Journal of Fertilization: In Vitro - IVF-Worldwide, Reproductive Medicine, Genetics & Stem Cell Biol
Open Access
ISSN: 2375-4508
ISSN: 2375-4508
Short Communication - (2021)Volume 9, Issue 3
Cryopreservation is a technique which preserves the different type of cells, tissues, and additional biological components through freezing the specimens in very low temperatures using various methods. As Assisted Reproductive Techniques develops, the cryopreservation process has had several milestones over the years aimed at improving preservation of gametes and embryos. Accordingly, there is an increased requirement to maintain cellular structure and function without affecting genetic stability. Approaches must, avoid potential damage and improve upon prior outcomes, but also attempt to address concerns contamination. The cryopreservation of gametes and embryos has continued to increase in recent years and the accompanying improvements have including applying novel cryoprotective agents and temperature control, in addition to the different carrier devices that have been developed, such as close and open systems. This review article will discuss the characteristic cryopreservation processes of gametes and embryos, such as slow-rate and vitrification. It will also discuss cryopreservation and liquid nitrogen quality control and common adverse events that may be encountered.
Liquid nitrogen; Cry storage; Quality control; Contamination; Vitrification; Gamete; Blastocyst
LN2:Liquid Nitrogen; ART: Assisted Reproductive Techniques; PPE: Personal Protective Equipment; IVF: In- Vitro Fertilization;STIs: Sexually Transmitted Infections; DMSO: Dimethyl Sulfoxide; ICSI: Intracytoplasmic Sperm Injection
Cryopreservation in General
Cryopreservation is the process of applying a very low temperature to maintain the entire structure of living cells and tissues for an extended time. According to the cell types or given cells amongst different mammalian species, there is high heterogeneity in cryobiological response and cry survival through the freezing and thawing cycle. Cryopreservation is based on chemical and biological reactions in living cells to dramatically decreased cell temperature. This event can point to the possible long-term preservation of cells and tissues. However, freezing can be lethal to several living organisms. Both intra- and extracellular ice crystals are produced and change the cells' chemical setting, pointing to cellular mechanical constraints and injury. The main obstacle for cells to overcome at low temperatures is the water-to-ice phase transition. Cell injury at fast cooling rates is linked to intracellular ice formation [1]. Cryopreservation methods can generally be classified into the following classes:
• Slow freezing.
• Vitrification requires the solidification of the aqueous milieu of the cell or tissue into a non-crystalline glassy phase.
• Non-freezing Subzero storage.
• Dry state preservation method.
The vital stage in cryopreservation is mixing CPAs with cells or tissues ere cooling; cooling the cells or tissues to a low temperature and storage; warming the cells or tissues, and removing CPAs from the cells or tissues thawing. Accordingly, the proper use of CPAs is necessary to advance the sample's viability to be cryopreserved. The CPA usually is a fluid that decreases the cryopreservation freezing injury to the cells and tissues. CPAs must be biologically approved, be able to enter the cells and have low toxicity. Several CPAs have been produced and are applied to decrease the quantity of ice developed at any given temperature, depending on the cell type, cooling rate, and warming rate. Various factors should be controlled to optimize the cryopreservation process such as the sample volume, cooling rate, warming rate, and CPA concentrations, and obtain the best survival rate of cells and tissues depending on the several cell types and context of tissues. CPAs can be divided into two categories: (1) cell membrane-permeating cryoprotectants, such as dimethyl sulfoxide (DMSO); and (2) nonmembrane-permeating cryoprotectants, such as 2-methyl-2,4- pentanediol the most commonly used CPA in cryopreservation is Glycerol, used with static vapour cooling or controlled rate freezer in 1:1 ratio (0.6M-0.75M or 12-15% solution). What is unique in Glycerol is that it's required for cell survival, through *Mixing extraordinarily well with water *Changing the structure of a hydrogenbonded network *Reduces lethal ice formation (intracellular/ extracellular). However, Glycerol can cause osmotic damage by initial shrinkage (H2O leaves), then swelling as H2O+CPA re-enters. Several advances cryopreservation methods have been developed to preserve various cell types including female and male gametes, in addition to day 3, day 5, or blastocytes embryo, which allow for low-temperature maintenance.
Cryopreservation of Sperm
The Cryopreservation of human spermatozoa was introduced in the 1960’s, which is consider now to be an integral part of assisted reproduction technologies (ARTs). Sperm cryopreservation methods are a principle in ART since it preserves male fertility radiotherapy or chemotherapy, leading to testicular failure or ejaculatory dysfunction. Semen cryo-storage appears to be the only proven technique that can allow these couples the possibility of having children in the future. Cancer therapy can damage, ending in subfertility or sterility due to gonad extraction or constant damage to germ cells induced by cancer treatment [2]. As discussed before cryopreservation can be applied through different methods, in sperm cryopreservation rapid and slow freezing can used in preserving sperms (Table 1).
Table 1: Summary of cryopreservation method of sperms using different techniques (Di Santo et al.).
| Method | Description |
|---|---|
| Slow freezing | This method involves a gradual sperm cooling over a period of 2–4 h in two or three stages, either manually or automatically through a semi programmable freezer. |
| Rapid freezing | This technique needs direct contact between the straws which contain the sperm and the nitrogen vapors for 8–10 min and immersion in liquid nitrogen at −196°C. |
| Cryopreservation of small numbers of spermatozoa | This method is used for epididymal and testicular spermatozoa. Different approaches have been explored to preserve small numbers of spermatozoa, such as microdroplets, ICSI pipette, and empty zona pellucida methods. |
Cryopreservation of Embryos/ Blastocysts
Embryo cryopreservation is the process which involves the freezing and storing of embryos for purposes like preserving embryos in the status of particular medical treatments, avoid the wastage of extra embryos also to narrow the risks of multiple pregnancies resulting from the transfer in uteri of many embryos [3]. Several studies assessed frozen blastocysts embryo transfer according to cleavagestage embryo transfer, different freezing strategies (vitrification vs slow freezing), and different vitrification and warming media. Accordingly, feasible protocols are required to represent embryos to freeze when they are most competent and least vulnerable [3]. The cryopreservation of embryos at the blastocyst stage has developed to be an essential part of the daily routine process in an IVF lab. There are several protocols and commercial kits that can be used for blastocyst vitrification [4] (Figure 1).
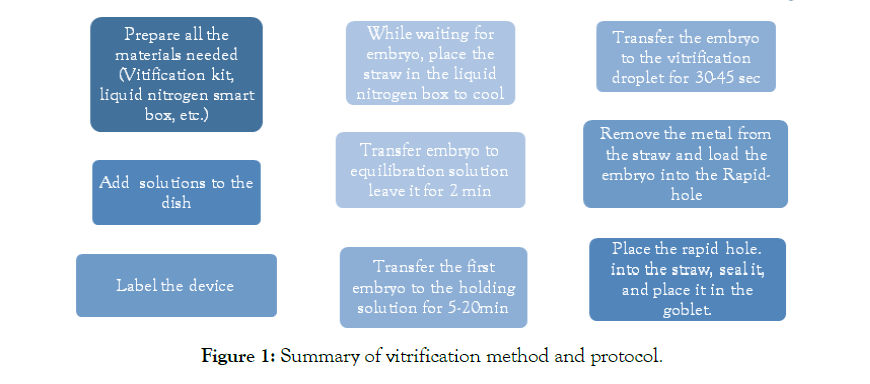
Figure 1: Summary of vitrification method and protocol.
Cryopreservation of Storage
Usually, there are two types of storage, the vapor-phase and Liquid phase. In the vapor-phase, there is no contact between liquid nitrogen and the sample, while in the liquid phase, there is contact between the liquid nitrogen and the sample. There are two types of liquid-phase storage, discontinuous immersion, where the sample will be in liquid nitrogen depending on, the nitrogen level, and the continuous immersion; the sample will be continuously in liquid nitrogen, thus requiring specific adjustment.Currently, vitrification protocols can either use a closed or open system for vitrification. In the closed systems, the samples are separated from liquid nitrogen throughout the entire cooling, storage and warming process. In contrast, open systems enable direct contact between the sample and liquid nitrogen [5]. Since the current open and close vitrification system show various levels of biosafety [6], and different liquid nitrogen can sometimes contain infective agents, open system vitrification can probably indicate a chance for infection and disease transfer. However, no disease transfer has yet been reported, and an estimated 600,000 to 1,000,000 vitrified embryos using open systems have been transferred [5]. Table 2 compare and contrast different types of open and close system vitrification.(Figure 2A, 2B)
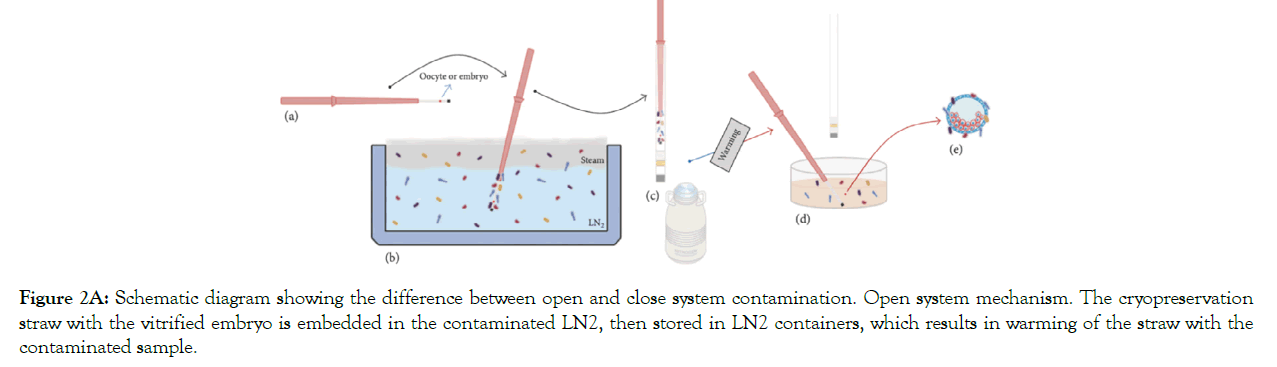
Figure 2A: Schematic diagram showing the difference between open and close system contamination. Open system mechanism. The cryopreservation straw with the vitrified embryo is embedded in the contaminated LN2, then stored in LN2 containers, which results in warming of the straw with the contaminated sample.
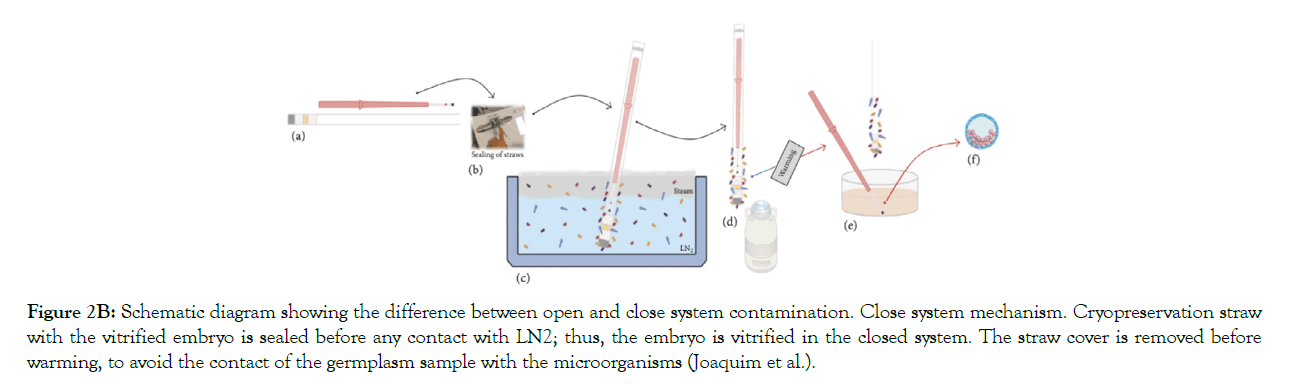
Figure 2B: Schematic diagram showing the difference between open and close system contamination. Close system mechanism. Cryopreservation straw with the vitrified embryo is sealed before any contact with LN2; thus, the embryo is vitrified in the closed system. The straw cover is removed before warming, to avoid the contact of the germplasm sample with the microorganisms (Joaquim et al.).
Table 2: Main types of open and close system vitrification (Vajta et al.).
| Type of Vitrification System | Characteristics of the System |
|---|---|
| Fully open system | Unprotected OPS, unsealed straws; and the Cryoloop stored in cryotubes. Extremely high cooling and warming rates. |
| Open cooling and closed storage systems | The carrier tool is inserted into a pre-cooled sterile container which is heat- sealed and can to resists the extremely temperature variation. Extremely high cooling and warming rates. |
| Semi-closed cooling | This system is connected to either open or closed storage systems, and are neither closed nor entirely biosafe. |
| Closed thin walled, narrow capillaries | The system is heat sealed before cooling, and opened only after warming |
| Carrier tools sealed into a container | The carrier tools are sealed into a container, that splits them safely while cooling, storage and warming. High security vitrification kit. |
Cryopreservation and Liquid Nitrogen
Historically, human ART clinics used to apply slow-cooling techniques for freezing embryos. More recently vitrification is the preferred option, allowing live offspring from human oocytes and embryos. Vitrification is a simple alternative to slow cooling, needing no expensive freezing equipment protocols; in vitrification liquid nitrogen (LN2) is applied, for rapid direct cooling. This rapid-cooling method inhibits ice crystal formation. However, since vitrification may require direct contact with LN2, the potential exists for mechanical injury and disease transmission risk through contact with contaminated LN2 during storage [7]. Liquid nitrogen is a cryogenic fluid essential for in vitro reproductive technologies widely used in human IVF, in the cattle industry in vitro embryo production, and for livestock breeding research purposes. LN2 is a liquid substance produced through an industrial process by means of a fractional distillation method. Air is liquefied and then distilled in order to separate the nitrogen gas. Its main characteristic is the ability to maintain the ultralow temperature of 196°C, well below the freezing point of the water (0°C), making it useful for several applications. One of them is the cell cryopreservation process, used ART to preserve gametes and embryos for the treatment of human infertility and fertility preservation issues, as well as in cry banking of animal gametes and embryos in the cattle industry. The cryogenic temperature slows chemical and physical reactions of the biomolecules and stops the samples from degrading for future. Nowadays, new LN2 tanks have been developed to be applied in various laboratories, liquid nitrogen (LN2) in several designs. The modern LN2 tank design is was applied to the first practical semen storage tank developed as a joint venture between the American Breeders Service and the Linde Division of the American Cyanamid Company in 1956 [8]. Until this time, it was more famous for livestock operators to use fresh or cooled extended semen. After semen cryopreservation showed reliable results in ART clinics, the cryopreserved semen was stored with dry ice—the LN2 storage systems available e frequent management, such as close monitoring and regular filling [8].
Liquid Nitrogen Supply and Staff Safety
Freezer storage that has temperate much higher than - 135 C is called 'glassy transformation temperature' and is sometimes suspected of harming the frozen cells or tissues. Therefore, sustenance of ultralow temperatures and a maintained supply of liquid nitrogen must be assured for specimens' safekeeping. ART clinics must guarantee that if the nitrogen supply fails for any reason, there are fail-safe techniques to cope. Supply failure can happen for various reasons; for instance, if the pressurized vessel vacuum breaks or releases its contents; the automated refrigerator over-fills; the LN2 supply company fails to deliver. Such circumstances can be solved and secured by; implementing contingency measures in places, like an extra supply in terms of nitrogen delivery vessels, proper written plans and training for all applicable staff for dealing with short supply cases [9]. ART clinics are usually too dependent on delivery from the nitrogen supply companies for routine filling of pressurized storage vessels. Controls must also anticipate likely delivery vehicle problems. For instance, heavy road traffic, accident or breakdown, and contingency may include having spare capacity, either by keeping a surplus in light pressurized vessels or keeping an external bulk tank, e.g. 1000 litres [9].
Liquid Nitrogen Dangerous Effects on Cryopreservation
In human reproductive cells such as sperms and oocytes or embryos cryopreservation, those cells can be contaminated by LN2 produced by frozen microorganisms' adhesion, possibly present in LN2, on the frozen specimen or the outer exterior of the carrier or container. It has now been confirmed that several pathogens could be detected in reproductive cells/tissue cry banks [10]. In some models of cryo containers, because of their design (straws, vitrification carriers, etc.) it’s difficult to avoid the possible contact with microorganisms in LN2/NV through the cryopreservation procedure or cry storage; accordingly, the organisms are consequently transmitted in the culture medium through the thawing procedure when any cryopreserved microorganism found in the LN2/NV reactivates after thawing. Cross-contamination with bacteria between samples was also demonstrated with semen pellets, where sterile samples have been found to be contaminated with Escherichia coli and Staphylococcus aureus. Furthermore, liquid nitrogen with various viruses can also be found in embryos to be infected. Accordingly, preserving semen and embryos in closed containers limit viral contamination. Additionally, it has been found that almost limited numbers, viral screening was made on spent culture media and liquid nitrogen applied to vitrify oocytes and embryos from infected women. No viral sequences were detected, proposing that the risk of cross-contamination is low; however, it was suggested that more reliable cryopreservation techniques should be developed to avoid any possible contamination [11]. Several components of embryo culture media and semen extenders can support micro-organisms at freezing temperatures such as milk, serum or serum albumin, sucrose, sorbitol and other sugars. Nevertheless, the standard CPs used in the embryo and sperms cryopreservation are glycerol, DMSO, ethylene glycol, propylene glycol, methanol etc.; for sufficient protection against bacteria and viruses’ cryoinjuries. For example, DMSO concentrations as low as 5% support samples against viruses and trauma of freezing [12]. However, non-enveloped viruses are not cleared out by freezing and thawing, even in protecting CPs. This is because some microorganisms can persist in association with germplasm and lead to disease transmission potential to recipients by embryo transfer. Thus, it's crucial to store and test the samples for health and safety and quality control of embryos for appropriate cryopreservation [12,13]. The LN2 tank's design (size, shape, composition elements, welds, and sealants) does not restrict structural integrity loss. Metal weakness and structural pressures can end in a progressive loss of space and gradually increase liquid nitrogen use. Gradual alterations in fluid nitrogen levels are normal. One of the most vulnerable sections of small-capacity tanks is the welds that connect the tank's neck to the anterior chamber and the top of the outer chamber (Figure 3). This neck suspends the tank's entire weight, which is almost 75 to 100 pounds. These neck welds are not found in large-capacity tanks.
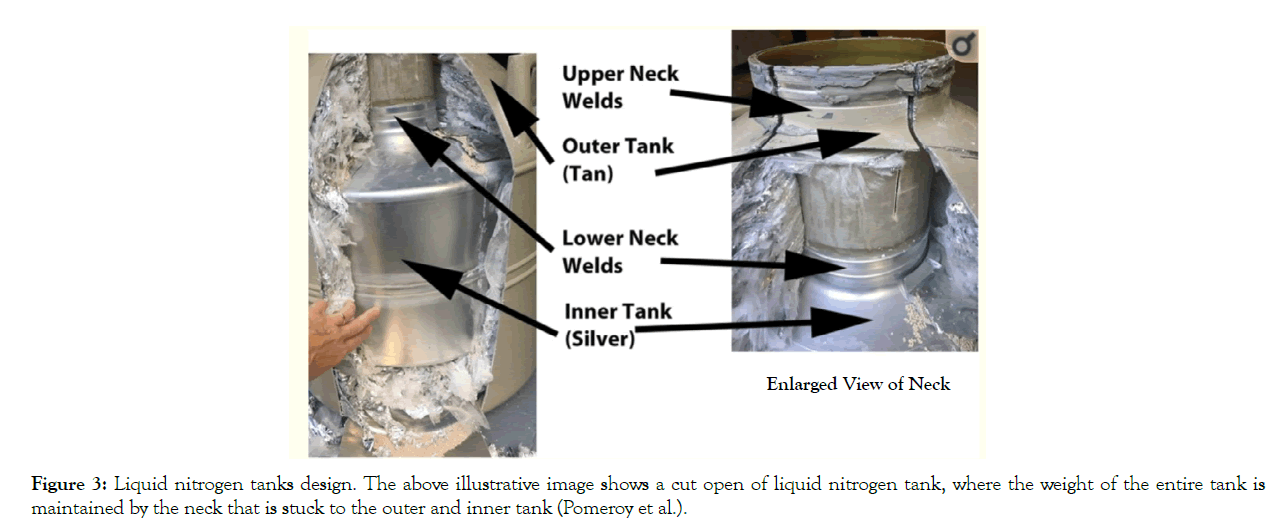
Figure 3: Liquid nitrogen tanks design. The above illustrative image shows a cut open of liquid nitrogen tank, where the weight of the entire tank is maintained by the neck that is stuck to the outer and inner tank (Pomeroy et al.).
Liquid Nitrogen Quality Control
The recommended LN2 containers dewans are 73 L, and highcapacity bulk vessels for long-term, unlimited storage. The more transportable LN2 containers are the most popularly used in IVF clinics; however, some have promoted the safe use of LN tanks for sperm and oocytes/embryos. A hypothetical hazard of microbial and viral cross-contamination among specimens is usually preserved in LN2 without securely sealed closed-device systems. Nevertheless, in clinical practice, such cross-contamination of gametes or embryos has never been confirmed under normal cry storage circumstances [14]. Cryogenic containers are made of both open-lid storage tanks and closed pressured supply tanks. Both types are composed of 2–3 layers of carbon and stainless steel or aluminum, in addition, copper- and nickel-alloys can be included sometimes. The layers are well-sealed to generate an "annular space" to depressurize via a pump to produce high vacuum conditions.The annular area can also be filled with an insulating element such as perlite, silica aerogel, or fiberglass, to reduce heat alteration into the inner tank cylinder to decrease vaporization, the conversion of liquid to gas when the boiling point is reached. The transition of thermal energy from the warm exterior to supercool interior is referred to as the "inleak" potential heat transfer. Therefore, the number of vacuum layers, type and amount of insulation, quality of welds, and freedom from defects can all affect the inleak potential of a tank and, for our practical purposes, the evaporation rate of LN2 [14]. Another vital aspect is the location of units', where an accurate evaluation of future storage needs is necessary for choosing and utilizing a cry storage area that fits operational needs. Such requirements must include an assessment of future cry storage usage, which consists of enough empty back-up tank resources. Additional critical safety considerations can consist of total square footage, and ventilation is essential considerations of safety. Moreover, a dedicated air handling system with a full turnover of fresh outside air is preferred, that is 100%out-take and 0% re-circulation. Almost in all IVF center, the above guidelines are challenging to manage in an ART clinic setting. Alternatively, attempts should be applied to vent residual nitrogen gas out of a building, especially in a low emergency O2situation [14]. LN2 filling is a critical aspect of LN2 quality control. Manual-fill of LN2 storage containers such as low-capacity dewan tanks (< 60–L) should be filled at least weekly through suitable safety precautions. The safety measures routinely apply to wear insulated thermal gloves and liners, protective eyewear, and other personal protective equipment (PPE). Checking the LN2 tank levels should be done at least weekly, ere all manual filling events. These measures aim to evaluate each tank's usage/ evaporation rate and decide if the liquid levels are in an acceptable range limit. Additionally, personnel must routinely make spotcheck evaluations, possibly measurements, that the LN2 levels are above canister/sample device levels whenever a sample is removed from or placed into a tank.
The Objective of the Society for Art Irifiv Science Research Group (IRIFIV-AISRG), Research foundation in Casablanca, Maintaining consistent and reliably high success rates is a monthly challenge for in IVF labs, the IRIFIV Fertility Center in Casablanca – Morocco Department of Reproductive Medicine and Reproductive Biology and Embryology, advocacy of interdisciplinary Department of Reproductive Medicine and Reproductive Biology and Embryology study, encompassing the areas of research, collections and publishing Articles.
Citation: Al-Ibraheemi A, Zakaria M, Senhaji WR,Zarqaoui M, BouticheR, Ennaji M, et al. (2021) Serious Impact of Deficiency of Liquid Nitrogen in Cryogenic Containers and Quality Control for Preservation of Gametes and Embryos. J Fertil In vitro IVF WorldwReprod Med Genet Stem Cell Biol 9:234.
Received: 15-Feb-2021 Accepted: 09-Apr-2021 Published: 16-Apr-2021 , DOI: 10.35248/2375-4508.21.9.234
Copyright: © 2021 Al-Ibraheemi A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.