
Journal of Clinical and Cellular Immunology
Open Access
ISSN: 2155-9899

ISSN: 2155-9899
Research Article - (2021)Volume 12, Issue 7
Background: Wheezing is the dominant pathological characteristic of asthma. It is estimated that 50% of all children have experienced wheezing at least once in their first six years of life. In the present study, we aimed to investigate the initial wheezing attacks of children and to determine whether there was a connection between transient wheezing attacks and persistent wheezing.
Methods: We studied the immune responses of children who initially presented with wheezing attacks. A total of 231 children with wheezing attacks at the time of admission were enrolled in the study. The study population consisted of 68 children with wheezing episodes. Children with recurrent wheezing who were introduced to inhale steroids after 12 months were further grouped into either persistent wheezing (PW) or transient wheezing (TW) groups.
Results: At the initial onset, cytokine analysis of the children revealed a marked increase in serum soluble and transmembrane forms of interleukin receptor-2 (ST2) in PW. Contrastingly, there were no changes in the patients’ serum levels of IL-4, IL-13, and IL-33. The serum ST2 in PW was not only increased as compared to TW. However, a significant increase in ST2 expression in PW children with recurrent wheezing attacks was observed.
Conclusion: The present study demonstrated that ST2 might be a useful index for predicting the prognosis of infantile asthma. Hence, elucidation of the mechanism of ST2 expression in childhood allergic diseases is essential.
Wheezing; Infantile asthma; Interleukins; Allergy; Inflammation
BA: Bronchial Asthma; GWAS: Genome-Wide Association Study; hMPV: human Meta Pneumo Virus; ICS: Inhaled Corticosteroids; IgE: Immunoglobulin E; IL-1RL1: Interleukin-1 Receptor-Like 1; IL33: Interleukin-33; ILC2s: Group 2 Innate Lymphoid Cells; PW: Persistent Wheezing; RSV: Respiratory Syncytial Virus; TW: Transient Wheezing
Asthma is a common chronic inflammatory lung disease that recurrently exacerbates inflammation in the lower respiratory tract and narrows the airways of the lungs [1,2]. Chronic inflammation in the airways is a significant health burden that causes swelling, wheezing, shortness of breath, coughing, and chest tightness [1,2]. Asthma is mainly associated with several otolaryngologic diseases, such as allergic rhinitis, chronic rhinosinusitis, and obstructive sleep apnea [3]. As a result of the severe increase in the prevalence of asthma globally, it is estimated that almost 2.5 million patients die annually [3-5]. In particular, the incidence of asthma mainly involves children and teenagers in urban areas of middle-and low-income countries. However, the rise in asthma prevalence is now leveling off among children and adolescents in Australia and North-West Europe [5]. Inhaled corticosteroids are the most commonly recommended safe first-line therapy for persistent and mild asthma in both adults and children [6]. However, most of the time, asthmatic patients fail to receive maximum treatment benefits due to the unique skills required for drug inhalation [4].
Asthma is characterized by reversible airway obstruction and hyperreactivity of the airways due to various stimuli [1,2]. Wheezing is the dominant pathological characteristic of asthma. Asthma is characterized by bronchoconstriction, hypersecretion of mucus, and mucosal edema. These mechanisms result in the narrowing of the caliber of the airways [1,2]. It is estimated that 50% of all children have experienced wheezing at least once in their first six years of life [7]. In fact, wheezing is more frequent in infants and toddlers. Initial episodes of wheezing are associated with respiratory infections, such as viral infections, which may lead to transient wheezing. Moreover, it is more frequent in infants and toddlers who are asymptomatic between colds [8]. The remarkable cause of the late development of recurrent wheezing and asthma is bacterial colonization of the newborn airway [7]. Children who experienced transient wheezing in early childhood or who presented with persistent wheezing are classified as immunoglobulin E (IgE)- associated and/or atopic or nonatopic [7].
The development of wheezing and subsequent asthma is associated with interleukin-33 (IL33)-interleukin-1 receptor-like 1 (IL-1RL1) pathway polymorphisms. In particular, persistent wheezing is affected by IL-33 single-nucleotide polymorphism [9]. Of the members of the family of IL-1, IL-33, a unique cytokine, plays multiple roles in tissue homeostasis, prevention of parasitic infection, and induction of allergic inflammation [10-13]. In addition, IL-33 is passively released from the cell nuclear region during cell necrosis or cell damage to function as an immune system alarm during infection, physical stress, or trauma [10-13]. IL-33 instigates type-2 innate immunity via the activation of group 2 innate lymphoid cells (ILC2s) through its receptor, ST2, which causes allergic inflammation [10-13]. Moreover, IL- 33-ST2 receptor binding activates allergic inflammation-related eosinophils, basophils, mast cells, and macrophages [10-13]. The results of a genome-wide association study (GWAS) indicated that the polymorphisms of ST2 were strongly associated with allergic diseases and asthma [14]. The involvement of IL-33 and ST2 in infantile asthma in children has previously been examined by Savenije et al. [8] and Savenije et al. [14].
The underlying mechanism of IL-33-ST2-mediated allergic inflammation-linked wheezing remains unclear. Thus, we aimed to investigate the initial wheezing attacks in children, to determine whether there was a connection between transient or episodic wheezing attacks and persistent wheezing, to study the immune responses of children who presented with initial wheezing attacks, and to determine which factors were present in children who subsequently developed recurrent wheezing.
Participants
This study was approved by the local ethics committee of the Juntendo University (approval number 643-30.438) and was carried out in accordance with the principles embodied in the Declaration of Helsinki of 1965 (as revised in Brazil 2013). Written informed consent was obtained from all of the patients and/or their parents. A total of 231 children with a wheezing attack at the time of admission (male: female ratio: 1.13) were enrolled in the study from January 1, 2014, to December 31, 2018.
Study population
Children with one or more of the following conditions were excluded from this study: fever, atopic dermatitis, food allergy, premature birth or low birth weight, laryngomalacia, gastroesophageal reflux disease, tracheobronchomalacia, and vascular ring. Children who had not been previously diagnosed with bronchial asthma (BA) and whose expiratory wheezing was detected by a pediatrician during hospitalization were included in the study. The patients’ age, sex, virus identified on admission, and number of days hospitalized were recorded (Figure 1). The use of inhaled corticosteroids (ICS) was determined based on the diagnosis of infantile asthma in all participants who underwent a follow-up check-up within 12 months after discharge. Children with recurrent wheezing were further grouped into persistent wheezing (PW) or transient wheezing (TW) groups.
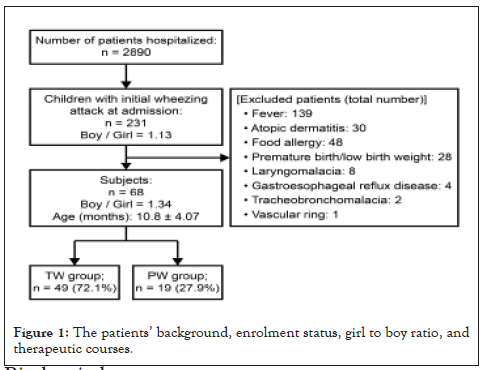
Figure 1:The patients’ background, enrolment status, girl to boy ratio, and therapeutic courses.
Biochemical measures
Peripheral venous blood samples from all children were obtained for biochemical measurements on the first day of their hospitalization. In the PW group, a 12-month follow-up check-up was conducted at the time of non-wheezing attacks during outpatient visits. Serum IgE and TARC (thymus and activation-regulated chemokine) were analyzed to determine atopic allergic inflammation-mediated wheezing. The serum levels of pro-inflammatory cytokines such as interleukins-4 (IL-4), IL-13, IL-33, IFN-γ, and ST2 were determined using ELISA (GenWay, Biotech Co. Ltd, San Diego, CA, USA).
Statistical analyses
Data were analyzed using Prism 8 (GraphPad Software Inc., La Jolla, CA, USA). Values for continuous variables were expressed as either mean ± standard deviation or median (interquartile range) based on the data’s normality of distribution. The student’s t-test was conducted to compare normally distributed variables. The Wilcoxon signed rank-sum test was used to examine the differences between continuous variables within the study groups. The Mann- Whitney U test was performed to analyze the relationship between the study groups. The statistical significance was set at p<0.05.
A total of 231 children who presented with a wheezing attack at the time of admission (Male: Female ratio: 1.13) between January 1, 2014 and December 31, 2018 were enrolled in the study. The study group consisted of 68 children (mean age: 10.8 ± 4.07 months; boy to girl ratio=1.34). The administration of ICS in the 12 months after discharge were done in 19 children with infantile asthma, in the persistent wheezing (PW) group (27.9%), and in the rest of transient wheezing (TW) group. The backgrounds of the PW and TW are shown inTable 1. The mean age of the PW group was 11.9 ± 3.79 months. The boy to female ratio was 1.38. Rapid antigen testing for respiratory syncytial virus (RSV), human metapneumovirus (hMPV), and Flu was performed on 40% of the patients upon admission, and these viruses were confirmed to be present in the patients (Figure 2).
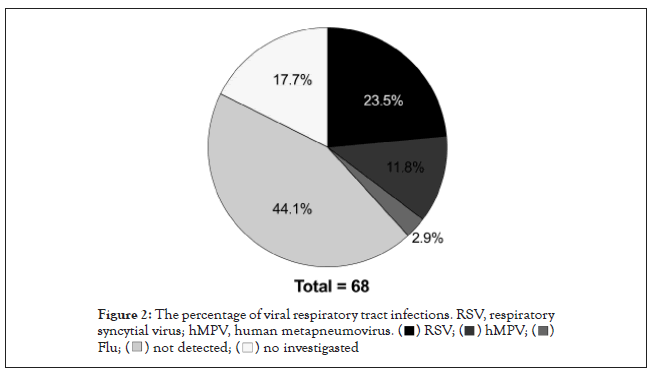
Figure 2:The percentage of viral respiratory tract infections. RSV, respiratory
syncytial virus; hMPV, human metapneumovirus.
| TW group | PW group | p value | |
|---|---|---|---|
| n=49 | n=19 | ||
| Age* | 13 (2-31) | 12 (4-23) | ns† |
| Sex (boy to girl ratio) | 1.33 | 1.38 | ns† |
| Virus identified on admission (n=56) | 43 | 13 | ns‡ |
| Hospitalization* (day) | 6 (3-11) | 5.5 (4-14) | ns† |
Note: *Median (interquartile ranges), †Mann-Whitney test, ‡Fisher's exact test. TW: Transient Wheezing; PW: Persistent Wheezing; ns: no significant.
Table 1: The backgrounds of patients.
The results of the therapeutic course analysis revealed that systemic steroid administration was done in 24 patients (35.3%). The average duration of hospitalization was 7.07 ± 1.70 days. The oxygen demand was 40 (58.8%). The mean administration duration was 2.35 ± 1.28 days. Among the 24 patients treated with steroids, 13 patients were in the PW group. There was no statistical difference between the PW and TW groups. The percentage of viral respiratory tract infections revealed that 24% had RSV; 13% hMPV; and 3% had influenza virus (Figure 2). There was no difference in the percentage of viral pathogens between the PW and TW groups.
Pro-inflammatory cytokine analysis in PW children with initial wheezing attack revealed that no statistically significant differences were observed in the serum levels of IL-4, IL-13, INF-γ, and IL-33 in the PW and TW groups. These pro-inflammatory cytokines during the initial wheezing attack (Figure 3) were concerning. In addition, IgE and TARC analyses showed no statistically significant difference between the groups. In contrast, children who presented with an initial wheezing attack also had elevated serum levels of ST2. Interestingly, children with recurrent wheezing also presented with elevated ST2 from the initial onset in the PW group compared with that of the TW group. Especially in PW children, ST2 levels increased even higher after 12 months (Figure 4). The Wilcoxon t-test analysis revealed a statistically substantial increase in ST2 expression in children with recurrent wheezing attacks.
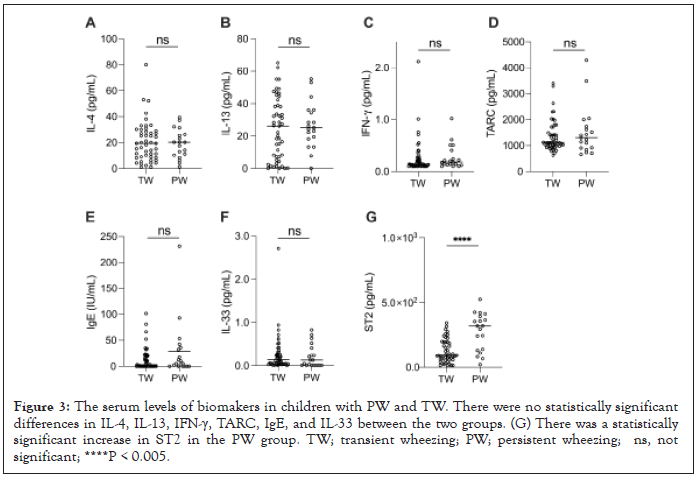
Figure 3:The serum levels of biomakers in children with PW and TW. There were no statistically significant differences in IL-4, IL-13, IFN-γ, TARC, IgE, and IL-33 between the two groups. (G) There was a statistically significant increase in ST2 in the PW group. TW; transient wheezing; PW; persistent wheezing; ns, not significant; ****P < 0.005.
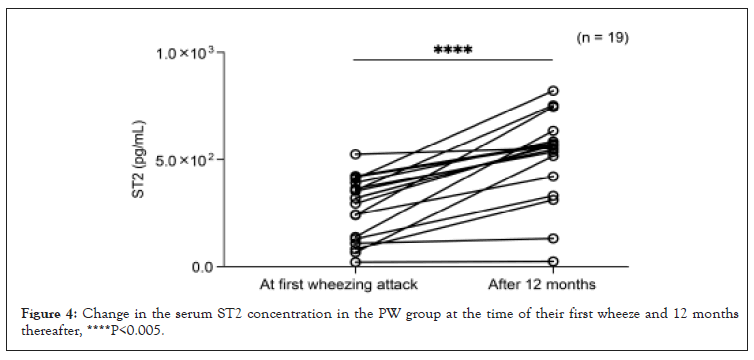
Figure 4:Change in the serum ST2 concentration in the PW group at the time of their first wheeze and 12 months thereafter, ****P<0.005.
In the present study, we evaluated the different proinflammatory cytokines and their receptors, which could help clinicians, predict the prognosis of infantile asthma. We investigated the proinflammatory cytokines (IL-4, IL-13, and IL33) and their association with the pathogenesis of childhood wheezing. ST2 is correlated with the development of recurrent wheezing [15]. Furthermore, it is implicated to play a role in the pathogenesis of inflammatory allergic diseases. Several studies have already demonstrated that serum ST2 levels are higher in BA patients [16,17]. In children, there have been several reports of elevated serum ST2 concentrations during acute exacerbations of BA [18]. It is interesting to note that in the initial wheezing attack without a diagnosis of BA, serum IL-33 was not elevated while serum ST2 concentrations were elevated in this study, and in the group that subsequently received a diagnosis of BA (i.e., PW group), ST2 concentrations were high from the infancy stage. Overall, our findings provide clinical evidence that examination of the expression of ST2 receptor levels is a more appropriate predictor of infantile asthma.
IL-4 induces the production of IgE antibody and differentiation of Th2 cells. The IL-13 receptor is consistently expressed in bronchial epithelial cells and smooth muscle cells [19]. A recent report provides evidence that the four-locus gene model, which consists of L13 rs20541, IL4 rs2243250, ADRB2 rs1042713, and FCER1B rs569108, is used to determine the risk of developing asthma and atopy in Chinese Han children [20]. Similarly, in African-American infants with a family history of atopy, IL-4 C-589T polymorphism was associated with a 10-fold increased risk of having wheezing without a cold [21]. Another clinical report indicated that a significant correlation between serum IL-13 levels in infants and the number of wheezing episodes existed. Moreover, an elevated serum IL-13 and a positive history of allergy have a functional significance in the recurrence of acute bronchiolitis [22]. Severe RSV disease is linked to an increased Th2 response resulting from the overexpression of IL-4, which subsequently leads to wheezing [23]. There was no change in IgE levels or the production of IL-4 and IL-13 in transient and recurrent wheezing. IL-4 produced from basophils activates NH cells in the lungs, and this interaction induces allergic diseases [24]. IL-4 and IL-13 are Th2-type inflammatory cytokines that increase as a response to bronchial inflammation. Cysteine protease, when acting as an antigen that presents with wheezing attacks, strongly induces allergic inflammation.
In line with the above findings, IL-4 and IL-13 might be the key cytokines that prolong the symptoms, but our results showed no statistical difference between the TW and PW groups. The wheezing attacks that can be observed in infants may not be explained by Th2-type immune responses or innate immune responses alone since they may also be related to cellular damage caused by substances called pathogen-associated molecular patterns (PAMPs), including allergens and viral infections.
We hereby discuss the immune responses associated with IL-33 and ST2. IL-33 is constitutively expressed in various cells, such as endothelial, epithelial, and mast cells, of barrier tissues, which contribute to the pathogenesis of inflammatory allergic diseases. Genetic variation of the IL-33 locus is strongly associated with an increased susceptibility to allergic sensitization in childhood and the development of wheezing phenotypes [25]. A recent clinical report indicated the interaction between IL-33 and CD4 (+) CD25 (+) Foxp3 (+) regulatory T cells is implicated in the pathogenesis of asthma in children [26]. Moreover, single nucleotide polymorphisms of the IL-33-IL1RL1 pathway are linked to intermediate-onset wheezing and asthma through sensitization in early childhood [9]. ST2 is a well-replicated asthma gene associated with eosinophilia. Another clinical study indicated that ST2 in wheezing preschool children has a predictive value for the development of eosinophilic airway inflammation in children with asthma [27]. Phylogenetic analysis showed co-circulation of hMPV, and the genotype ST2 was found.
Cysteine protease, an antigen that presents with wheezing attacks, strongly induces allergic inflammation. IL-33 is released from tracheal epithelial cells damaged by this enzyme, which in turn results in the induction of asthmatic symptoms. IL-33 and ST2 levels were elevated during wheezing attacks, but children who developed persistent wheezing did not present with differences in IL-33 at the initial admission. Nevertheless, ST2 was notably elevated. It is conceivable that higher expression of ST2 in inflammatory cells is associated with increased inflammation and frequency of wheezing due to IL-33 released during acute exacerbations. The finding of serum ST2 levels from infancy may be related to the phenomenon of increased susceptibility to repetitive wheezing, which may be due to epigenetic changes in ST2 expression genes and other factors that regulate these mechanisms.
In contrast to IL-33, a cytokine localized in the nucleus that enhances the immune response upon tissue damage, ST2 may be expressed due to atopic diathesis. Cases of infantile asthma that progress to persistent wheezing have a latent high expression of ST2 and may suffer from wheezing through IgE.
This study has limitations. Conducting ELISA to determine the serum IL-33 level was difficult. Moreover, it might have not necessarily measured “active" cytokines. Thus, we could not say that IL-33 had no involvement in PW and TW children. It was also controversial whether serum ST2 measured soluble ST2. In addition, it was unclear whether the ST2 measured in this study was associated with ST2 expression.
The function of ST2 has not been elucidated. ST2 may be important in allergic diseases such as persistent wheezing. The children who developed infantile asthma (PW group) in our study had an elevated serum ST2 when they presented with their wheezing attack for the first time compared with children with transient wheezing (TW group). Serum ST2 was further elevated in blood tests 12 months later. If soluble ST2 was detected, soluble ST2 bound to receptors and regulated their expression, which might have affected the prognosis of patients with infantile asthma.
We found that ST2 was elevated in persistent wheezing attacks. However, ST2 was also notably increased in the initial measurements of children who eventually developed recurrent wheezing. Although there was no difference in the level of IL-33 in the serum between the transient and prolonged wheezing groups, the phenomenon of the ST2 difference in its receptor might result in a potential predisposition to predict the future development of BA. Patients who developed recurrent wheezing from infantile asthma have a high latent expression of ST2, which might be involved in the onset of wheezing through IgE.
In considering the prognosis of infantile asthma, various factors should play a role in making therapeutic choices. Furthermore, biomarkers have a significant value in disease management. This study demonstrates that ST2 may be a useful index for predicting the prognosis of infantile asthma. Moreover, elucidation of the mechanism of ST2 expression in childhood allergic diseases is essential.
The authors would like to thank the members of the Juntendo Shizuoka Medical Research Center for Disaster for technical assistance with the experiments. Editorial support in the form of medical writing, assembling tables, creating high-resolution images based on the authors’ detailed directions, collating the authors’ comments, copyediting, fact checking, and referencing were provided by Editage, Cactus Communications. Finally, we would like to thank Ms. Yukiko Ichida for her secretarial assistance.
This work was supported by JSPS KAKENHI [grant number 19K17377] and a Grant-in-Aid for Special Research in Subsidies for the ordinary expenses of private schools from The Promotion and Mutual Aid Corporation for Private Schools of Japan. The funders had no role in the study design; collection, analysis, or interpretation of data; or writing the report.
The authors declare no conflicts of interest.
Conceived and designed research
Yosuke Baba
Performed experiments
Yosuke Baba, Hiromochi Yamada, Toshiyuki Yoneyama
Analyzed data
Yosuke Baba, Susumu Yamazaki, Kazuki Miyabayashi
Interpreted results of experiments
Yosuke Baba, Eisuke Inage
Prepared figures
Akina Matsuda, Yuri Takaoka, Kazuki Miyabayashi
Drafted manuscript
Yosuke Baba
Edited and revised manuscript
Yosuke Baba, Yoshikazu Ohtsuka
Approved final version of manuscript
All authors
Citation: Baba Y, Matsuda A, Takaoka Y, Miyabayashi K, Yamada H, Yoneyama T, et al. (2021) Role of IL-1RL1/ST2 in Infantile Asthma. J Clin Cell Immunol.12:634.
Received: 05-Nov-2021 Accepted: 19-Nov-2021 Published: 26-Nov-2021
Copyright: © 2021 Baba Y, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.