LA (Lanco), LL (Los Lagos), MF (Máfil), MQ (Mariquina), PC (Paillaco), PG (Panguipulli), and VD (Valdivia).
| Plots Communes | Trees/ha | Trees/plot | Attacked Trees | Nº damage signs | Basal Area/plot | Mean dbh (cm) | Elevation (masl) | Slope Range (%) | Density factor |
|---|---|---|---|---|---|---|---|---|---|
| CO01 | 1575 | 63 | 1 | 1 | 18.47 | 10.13 | 124 | 20 | Closed Stand |
| CO02 | 775 | 31 | 0 | 0 | 20.54 | 16.52 | 51 | 35 | - |
| CO03 | 375 | 15 | 6 | 6 | 24.06 | 26.50 | 87 | 25 | Open Stand |
| LA01 | 675 | 27 | 4 | 5 | 53.67 | 30.63 | 183 | 30 | Closed Stand |
| LA02 | 800 | 32 | 0 | 0 | 33.77 | 21.53 | 183 | 30 | Closed Stand |
| LA03 | 800 | 32 | 5 | 6 | 62.89 | 29.89 | 230 | 10 | Closed Stand |
| LL01 | 1425 | 57 | 1 | 1 | 34.18 | 13.32 | 404 | 20 | Closed Stand |
| LL02 | 775 | 31 | 3 | 4 | 39.11 | 23.13 | 101 | 10 | Open Stand |
| LL03 | 775 | 31 | 1 | 1 | 37.63 | 23.40 | 148 | 33 | Closed Stand |
| LL04 | 900 | 36 | 2 | 2 | 53.37 | 24.94 | 158 | 0 | Closed Stand |
| LL05 | 2075 | 83 | 11 | 12 | 68.65 | 19.04 | 220 | - | Closed Stand |
| LL06 | 800 | 32 | 1 | 1 | 34.49 | 21.09 | 295 | 5 | Closed Stand |
| LL07 | 1350 | 54 | 0 | 0 | 30.47 | 15.15 | 277 | 30 | Closed Stand |
| MF01 | 925 | 37 | 4 | 5 | 42.78 | 21.80 | 202 | 50 | Closed Stand |
| MF02 | 1275 | 51 | 1 | 1 | 49.27 | 18.15 | 239 | 37 | Closed Stand |
| MF03 | 1350 | 54 | 7 | 9 | 61.11 | 21.09 | 113 | 35 | Closed Stand |
| MQ01 | 725 | 29 | 3 | 7 | 32.45 | 20.72 | 139 | 20 | Open Stand |
| MQ02 | 875 | 35 | 5 | 6 | 32.12 | 19.77 | 125 | 20 | Open Stand |
| MQ03 | 1100 | 44 | 6 | 6 | 32.53 | 17.77 | 160 | 33 | Closed Stand |
| MQ04 | 550 | 22 | 3 | 3 | 27.04 | 23.95 | 209 | 20 | Closed Stand |
| PC01 | 1375 | 55 | 5 | 6 | 16.23 | 10.93 | 132 | 18 | Closed Stand |
| PC02 | 1425 | 57 | 9 | 13 | 46.62 | 18.35 | 79 | 15 | Closed Stand |
| PC03 | 2050 | 82 | 16 | 17 | 22.74 | 11.27 | 89 | 20 | Open Stand |
| PG01 | 550 | 22 | 13 | 19 | 36.10 | 28.30 | 253 | 2 | Open Stand |
| PG02 | 925 | 37 | 7 | 7 | 58.84 | 25.68 | 281 | 15 | Closed Stand |
| PG03 | 775 | 31 | 1 | 1 | 43.86 | 24.18 | 471 | 30 | Closed Stand |
| PG04 | 600 | 24 | 2 | 2 | 48.23 | 30.46 | 348 | 28 | Open Stand |
| PG05 | 525 | 21 | 6 | 7 | 60.72 | 37.05 | 270 | 30 | Closed Stand |
| PG06 | 925 | 37 | 0 | 0 | 45.43 | 23.53 | 539 | 15 | Closed Stand |
| PG07 | 1225 | 49 | 2 | 2 | 56.66 | 21.19 | 524 | 40 | Closed Stand |
| PG08 | 525 | 21 | 0 | 0 | 37.29 | 28.12 | 464 | 15 | Open Stand |
| PG09 | 1175 | 47 | 0 | 0 | 55.04 | 22.98 | 600 | 15 | Closed Stand |
| VD01 | 2175 | 87 | 0 | 0 | 37.10 | 13.06 | 266 | 5 | Closed Stand |
| VD02 | 1650 | 66 | 13 | 16 | 35.76 | 14.65 | 34 | 10 | Open Stand |
| VD03 | 1975 | 79 | 20 | 27 | 51.09 | 16.91 | 39 | 2 | Closed Stand |
| VD04 | 2275 | 91 | 6 | 8 | 39.24 | 12.58 | 28 | 30 | Closed Stand |
Climate and soils
The Valdivia Province presents temperate rainy climate with Mediterranean influence, with an average annual relative humidity of 76%, and an average annual precipitation of 1780 mm [23]. There are three major soil groups: organic-rich andisols, aquic andisols and red clay. Organic-rich andisols occupy about 86% of the study area and correspond to andesitic-basaltic volcanic soils. In general, these soils are deep to very deep with good drainage [24,25].
Variables collected in the field
Each plot was georeferenced and assessed for slope, elevation, tree stocking and for a visual density factor to define open-stands and closed-stands. Information on flora composition and biodiversity was generated by two vegetation censuses based on plots of 64 m2. Censuses were carried out by following the phytosociological principles of the South-European School [26]. Field records included the species list and the abundance-dominance score per species (degree of cover), which was estimated visually by using [27]. Sampling points were selected according to physiognomic and floristic homogeneity. Lists of plant species were analyzed to estimate two indicators: the Shannon-Weaver index of understory species and the Importance Value Index (IVI) of C. quila. The Shannon-Weaver index is a diversity measure that accounts for both evenness and abundance of species present in the community. IVI is an efficient indicator to compare species in terms of their importance within the community [28].
At tree-level, each N. obliqua individual was evaluated for social status, diameter at breast height (dbh), height and form. Infestation was recorded as attacked or non-attacked tree, by detecting external signs such as sawdust drain orifices and emergence orifices in the stem.
Data analysis
At tree-level, infestation was managed as a dichotomous variable 1/0, where 1 represented presence of damage signs (infestation) and 0 their absence (no infestation). Trees were grouped according to infestation and compared on dbh using a t-test. We fitted a hierarchical linear mixed model with a logistic link function. The fixed-effects included a factor for stand density (Open Stand versus Closed Stand) and estimated tree height as a covariate. The random effects considered a separate intercept (in the logit scale) for each plot. Residuals were assumed to be independent of each other and to follow a binomial distribution.
All models were fitted using the lme4 package [29] in the R Statistical System (R Core Team). R code and data are available as supplementary materials.
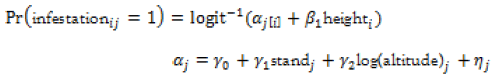
, with 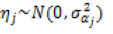
Stand density and altitude were fitted at the plot level, while total height entered the model at the tree level.
Infestation and damage signs
A total of 1602 trees were evaluated, and 164 of them showed damage signs produced by P. chilensis. The damage was concentrated in the commune of Valdivia (51 signs in 39 trees). The infestation per plot ranged from 1.5 to 59.1% among the 36 plots. Average infestation, at the commune level, did not exceed 14%. The lowest infestation was observed in Los Lagos (5.18%), Máfil (8.56%) and Lanco (10.13%) communes, whereas the highest occurred in Valdivia (12.87%), Mariquina (12.90%), Panguipulli (13.56%) and Corral (13.83%) (Table 1).
According to Table 1, plots had similar slope; however, variables such as average dbh, and elevation were highly variable among plots. Average infestation was 12%, and only 4 plots presented infestation levels higher than 25%, two in Panguipulli (59.1% and 28.5%), one in Corral (40%) and one in Valdivia (27.3%).
Tree-level damage models
A t-test assessed statistical differences between mean dbh of infested trees and non-infested trees. That difference was statistically significant (p< 0.05). A mixed-effects logistic regression model was fitted to explain P. chilensis infestation at tree level (Table 2). Elevation, dbh, C. quila IVI, Shannon-Weaver index, estimated tree height and stand density factor were also tested as explanatory variables but did not help explaining infestation.
Table 2: Fixed-effects coefficients in the logit scale. The random effect plot variance is 1.08.
| Variables | Estimate | Std. Error | z-value | Pr (>|z|) |
|---|---|---|---|---|
| Intercept | -2.46951 | 0.56227 | -4.392 | 1.12e-05 |
| Estimated tree height | 0.06192 | 0.01885 | 3.284 | 0.00102 |
| Stand density factor | -124.325 | 0.46479 | -2.675 | 0.00748 |
Figure 2 summarizes the model with the probability of damage increasing with tree size. The left panel displays only the fixed effects of the model, using red for open stands and blue for the closed stands. The right panel introduces the plot random effects, showing the large between-plot variability for open (red) and closed stands (blue).
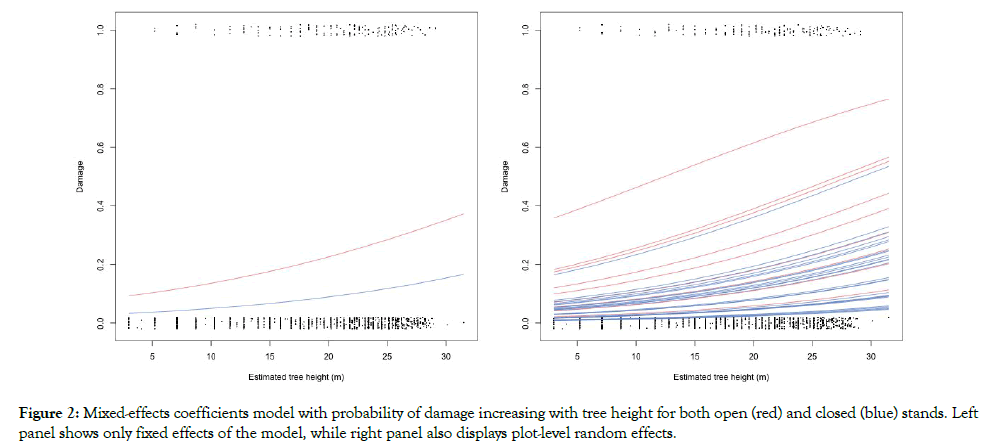
Figure 2: Mixed-effects coefficients model with probability of damage increasing with tree height for both open (red) and closed (blue) stands. Left panel shows only fixed effects of the model, while right panel also displays plot-level random effects.
Elevation was not statistically significant to explain the probability of a tree being attacked by P. chilensis. Concerning tree attributes, estimated height was better predictor than tree dbh; although most of studies so far indicate than tree dbh have significant effect on xylophagous damage. Thus, probability of attack increases as tree dbh increases [2]. Results indicated the probability of a tree being attacked increased significantly with tree height (0.06%). Density factor, which describes how closed is a stand, was also significant to explain P. chilensis attack. Our results indicated that the probability of infesting a tree decreased significantly with stand density, matching the trend observed by Baldini and Oltremari [20] when looking for factors influencing xylophagous infestation.
Infestation levels were occasionally over 12% in the 36 plots, and just 4 plots in communes of Panguipulli, Corral and Valdivia presented infestation higher than this percentage, which represent 11% of the total plots. Thus, our study does not support the idea of a generalized xylophagous infestation problem in Valdivia Province. On the other hand, these results differ significantly from those obtained in previous studies, in Los Ríos Region, by Díaz [17] who reported levels of infestation of 22.4%, 43.5% and 9.5% respectively. These results could be explained because the selected places had high infestation levels, or due to variations on insect population cycles.
We consider that most of the forests analyzed in this study are growing with a natural health, according to the expected host-guest relationship between two native species [1]. However, it became interesting to assess site, flora and tree variables that explain the variability of infestation and damage signs, as a way to contribute to the knowledge of this insect, and to make suggestions about silvicultural practices that could reduce P. chilensis damage. Using a mixed-effects logistic model helped identify variables that contribute to increase the probability of a tree being attacked. As a result, estimated tree height was highly associated with this probability (Table 2). This study confirms that the most severe attack of P. chilensis occurs in dominant and codominant trees, which are the largest trees of a stand [2,17]. Most of studies concerning to P. chilensis report that dbh would be the tree variable that mostly explains P. chilensis attack; thus, both dbh and tree height would be plausible variables to represent damage trends.
Stand density had a significant importance decreasing the probability of a tree being attacked. Suárez et al. [2] Tried the same predictor when analyzing infestation patterns of P. chilensis in Valdivia, Osorno and Llanquihue Provinces; however, their results indicated that this predictor was not significant (p>0.05). Stand density is associated with temperature and light decreasing effects, which affect habitat quality of organisms like wood-boring insects, since their reproductive behavior is stimulated by sunny exposure [30]. Stand manipulation by silviculture has been reported as a sustainable method to control insect damage [31-33]. However, it is necessary to first understand infestation patterns, because insects have different infestation strategies. A study developed in Canada by Gebeyehu and Wingfield [34] showed that the weevil Pissodes nemorensis Germar (Col: Curculionidae) moves around very little in shaded pine stands, since shade has a negative influence on the insect’s visual response to attractive pine leads. In the same way, our results suggest a negative relationship between infestation and stand density factor (Table 2). Thus, we hypothesize that dense stands and shaded habitats would preclude P. chilensis development, because temperature and radiation levels that prevail in these conditions, limit feeding, oviposition and larvae development. Accordingly, we suggest creating low temperature and more cover shade conditions by keeping stand density over 500 stem per hectare. In this way, thinning practices must be approached with a tree health criterion based on protocols designed to identify P. chilensis damage [35].
Elevation, flora biodiversity index and C. quila IVI were not significant at explaining the probability of a tree being attacked by P. chilensis. We hypothesized that increasing elevation would be associated with decreasing damage since the temperature decreasing effect is shown to affect P. chilensis infestation. Interactions between plants and herbivores vary with altitude; thus, herbivory at lower elevations can even limit the distribution range for some plant species [36,37]. Previous studies on P. chilensis showed an inverse relationship between damage and elevation. Thus, Díaz [17] climatic conditions; particularly temperature, limited P. chilensis infestations in higher altitude. In addition, at lower altitude anthropic alteration increases, which means best trees were felled and attacked trees in the stand are provoking infestation increase. Concerning flora biodiversity, which was represented by Shannon- Weaver index, we hypothesized that trees growing in more biodiverse environments would present less damage since insects’ herbivory, with very specific hosts, has shown to decrease in the presence of high vegetation biodiversity. In fact, Altieri and Landis et al. [38,39] explain that high biodiversity in plants sustains more parasitoids, predators and natural enemies. We also hypothesized that C. quila IVI would have negative effects on P. chilensis due to the microclimatic conditions that this Bambuseae creates by reducing temperature and light, which is comparable to maintain high stocking. This understory species has shown to obstruct the establishment and growth of trees, especially Nothofagus, but there are not studies that relate to their effect on xylophagous infestation [18].
Results also indicated that the most frequent attack of P. chilensis is in dominant and codominant trees, which imposes a complex scenario to put forward silviculture suggestions, because infestation increases with decreasing stand density. Therefore, in keeping denser stands, log production will be concentrated in smaller diameters, which have lower prices.
As height and diameter growth, and P. chilensis infestation are inversely related to stand density, stocking decisions will generate volume-damage tradeoffs that will affect the value recovery of the forest. An alternative silvicultural approach for balancing production and forest health is the application of uneven-aged management strategy, which has shown it can contribute to good ecosystem health, diversity and sustainability [40]. The advantages of uneven-age management are providing income at more frequent intervals, minimizing regeneration costs and offers wildlife habitat and recreational services. The main disadvantage of this approach is the complexity of achieving a balanced and sustained yield of forest products.
Although P. chilensis is native, its attack to Nothofagus forests beyond the natural balance between host and insect, could affect value recovery and sustainability; however, this study indicates that infestation in Valdivia Province is under 12%, which is lower than previous values reported in the literature.
This study confirm that stand density (stocking) is a statistically significant factor to explain the probability of a tree being attacked by P. chilensis, which suggests the possibility of managing stocking to reduce damage in natural forests and applying such learning when establishing plantations based on Nothogafus species.
In testing climatic factors, altitude was not significant to explain the probability of a tree being attacked by P. chilensis; nevertheless, previous studies showed an inverse relationship between damage and elevation. For example, Díaz [17] indicated that, at higher altitude, temperature limits P. chilensis infestations. On the other hand, at lower altitude anthropic alteration is higher and forests are characterized by presenting a high proportion of attacked trees inducing increased infestation.
There is no single strategy able to confront phytosanitary challenges on Nothofagus forests. From a silviculture point of view, sustaining uneven aged structure, favoring flora diversity and maintaining high tree stocking have been argued to reduce damage of xylophagous insects. Donoso [41] points out that diverse systems are the best guarantee to maintain the balance between prey and predators, thus avoiding the proliferation of pests and positively impacting forest health.
Our study did not find significant relationships between attack and flora diversity; in addition, sampled stands corresponded to secondgrowth forests with even-aged structure; so, it was not possible to study differences between uneven and even-aged structure. In the same way, our results do not evidence that P. chilensis is representing problems to crops or ecosystem services such as water quality neither is a threat to other native insects.
This study was funded by the Natural Forest Grant of Chilean Forest Service (FIBN 006/2012, CONAF). We thank Ronnie Reyes and Jessica Pérez for their assistance in field work.
Citation: Alzamora RM, Apiolaza LA, Ruiz C, Lanfranco D (2020) Site, Tree and Silvicultural Factors Influencing the Infestation of Xylophagous Insects on Nothofagus Forests. 9:226. doi: 10. 35248/2168-9776.20.9.226
Received: 19-Apr-2020 Accepted: 15-May-2020 Published: 22-May-2020 , DOI: 10.35248/2168-9776.20.9.226
Copyright: ©2020 Alzamora RM, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.