Research Article - (2023)Volume 7, Issue 2
As a significant economic insect, silkworms have prominent advantages in life science, including low breeding cost, large progeny size, short growth cycle, and clear genetic background. Importantly, silkworms can provide abundant raw materials to prepare biomaterials for application in various fields, such as biomedicine, cell culture, cosmetics, and functional food. Silkworms can be divided into wild-type or silk fibroin-deficient mutants according to whether they synthesize and secrete silk fibroin. Here, we presented an overview of our silkworm varieties resources, especially silk fibroin-deficient mutant silkworms. Next, we optimized various extraction methods of sericin and summarized the characteristics and advantages of sericin. Finally, we developed a series of sericin-based biomaterials for promising applications to a diverse set of needs.
Sericin; Fibroin-deficient silkworm; Hydrogel; Wound dressing
Resources of sericin and cultivation of silkworm varieties
Sericin, a natural protein secreted by silkworms [1], consists of 18 amino acids. Sericin has high yields (annual output of about 50,000 tons worldwide), benefiting from silkworm varieties' rich resources. There are thousands of silkworm varieties in the world, and our institute (Sericultural Research Institute, Chinese Academy of Agricultural Sciences) has more than a thousand species resources. Varieties of silkworms provide us with different properties of sericin. Silkworms can be divided into wild-type or silk fibroin-deficient mutants according to whether they synthesize and secrete silk fibroin. The wild-type silkworms secrete two major protein components, silk fibroin and sericin. Sericin glued together silk fibroin to form cocoons, of which silk fibroin accounts for about 70-75% [2]. Another variety, silk fibroin-deficient mutant silkworms can only secrete sericin but not silk fibroin due to the absence of posterior silk glands that synthesize silk fibroin [3]. Cocoons produced by these varieties are favored for separating and extracting sericin with excellent properties. However, this kind of mutant silkworm has low yields of sericin, poor resistance against disease, and breeding difficulty [4]. Therefore, our research lab focused on breeding new silk fibroin-deficient mutants to overcome the above defects. We obtained silk fibroin-deficient mutants with high yield, disease resistance, large cocoon, and small pupa by using silk fibroin-deficient mutant varieties, Nuclear Polyhedrosis Virus (NPV) resistance varieties, high-yield and high-quality practical varieties as parents through silkworm genetic breeding techniques (cross and backcross) for dozens of generations. The cocoon layer ratio of sericin produced by new mutant silkworms has been increased from about 1% to 5%. These superior varieties have laid a solid foundation for the exploitation and utilization of sericin. Figure 1 presented the silk glands, cocoons, and microstructures of silks from different species of wild-type silkworms and fibroin silkworms in our research group.
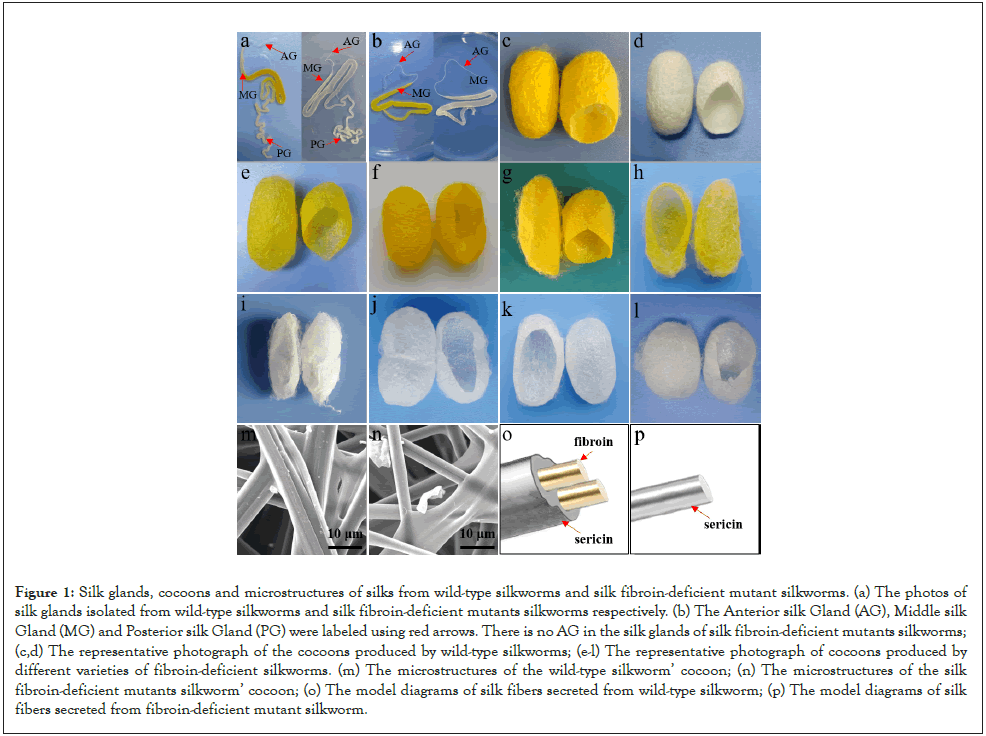
Figure 1: Silk glands, cocoons and microstructures of silks from wild-type silkworms and silk fibroin-deficient mutant silkworms. (a) The photos of silk glands isolated from wild-type silkworms and silk fibroin-deficient mutants silkworms respectively. (b) The Anterior silk Gland (AG), Middle silk Gland (MG) and Posterior silk Gland (PG) were labeled using red arrows. There is no AG in the silk glands of silk fibroin-deficient mutants silkworms; (c,d) The representative photograph of the cocoons produced by wild-type silkworms; (e-l) The representative photograph of cocoons produced by different varieties of fibroin-deficient silkworms. (m) The microstructures of the wild-type silkworm’ cocoon; (n) The microstructures of the silk fibroin-deficient mutants silkworm’ cocoon; (o) The model diagrams of silk fibers secreted from wild-type silkworm; (p) The model diagrams of silk fibers secreted from fibroin-deficient mutant silkworm.
Fluorescent materials with high biocompatibility and bioactivity are widely applied in tracing and labeling [5]. When they act as an additive, the fluorescence will interfere with some test items. For different practical applications, we selected silk fibroin-deficient mutant silkworms through genetic breeding technology, which can produce sericin with strong fluorescence or weak fluorescence at different wavelengths as shown in Figure 2.
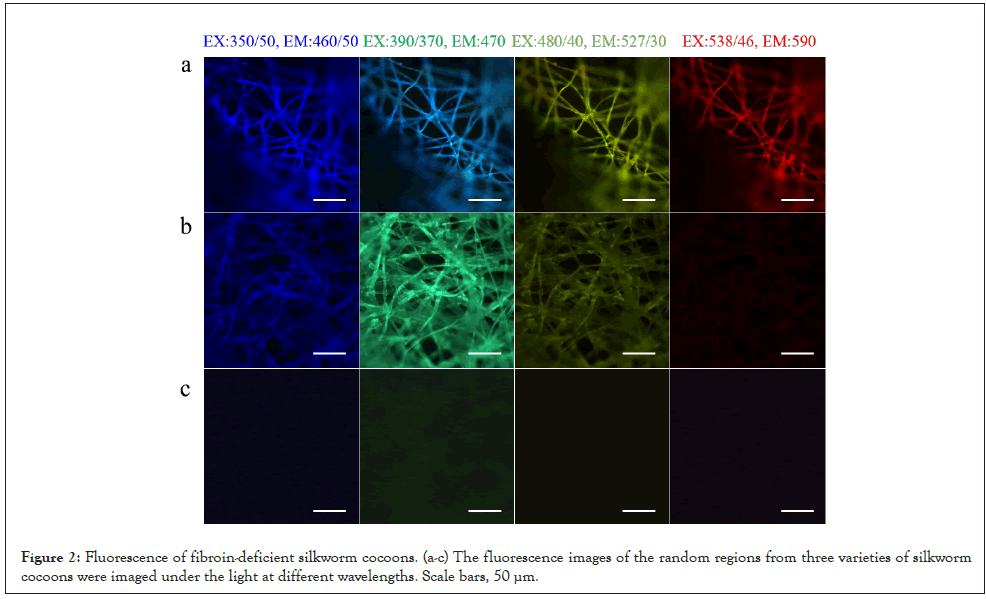
Figure 2: Fluorescence of fibroin-deficient silkworm cocoons. (a-c) The fluorescence images of the random regions from three varieties of silkworm cocoons were imaged under the light at different wavelengths. Scale bars, 50 μm.
Artificial feed rearing of silkworms is an important development direction of silkworm rearing [6]. This silkworm-rearing mode is conducive to the formation of uniform and stable silkworm cocoons through the standardized control of the environment. The uniformity and stability of material properties are very important for their application in biomedicine. For this reason, the anti-diseases silk fibroin-deficient mutant silkworms were employed to cross silkworms with high adaptability of artificial feed. We obtained a new silk fibroin-deficient mutant silkworm with adaptability to artificial feed through successive selection and backcrossing, which can produce the uniformity cocoons with high cocoon layer ratios as shown in Figure 3.
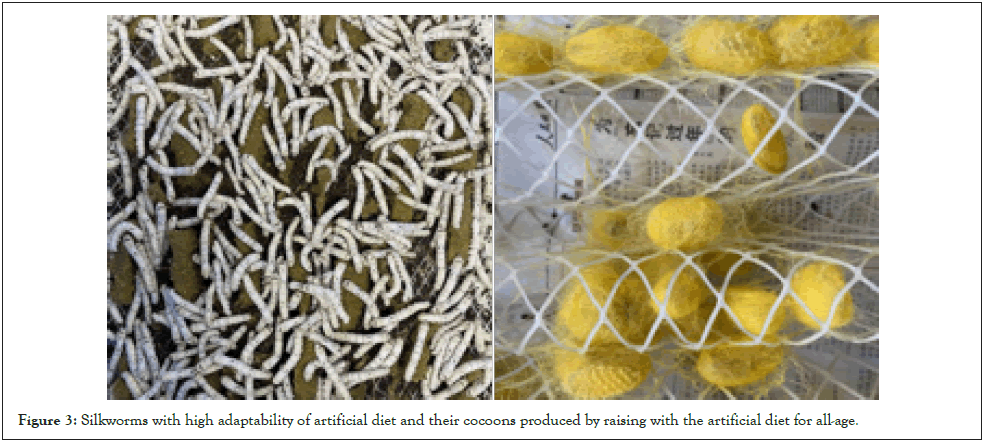
Figure 3: Silkworms with high adaptability of artificial diet and their cocoons produced by raising with the artificial diet for all-age.
The quality of silkworm cocoons depends on the rearing and mounting conditions. To obtain high-quality pure sericin cocoons, we use high-quality silkworm feed (mulberry leaves or artificial feed) to rear silkworms under standardized feeding conditions [7]. Considering that the temperature, humidity, and airflow (especially the humidity) in the mounting conditions can affect the properties of sericin, we optimized the mounting conditions and realized the standardization of the parameters to maintain the uniformity and stability of silkworm cocoons. In addition, we found that the silk secretion environment of silkworms influences the properties of cocoons during the production process of sericin raw materials, especially, environmental humidity significantly affected the secondary structure composition of sericin in silkworm cocoons. Based on our research, it is appropriate to control the environmental temperature of silkworm silk production at 23-28°C and humidity at 60-70%. Finally, the sick and injured individuals were first removed to prevent contamination of the cocoons in the cocoon-picking process.
Characteristics and advantages of sericin from fibroindeficient mutant silkworms
In general, extracting sericin from wild silkworm cocoons mainly adopts the methods of high-temperature and high-pressure, urea, sodium carbonate, and acid extraction [8]. However, these relatively rough methods will seriously damage the natural structure of sericin, leading to the degradation of sericin. In addition, it is difficult to completely remove the chemical agents introduced by the above methods, thus resulting in seriously restricting the practical applications of sericin [9].
Sericin extracted from silk fibroin-deficient mutant silkworm cocoons has many advantages. Firstly, sericin with a low degradation degree can be extracted from cocoons by a relatively mild method of LiBr [10]. Secondly, the solubility of sericin in fibroin-deficient mutant cocoons is much higher than that in wild-type cocoons in hot water. Sericin in mutant cocoons was dissolved completely at 121°C for 20 minutes, while wild-type cocoons could dissolve only a fraction. It is well known that sericin is comprised of various bioactive peptides [11]. Therefore, the ingredients of sericin isolated from fibroin-deficient cocoons are different from that of wild-type cocoons, even when using the same extraction method. Thirdly, the contents of the free amino group in sericin extracted from mutant cocoons are much higher than that from wild-type cocoons. For example, the content of free amino end groups in the sericin isolated from fibroin-deficient silkworm cocoons (180 Nd-s) was as high as 12 times that of wild-type silkworms cocoons.
Furthermore, based on the characteristics of sericin secreted by mutant silkworms, we established or optimized various extraction methods of sericin and obtained sericin for meeting different needs, which greatly expanded the application of sericin as shown in Figure 4. At present, dozens of organizations have used our sericin for basic research or product development.
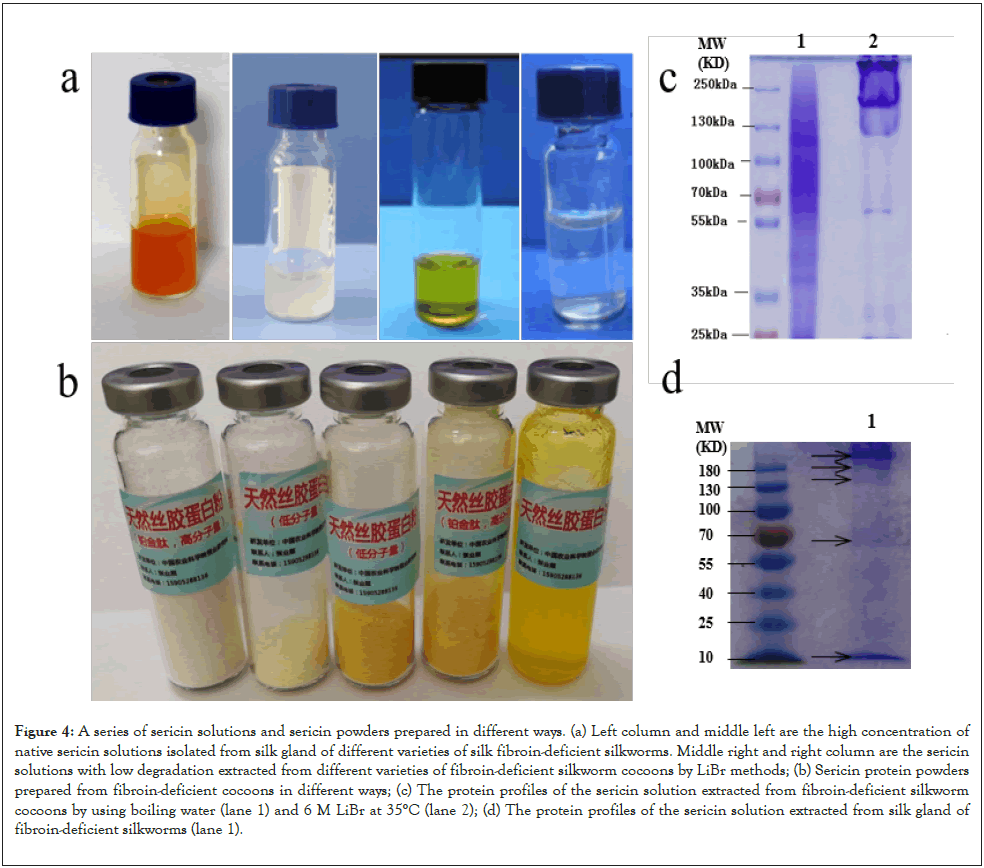
Figure 4: A series of sericin solutions and sericin powders prepared in different ways. (a) Left column and middle left are the high concentration of
native sericin solutions isolated from silk gland of different varieties of silk fibroin-deficient silkworms. Middle right and right column are the sericin
solutions with low degradation extracted from different varieties of fibroin-deficient silkworm cocoons by LiBr methods; (b) Sericin protein powders
prepared from fibroin-deficient cocoons in different ways; (c) The protein profiles of the sericin solution extracted from fibroin-deficient silkworm
cocoons by using boiling water (lane 1) and 6 M LiBr at 35°C (lane 2); (d) The protein profiles of the sericin solution extracted from silk gland of
fibroin-deficient silkworms (lane 1).
The extraction methods of sericin from mutant cocoons are as follows:
• The cocoons were crushed at a low temperature and then sieved to 100 mesh, which could accelerate the hydration of sericin, shorten the time of sericin dissolution, and further reduce the degradation of sericin.
• Low molecular weight sericin was obtained by the hightemperature and high-pressure method or enzymolysis method.
• The concentration of sericin reaches 16% (w/v) by extracting sericin from silk glands of silkworms directly at the mature stage in 5th instar.
Preparation and application of sericin-based biomaterials
Activity study of sericin: With the rapid development of biomedicine industry, the scale of cell culture is growing as well as the market demand for cell culture medium. Fetal Bovine Serum (FBS), as one of the main components of culture medium, has occupied tens of billions of dollars in the market [12,13]. However, there are many problems existed in the expansion and application of FBS, such as high prices, the risk of viral infection, animal ethics issues, and insufficient resources. Hence, developing inexpensive, safe, and effective FBS substitutes for cell culture has become an important research topic and hot issue in cell culture fields [14]. In this contribution, we provide FBS substitutes of sericin protein without toxic and harmful substances by extracting sericin from three silk fibroin-deficient mutant cocoons through high-temperature and high-pressure or enzymolysis methods. We then systematically evaluated the effects of sericin protein and its hydrolysate on culturing several cell lines, including cell adhesion, cell viability, cell growth, and proliferation. The results showed that sericin and its hydrolysate from different varieties of cocoons could greatly promote cell proliferation. Moreover, we compared the function of sericin and FBS on cell culture. The results of cell viability showed that sericin protein and its hydrolysate groups were superior to the control (10% FBS), and there was no significant difference in cell morphology between the two groups as shown in Figure 5.

Figure 5: The cell viabilities and morphologies of NIH3T3 cells cultured using high sugar DMEM medium containing 10% Fetal Bovine Serum (FBS)
or 0.2% (w/v) sericin isolated from cocoons of 3 silkworm varieties (1201, 1301, and 1442).
Note: (a) Cell viabilities; (b) Morphologies
Study on sericin protein in cosmetics filed: Sericin attracted great attention in the field of cosmetics due to its natural biological activities, including moisturizing, antibacterial, antioxidation, anti-inflammatory, inhibition of tyrosinase, and polyphenol oxidase activities [15,16]. Sericin contains various amino acids with hydrophilic side groups (about 80%), such as serine (30 to 33%), which has a super water absorption capacity. The sericin may also prevent the loss of water by forming a smooth and soft film on skin surface [17]. We have developed a range of skincare products by adding high-quality sericin powder or sericin solution to cosmetic formulations, including masks, toners, moisture lotions, face creams, and cleansers as shown in Figure 6.

Figure 6: A series of sericin-based cosmetics including masks, toners, moisture lotions, face creams, and cleansers were developed by our research group.
The natural wound dressing of sericin: Sericin fibers with nondestructive structures have excellent physicochemical properties and biological activities, while it is attractive and challenging to develop a bioactive wound dressing based on native sericin fiber [18]. We first designed and made a cubic base to provide a spinning platform for silkworms. Then we selected the silk fibroin-deficient mutant silkworms, adopted the directional genetic breeding technology by regulating the spinning behaviors of silkworms, and successfully bred new silkworm species for spinning a flat and uniform natural sericin fiber scaffold as shown in Figure 7. Excitingly, the obtained flat and uniform natural sericin fiber scaffold has original unique properties (such as natural structures and bioactivities) of natural sericin. Moreover, the sericin fiber scaffold has a porous fibrous network structure with high porosity, thus achieving excellent air permeability. Furthermore, the sericin fiber scaffold presents softness, high mechanical strength, and high water absorption capacity. More importantly, the sericin fiber scaffold has good biocompatibility, which can support cell growth, proliferation, and migration [19]. The above parameters of this sericin fiber scaffold fit well with the ideal wound dressing. When tested in a mouse model of full-thickness skin wounds, the sericin fiber scaffold effectively promoted wound healing.
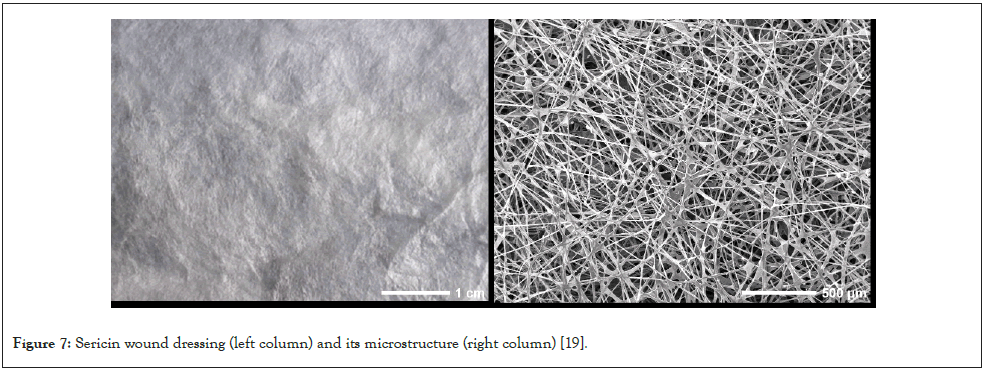
Figure 7: Sericin wound dressing (left column) and its microstructure (right column) [19].
Various sericin hydrogels prepared by sericin protein: Hydrogels are a class of Three-Dimensional (3D) polymer networks, which can adjust their physicochemical properties to meet specific needs under different conditions. As a type of promising materials, they have been widely applied in the biomedical fields ranging from biological mechanism studies to disease therapies and tissue regeneration [20,21]. As early as 2014, we prepared injectable and fluorescent sericin hydrogels by crosslinking glutaraldehyde with low-degradation sericin extracted with the LiBr method from silk fibroin-deficient mutant cocoons as shown in Figure 8a [10]. Considering the toxicity of crosslinkers and the poor transparency of the prepared sericin hydrogels, we extracted sericin from the white fibroindeficient mutant cocoons and used the ultrasonic method to prepare an injectable sericin hydrogel with high transparency, good elasticity, and high biocompatibility as shown in Figure 8b [22]. The difficulty in sterilization of silkworm cocoons and the extracted sericin protein solution’s failure to pass the filtration membrane (0.22 μm), while the existing sterilization methods of sericin hydrogels will cause adverse effects on the material. Therefore, we synthesized a sterile self-assembled sericin hydrogel by a simple two-step method as shown in Figure 8c [23]. In the first step, the silk fibroin-deficient silkworm cocoons were ground into powders to improve water infiltration, followed by adding water to powders and extracting sericin protein solution under high-temperature and high-pressure. A high extraction rate of sericin (up to 95%) and a high concentration of sterile sericin solution (up to 15 % w/v) were obtained directly under high-temperature and high-pressure conditions. In the second step, the collected sericin solution self-assembles to form hydrogel at room temperature. The gelation time of these kinds of sericin hydrogels can be controlled within a few minutes to hours by adjusting the concentration, pH, and temperature of sericin protein solution. Besides, these hydrogels without toxic crosslinkers formed by self-assembly in a sterile environment can be directly used as biomaterials for cell culture or tissue engineering. Moreover, sericin hydrogels have good biocompatibility, cell adhesion, mild forming conditions, and simple preparation processes. It is promising for development and application in biomedical fields.
In many cases, a key limitation to the application of hydrogels is their relatively poor mechanical strength [24]. For this reason, we tried to isolate non-degradable sericin with high concentration (16% w/v) from silk fibroin-deficient mutant silkworms and then prepared a strong sericin hydrogel with high elastic modulus (310 kPa) by crosslinking sericin with H2O2 [25]. This hydrogel has excellent elasticity, good cytocompatibility, and controlled drug delivery as shown in Figure 8d. In addition, we combined sericin with alginate or polyacrylamide to prepare two kinds of interpenetrating double-network hydrogels with excellent properties, namely sericin-alginate hydrogel as shown in Figure 8e [26] and sericin-polyacrylamide hydrogel as shown in Figure 8f [27].
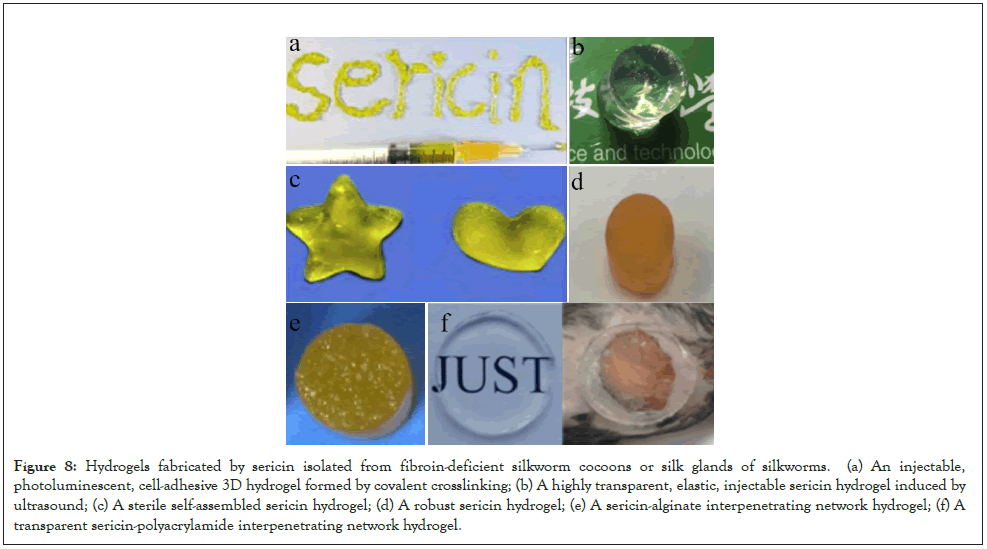
Figure 8: Hydrogels fabricated by sericin isolated from fibroin-deficient silkworm cocoons or silk glands of silkworms. (a) An injectable, photoluminescent, cell-adhesive 3D hydrogel formed by covalent crosslinking; (b) A highly transparent, elastic, injectable sericin hydrogel induced by ultrasound; (c) A sterile self-assembled sericin hydrogel; (d) A robust sericin hydrogel; (e) A sericin-alginate interpenetrating network hydrogel; (f) A transparent sericin-polyacrylamide interpenetrating network hydrogel.
The above sericin-based hydrogels have various properties, including a simple fabrication process, sterility, injectability, high mechanical strength, satisfactory bioactivities, etc., thus having a wide potential application in the biomedical field.
Sericin has excellent biological activity and abundant resources. Especially the presence of mutant varieties of silkworms, bringing great conveniences to our development and application of sericin. The optimization of sericin extraction process, the further study of sericin biological activity, and the exploitation of new sericin-based materials, sericin will expand its application in the fields of biomedicine, cell culture, cosmetics, and functional food. In summary, sericin has promising applications and commercial value in the future.
This work was supported by the Natural Science Foundation of Jiangsu Province of China (Grant No. BK20221291), China; the earmarked fund for CARS-18; Postgraduate Research and Practice Innovation Program of Jiangsu Province (Grant No. KYCX21_3511, KYCX22_3767), China.
The authors declare no competing financial interests.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Wei Y, Li Y, Zhang G, Zhang Y (2023) Sericin from Fibroin-Deficient Silkworms Served as a Promising Resource for Biomedicine. J Pharma Reports. 07: 175.
Received: 17-Apr-2023, Manuscript No. JPR-23-23576; Editor assigned: 20-Apr-2023, Pre QC No. JPR-23-23576 (PQ); Reviewed: 05-May-2023, QC No. JPR-23-23576; Revised: 12-May-2023, Manuscript No. JPR-23-23576 (R); Published: 19-May-2023 , DOI: 10.35248/JPR.23.7.175
Copyright: © 2023 Wei Y, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.