Journal of Psychology & Psychotherapy
Open Access
ISSN: 2161-0487
ISSN: 2161-0487
Research Article - (2023)Volume 13, Issue 2
Background: Functional retentive overflow incontinence (Retentive FI) is the most common cause of fecal soiling in children. Based on the clinical experiences, patients with retentive FI and comorbid psychiatric disorders, were accelerated in their treatment of fecal incontinence when they were being treated with risperidone for their psychiatric comorbidities, therefore this study was conducted to evaluate the effect of risperidone in the treatment of retentive FI in children and adolescents.
Method: In this double blind randomized clinical trial, 170 patients aged 4 years-16 years eligible for the study were randomly divided into two groups receiving Risperidone (n=70) and placebo (n=70). About half of these patients had newly diagnosed psychiatric disorders and were drug naïve, this was considered in their division. Participants received a daily dose of 0.25 mg-0.5 mg every 12 hours of Risperidone syrup (intervention group) or maltodextrin (placebo group) for 12 weeks. Sociodemographic data including age, gender, weight, height, BMI and BMI z-score (equivalent BMI-for-age percentile), and socioeconomic status were recorded. nocturnal fecal incontinence, diurnal fecal incontinence and painful defecations information was collected from subjects.
Results: 136 participants (69 on risperidone and 67 on placebo) completed the intervention. Mean age of participant in the intervention and placebo groups were 7.2 years ± 2.4 years and 8.0 y ± 3.1 y, respectively. The mean number of nocturnal fecal incontinence (Ptrend=0.39), diurnal fecal incontinence (Ptrend=0.48) and painful defecations for participants with and without psychiatric comorbidities were not significantly different between the groups (P=0.49, P=0.47, respectively). While, a significant interaction was observed between time and psychiatric comorbidities (P<0.001) for diurnal fecal incontinence after treatment with Risperidone
Conclusion: Based on our findings in this study, Risperidone, used commonly for psychiatric disorders in children and adolescents, may be useful in the treatment of retentive fecal incontinence in the presence of psychiatric comorbidities, and along with other interventions.
Retentive fecal incontinence; Risperidone; Pediatric; Encopresis; Atypical antipsychotics; Fecal soiling
According to DSM-5 (Diagnostic and statistical manual of mental disorders fifth edition), encopresis is defined as voluntary or involuntary passage of stool in inappropriate places such as underwear, in a child with a developmental age of four years or older, after rule out of organic causes [1]. There are two types of encopresis, with and without constipation and overflow incontinence [2]. Functional retentive overflow incontinence (Retentive FI), also known as encopresis with constipation and overflow incontinence, is the most common cause of fecal soiling in children. 1.5% of children 7 to 8 years old have this problem, and it is three times more common in boys [3]. Patients often recover significantly, 30%-50% in a year and about 50%-75% after five years [4]. This disorder can lead to feelings of embarrassment and guilt in patients and can make them victims of bullying. Studies have shown that in 30 to 50% of cases, there are psychiatric disorders, especially Attention Deficit Hyperactivity Disorder (ADHD), Oppositional Defiant Disorder (ODD), mood and anxiety disorders, and poor school performance in children with fecal incontinence [5-8]. The bio psychosocial perspective conceptualizes underlying causes and course of fecal incontinence, and therefore, a comprehensive approach to evaluation and treatment is required [9].
Clinical experience shows that treatment of underlying psychiatric disorders, along with treatment of fecal incontinence, helps to better advance the treatment plan. In the comprehensive approach to the treatment of fecal incontinence, psych pharmacotherapy is recommended in some texts, and imipramine, methylphenidate, and atomoxetine, have been found to be effective in treating non retentive FI [10-12].
In the clinical experience of the psychiatrist in charge of this article, children and adolescents with retentive FI and comorbid psychiatric disorders were accelerated in their treatment of encopresis when they were being treated with Risperidone for their psychiatric comorbidities, although various studies have suggested that atypical antipsychotic drugs may cause fecal incontinence [11-13]. Risperidone, an atypical antipsychotic, is commonly used to treat many psychiatric disorders in all age groups, and exhibits its action as a serotonergic and dopaminergic receptor antagonist [14]. Therefore, this randomized clinical trial was conducted to evaluate the effect of Risperidone in the treatment of retentive FI in children and adolescents.
Study design
In this double blind randomized clinical trial, pediatric patients referred to the gastrointestinal clinic of two children's hospitals in Tehran were considered over a period of six months. To determine the sample size based on studies on the effect of Risperidone on mood and behavior symptoms, and also the expert opinions in the field of pediatric gastroenterology and child and adolescent psychiatry, the effectiveness of conventional therapies in the treatment of retentive FI in the short term, and the effectiveness of Risperidone in the treatment of retentive FI comorbid with psychiatric disorders was estimated at 25% and 50%, respectively. Using the percentage of treatment of fecal incontinence in both groups, and with a significant level of 95%, with the type I of error probability level of 5% (α=0.05), the type II error probability level of is 20% (β=0.20, power=80%) and assuming 10% of the possible loss, the sample size in each group 70 people, and a total of 140 people were identified as follow:
Patients’ selection: Inclusion criteria were willingness to cooperate and signing the informed consent form after full knowledge of the objectives and method of the study, age 4 years-18 years, and diagnosis of Retentive Fecal Incontinence (Retentive FI) according to ROME-IV diagnostic criteria. Exclusion criteria were having any histories of cardiovascular, hepatic, renal and metabolic diseases, morbid obesity, use of medications for psychiatric disorders, pregnant and lactating adolescents, smoking (more than one cigarette in the last week or more than 200 cigarettes in a lifetime), have any previous allergies to Risperidone, and noncompliance with medication.
Psychiatric and gastrointestinal assessments were performed by a child and adolescent psychiatrist using K-SADS, and a pediatric gastroenterologist using ROME-IV, respectively. Sociodemographic data including age, gender, weight, height, BMI and BMI z-score (equivalent BMI-for-age percentile), and socioeconomic status were recorded.
Randomization: 136 children and adolescents aged 4 years-16 years who meet the inclusion criteria were randomly divided into two groups receiving Risperidone and placebo. About half of these patients had newly diagnosed psychiatric disorders (with mild to moderate intensity) and were drug naïve and this was considered in their division. Considering that BMI Z-score and psychiatric disorders can have a great impact on the results of the study, to ensure the uniform distribution of these variables in the groups, random allocation by stratified randomization and using the Permuted block randomization method with quadruple and binary blocks were used. Based on the sample size of 136 subjects, the quadruple block or double block were produced using the online site [15,16]. These codes were inserted on the packages by the company receiving the supplements and placebos. Upon each person entering the study, based on the sequence generated, the drug package in which the code is recorded was assigned to the parents. Also, the random sequence generated during the study was unpredictable. The admission rate of patients after the intervention period was calculated using the following formula, and patients whose admission rate is less than 80% were excluded from the study. Acceptance rate=number of packages received at the beginning of the study/number of packages consumed at the end of the study*100 (Figure 1) [17].
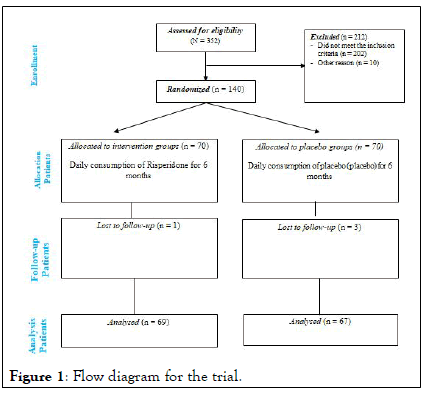
Figure 1: Flow diagram for the trial.
Interventions (pharmacological and nonpharmacological)
Participants received a daily dose of 0.25 mg-0.5 mg every 12 hours of Risperidone syrup (intervention group) or maltodextrin (placebo group) for 12 weeks. Due to the double blindness of the study, before starting the study, sets of packs containing Risperidone and placebo were prepared by someone other than the researchers, and the placebo syrup was similar in appearance to Risperidone syrup, therefore, none of the participants and researchers knew which of the two groups received Risperidone or placebo [18,19]. The drugs were given to the parents at the beginning of the study, and they were asked to bring empty packages of cans at the end of 3 months to check the acceptance of the intervention. In addition to polyethylene glycol, all the participants received family counseling and education for withholding behaviors and related behavioral interventions including regular toileting, use of diaries to track stooling, and reward systems for successful evacuations. Non pharmacological interventions for psychiatric comorbidities, including cognitive behavioral therapy, social skills training, Parent Management Training (PMT), and family therapy were performed by a child and adolescent mental health professional.
Statistical analysis
Quantitative variables were reported as mean (standard deviation) and qualitative variables were reported as numbers (percentage). To compare the mean of quantitative outcomes between the two groups, independent T-Test was used to compare the results between baseline and end of the intervention. Chi-square test or Fisher's exact test were used to compare qualitative factors between the two groups. The effect of Risperidone on quantitative variables was investigated by two-way repeated measure ANOVA and post adaptation covariance test for possible confounding factors. SPSS software version 16 was used to obtain statistical analyses, and significant levels for all tests were considered as P-value<0.05. Intention To Treat (ITT) analysis was performed as well. This study was approved by the research ethics committee of Shahid Beheshti university of medical sciences, Tehran, Iran (IR.SBMU.MSP.REC.1398.833). This prospective randomized controlled trial has been registered in Iranian registry of clinical trials at 19/06/2022 with IRCT number of IRCT20200203046352N1 [20].
136 participants (69 on Risperidone and 67 on placebo) completed the intervention. Demographic characteristics in each group are shown in Table 1. Mean age of participant in the intervention and placebo groups were 7.2 years ± 2.4 years and 8.0 years ± 3.1 years, respectively. No statistically significant differences were observed in weight, height, BMI Z-score and BMI (kg/m2) among the groups at baseline. While the number of girls between groups (girls in placebo=17 (36.2%) and intervention=30 (63.8%)) was different significantly. 34 subjects in Risperidone group and 35 in placebo group had psychiatric comorbidities and there was no statistically significant difference between the two groups regarding psychiatric comorbidity (P=0.43) [21].
| Variables | All subjects (n=136) | Risperidone (n=69) | Placebo (n=67) | P value a |
|---|---|---|---|---|
| Age (yrs.) | 7.6 ± 2.8 | 7.2 ± 2.4 | 8.0 ± 3.1 | 0.1 |
| Sex (girl) | 47 (34.6) | 17 (36.2) | 30 (63.8) | 0.02 |
| Weight (kg) | 26.9 ± 14.4 | 27.5 ± 16.0 | 26.1 ± 12.4 | 0.66 |
| Height (cm) | 121.1 ± 17.1 | 121.3 ± 16.8 | 120.7 ± 17.8 | 0.88 |
| BMI z-score | -0.11 ± 1.31 | 0.16 ± 1.30 | -0.26 ± 1.31 | 0.19 |
| BMI (kg/m2) | 16.3 ± 2.9 | 16.1 ± 2.5 | 16.1 ± 2.5 | 0.56 |
| SES b | ||||
| strong | 66 (48.5) | 32 (46.3) | 34 (50.7) | 0.78 |
| Moderate to weak | 70 (51.4) | 37 (53.6) | 33 (49.2) | 0.65 |
| Note: Data are mean ± SD or n (%). BMI: Body Mass Index; SES: Socioeconomic Status; A Calculated by ANOVA and chi-square tests for quantitative and qualitative variables; b Percent respectively | ||||
Table 1: Baseline characteristics of participants.
Table 2, shows gastrointestinal manifestations of participants at baseline. Values for constipation, fissures, hard stool, and compact feces were not significantly different among groups (P>0.05) [22]. While the percentage of hemorrhoids in patients with psychiatric disorders was significantly different between groups (P=0.025).
| Variable | Placebo (n=67) | Risperidone (n=69) | P value d |
|---|---|---|---|
| Constipation | |||
| With psychiatric disorders | 22 (46.8) | 25 (53.2) | 0.36 |
| Without psychiatric disorders | 21 (48.8) | 22 (51.2) | 0.31 |
| Hemorrhoids | |||
| With psychiatric disorders | 5 (100) | 0 (0) | 0.025 |
| Without psychiatric disorders | 3 (75.0) | 1 (25.0) | 0.34 |
| Fissures | |||
| With psychiatric disorders | 8 (53.3) | 7 (46.7) | 0.47 |
| Without psychiatric disorders | 4 (36.4) | 7 (63.6) | 0.2 |
| Hard stool | |||
| With psychiatric disorders | 22 (48.9) | 23 (51.1) | 0.56 |
| Without psychiatric disorders | 19 (46.3) | 22 (53.7) | 0.16 |
| Compact feces | |||
| With psychiatric disorders | 21 (48.8) | 22 (51.2) | 0.56 |
| Without psychiatric disorders | 20 (50.0) | 20 (50.0) | 0.46 |
| Note: Data are n (%). fP values have been calculated by chi-square tests | |||
Table 2: Gastrointestinal manifestations of participants at baseline.
Values for the mean number of nocturnal fecal incontinence (Ptrend=0.39), diurnal fecal incontinence (Ptrend=0.48) and painful defecations for participants with and without psychiatric comorbidities were not significantly different between the groups (P=0.49, P=0.47, respectively). When comparing changes in the number of diurnal and nocturnal fecal incontinence in terms of the presence of psychiatric comorbidities, a significant interaction was observed between time and psychiatric comorbidities (P<0.001) for diurnal fecal incontinence (Table 3).
| Variable | 1 Month | 2 Months | 3 Months | 4 Months | 5 Months | 6 Months | P value | P value | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Placebo (n=67) | Risperidone (n=69) | Placebo (n=67) | Risperidone (n=69) | Placebo (n=67) | Risperidone (n=69) | Placebo (n=67) | Risperidone (n=69) | Placebo (n=67) | Risperidone (n=69) | Placebo (n=67) | Risperidone (n=69) | ||||||
| Number of nocturnal fecal incontinence1 | |||||||||||||||||
| With a psychiatric comorbidity | 0.41 ± 1.50 | 0.32 ± 0.47 | 0.09 ± 0.29 | 0.24 ± 0.52 | 0.05 ± 0.21 | 0.08 ± 0.27 | 0.0 ± 0.0 | 0.04 ± 0.20 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.39a | 0.44b | |||
| Without a psychiatric comorbidity | 0.0 ± 0.0 | 0.33 ± 0.73 | 0.0 ± 0.0 | 0.24± 0.70 | 0.0± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.05 ± 0.21 | 0.0 ± 0.0 | 0.10 ± 0.30 | 0.0 ± 0.0 | 0.0 ± 0.0 | |||||
| Number of diurnal fecal incontinence1 | |||||||||||||||||
| With a psychiatric comorbidity | 8.24 ± 3.53 | 7.0 ± 2.34 | 5.42 ± 3.82 | 3.75± 3.33 | 1.80 ± 1.43 | 1.50 ± 1.88 | 1.19 ± 1.50 | 0.91 ± 1.44 | 0.16 ± 1.16 | 0.29 ± 0.85 | 0.52 ± 1.36 | 0.41 ± 1.01 | 0.48a | <0.001b | |||
| Without a psychiatric comorbidity | 2.31 ± 1.93 | 5.55 ± 3.59 | 3.23 ± 2.80 | 2.31 ± 2.51 | 2.0± 2.19 | 1.13 ± 1.78 | 1.30 ± 1.70 | 0.59 ± 1.09 | 1.07 ± 1.32 | 0.54 ± 1.18 | 0.38 ± 0.76 | 0.09 ± 0.29 | |||||
| Painful defecations2 | |||||||||||||||||
| With a psychiatric comorbidity | 20 (45.5) | 24 (54.5) | 10 (38.5) | 16(61.5) | 4 (44.4) | 5 (55.6) | 0 (0.0) | 0 (0.0) | 1 (50) | 1 (50) | 1 (100) | 0 (0.0) | 0.49 c | ||||
| Without a psychiatric comorbidity | 16 (42.1) | 22 (57.9) | 22 (55.8) | 19(44.2) | 3 (42.9) | 4 (57.1) | 0 (0.0) | 1 (100) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (100) | 0.47 c | ||||
| Note: aP values calculated by Repeated measures for time*group and adjusted for sex; socioeconomic status and significant values of Table 2; bP values calculated by Repeated P2 Data are n (%); 1Data are mean ± SD values calculated by chi-square tests measures for time*psychiatric comorbidities and adjusted for sex, socioeconomic status and significant values of Table 2; cP values calculated by chi-square tests; 1Data are mean ± SD; 2Data are n (%) | |||||||||||||||||
Table 3: Mean values of diurnal and nocturnal fecal incontinence and percentage of painful defecations in participants at baseline and after 6 months.
In pediatric patients with fecal incontinence, psychiatric comorbidities especially ADHD and ODD have been reported more frequently, and some studies showed that comorbid internalizing and externalizing psychiatric disorders are a predictor of poor outcome in children with fecal incontinence. Based on the clinical experiences, in addition to educational, psychological and behavioral interventions for patients with fecal incontinence and comorbid psychiatric disorders, psychopharmacologic interventions have sometimes been effective in this multidisciplinary treatment program. Atypical antipsychotics are prescribed for various psychiatric disorders such as psychotic, mood, anxiety, tic, obsessive-compulsive, and disruptive behavior disorders. The reason for using Risperidone as one of the atypical antipsychotics in our study was efficacy in symptom reduction in many child and adolescent psychiatric disorders. To our knowledge, this is the first double blind randomized clinical trial performed with Risperidone for the treatment of retentive fecal incontinence in a clinical setting. According to the results of our study, in the presence of psychiatric comorbidities, Risperidone added to other traditional interventions significantly reduced the frequency of fecal incontinence during the day (P<0.001). With gradual discontinuation of Risperidone at the end of the third month of study, fecal soiling gradually increased during the next three months of follow-up. Risperidone has high affinity for dopamine D2 and serotonin 5-HT2A, adrenergic α1- and α2-and histaminergic H1 receptors, and moderate affinity for serotonin 5- HT1C, 5-HT1D, and 5-HT2A receptors. It is not clear whether the mechanism of action of Risperidone on retentive FI is central or peripheral. Dopamine inhibits colonic movements and prolongs gastrointestinal transit time and the efficacy of risperidone as a dopamine antagonist in retentive FI may be due to this fact. Also, risperidone has possible analgesic effect.
In reviewing the literature, a relationship between atypical antipsychotics and fecal incontinence has been mentioned. In a systematic review in 2021, Arasteh, et al., pointed out those atypical antipsychotic drugs can cause fecal incontinence, which may be due to α1-adrenergic blockade, sedative effects, and blockage of the pudendal reflexes. Among atypical antipsychotic medications, Risperidone has less sedation. In our study the most common adverse effects observed with Risperidone were overweight and mild sedation. There are studies in support of Imipramine, Methylphenidate, and Atomoxetine, which suggest that these medications may be helpful in treating fecal incontinence. Imipramine is a Tricyclic Antidepressant and has an anticholinergic effect similar to Loperamide by reducing gastrointestinal motility and increasing sphincter tone. In some texts it is mentioned that Imipramine can be useful in treating non-retentive FI, but due to cardiovascular complications, Tricyclic Antidepressants should not be prescribed as usual. In a 2013 case report, Yılmaz, et al., noted that Methylphenidate leads to recovery from fecal incontinence and in a 2012 study, Huang and Chien showed that inhibition of gastric emptying and intestinal transit induced by amphetamine is due to an effect on the dopaminergic system (via D1 and D2 receptors) and to some extent adrenergic receptors. Golubchik, et al., in an article in 2013 pointed out that direct impact of methylphenidate, imipramine, and atomoxetine, on self-organizing skills, impulse control, and executive functioning, may enable children to recognize and respond to internal cues for defecation, helping them in the treatment process.
The effects of Atomoxetine in the treatment of fecal incontinence have been reported in the literature, however, constipation is a side effect of Atomoxetine, such as risperidone, and administration of Atomoxetine to patients with ADHD and comorbid fecal incontinence can improve or worsen fecal incontinence.
These findings may shed light on the effects of the central nervous system medications on the gastrointestinal tract, and given the high comorbidity of psychiatric disorders with fecal incontinence, it may be necessary to develop a comprehensive treatment plan, considering the benefits of psych pharmacotherapy.
Due to the high prevalence of psychiatric comorbidities in retentive FI, a comprehensive psychiatric evaluation is recommended, especially when there is a suspicion of mood and behavior disorders and attention problems. Based on our findings in this study, Risperidone, used commonly for psychiatric disorders, may be useful in the treatment of fecal incontinence in the presence of psychiatric comorbidities, and along with other interventions. Future studies should be conducted multicenter with large sample size to provide evidence based results.
The authors gratefully acknowledge the pediatric gastroenterology, hepatology, and nutrition research center, research institute for children's health, shahid beheshti university of medical sciences.
G.Z,A.H., and S.F contributed in conception, design, and statistical analysis. G.Z., A.H., S.F., L.T., K.P contributed in data collection and manuscript drafting. G.Z and A.H., supervised the study. All authors approved the final version of the manuscript.
No funding
Data is available upon request from the corresponding author for the article due to privacy/ethical restrictions.
This study was approved by the research council and ethics committee Shahid Beheshti university of medical sciences. We confirm that all methods were carried out in accordance with relevant guidelines and regulations. Also, we confirming that informed consent were obtained from a parent and/or legal guardians of the participants (control as well as patients).
Not applicable.
We, the authors, declare that we had no competing interests.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Zahed G, Fatahi S, Tabatabaee L, Prabahar K, Hosseini A (2023) Risperidone for the Treatment of Retentive Fecal Incontinence in Children and Adolescents: A Randomize Clinical Trial. J Psychol Psychother. 13:440.
Received: 24-Aug-2022, Manuscript No. JPPT-22-18989; Editor assigned: 26-Aug-2022, Pre QC No. JPPT-22-18989 (PQ); Reviewed: 09-Sep-2022, QC No. JPPT-22-18989; Revised: 13-Jan-2023, Manuscript No. JPPT-22-18989 (R); Published: 20-Jan-2023 , DOI: 10.35248/2161-0487.23.13.447
Copyright: © 2023 Zahed G, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.