Journal of Women's Health Care
Open Access
ISSN: 2167-0420
ISSN: 2167-0420
Research Paper - (2019)Volume 8, Issue 5
Background: Lower extremity peripheral artery disease (PAD) affects at least 8.5 million people nationwide, causing 143,000+ hospitalizations and 40,000 amputations annually. Women with PAD have a higher chance of being asymptomatic or present with atypical symptoms. Fewer than half of affected women know they have PAD, and studies illustrate an expected increase in under-diagnoses in these women. Women with PAD are two to three times more likely to experience stroke or myocardial infarction (MI). This is the first report on the clinical feasibility of using a specialized volume plethysmography system (VPS) as operated by a medical aide in the primary care setting to perform PAD testing on female patients.
Methods: In 2018, consecutive female patients in primary care settings were evaluated for the presence or absence and severity of PAD. Beforehand, the patients completed a self-administered questionnaire to identify gender, age, PAD symptoms, and atypical cardiovascular factors. Medical aides performed the test as part of routine clinical practice and presented results to primary care physicians who made the diagnosis based upon test findings.
Results: Of the 68,402 patients who met the American Heart Association/American College of Cardiology criteria and were tested in the primary care setting, 26,576 or 38.9% had moderate to severe PAD. These patients were frequently asymptomatic, hypertensive, hyperlipidemic, diabetic, and/or had a history of tobacco smoking.
Conclusion: VPS is an accurate, reproducible, cost-effective, and clinically feasible in-office or home visit test allowing for detection of PAD in women earlier in the disease process.
Peripheral artery disease; Cardiovascular risk factor; Advanced age; Cigarette smoking; Diabetic; Hyperlipidemic; Hypertensive; Lower extremity; Amputation
Ankle-brachial Index (ABI); American College of Cardiology (ACC); American Heart Association (AHA); Critical Limb Ischemia (CLI); Peripheral Artery Disease (PAD); Primary Care Physician (PCP); Volume Plethysmography System (VPS); Myocardial Infarction (MI)
Lower extremity peripheral artery disease (PAD) affects at least 8.5 million people nationwide and more than 200 million worldwide [1,2]. It is responsible for causing over 143,000 hospitalizations and 40,000 amputations annually [3]. Despite its high economic burden of $290 billion each year [4,5] to the United States health care system along with its high prevalence and associated morbidity, mortality, and costs, PAD remains frequently under-diagnosed [6].
The female patient with symptomatic PAD may suffer from painful cramping or heaviness during exercise, cold feet, sores on the toes, feet, legs that do not heal, gangrenous or dead tissue [7]. Women are more likely to be asymptomatic or present with atypical symptoms. Fewer than half affected actually know they have PAD, and recent studies illustrate an expected increase in under-diagnoses in these women. Inattention to the diagnosis of PAD is of particular concern, as women with PAD are two to three times more likely to have a stroke or MI than those without the disease [5,6].
Although they are a population at risk for cardiovascular disease, women have been under-represented in several PAD revascularization trials [2,4,6]. Women were more than 1.5 times as likely as men to require re-intervention after endovascular therapy for PAD which may indicate a bias to neglect the needs of earlier diagnosis and medical care for the female population. Moreover, older women with PAD who present with critical limb ischemia (CLI), are less likely to undergo surgery, but are more likely to undergo amputations [2,3].
The risk factors for development of PAD include diabetes mellitus, cigarette smoking, obesity, chronic kidney disease, advanced age, hyperlipidemia, and hypertension [6]. Recent studies identified additional comorbidities prevalent for women, such as depression and inflammation [2].
This is the first report analyzing the routine use of volume plethysmography in a female population to do PAD testing. VPS is a blood form waveform visualization and evaluation tool that can identify obstructions in the lower extremities including the anterior tibial and posterior tibial arterial distributions [4,5,8]. VPS is used in primary care clinics to identify patients at risk for cardiovascular events and aids in the earlier diagnosis of PAD [5,8,9].
As part of this study, routine office staff performed a VPS in less than five minutes [5,8] using a digital transducer placed sequentially on the extremities, without the difficulties inherent to the blood pressure cuff, ankle-brachial index (ABI) Doppler systems, which are time-consuming, use bulky equipment, and require experienced technical staff [5]. This analysis reports on the clinical feasibility of using an easily deployable VPS as operated in the primary care setting to perform PAD testing on female patients who met the AHA/ACC criteria [10], which identify whom should be tested [6]. When in the hands of generalists and routine staff, the specialized VPS is intended to provide relative ease and speed for the detection of flow obstruction [4,8,9].
From January 2018 through December 2018, a cohort of 68,402 consecutive female patients meeting AHA/ACC criteria for PAD testing were evaluated for the presence or absence and severity of PAD at primary care practices located in the United States.
All patients completed a self-administered questionnaire [5,9] to identify PAD symptoms, age, and cardiovascular risk factors. A medical aide conducted each evaluation without the need for a technologist or physician present [4,8]. The test was performed bilaterally on both upper and lower extremities using the FDAcleared VPS [8,9].
The test results were interpreted in terms of the presence and degree of flow obstruction, with flow obstructions of 0.60-0.89 rated as moderate, 0.40-0.59 rated as significant, and 0.00-0.39 rated as severe. The PCP made the diagnosis based on the test results and clinical judgment.
All data were de-identified and stored on a database, and no private information was accessed. As such, this retrospective analysis did not require an approval of an Institutional Review Board and no application for approval was submitted.
The medical aides who performed the tests included the clinical factors on the test forms from the patient questionnaires completed in the examination room. There was no protocol in the study to collect such data in a manner suitable to enable statistical analysis, such as investigating for confounding factors. There was also no data collected on patients who failed to meet the ACC/AHA criteria for testing [10], including how many were in this category who did not meet the criteria.
The patient mean age in the cohort (n=68,402) was 71.6 years (11.5 standard deviation), and all were women. Of the population studied, only 4,206 patients or 6.1 % of patients presented with claudication symptoms.
Medical aides successfully tested all patients who met the AHA/ ACC criteria and all patients completed testing. Of the 68,402 patients, there were 26,576 or 38.9% who were identified as having moderate to severe PAD (Figure 1).
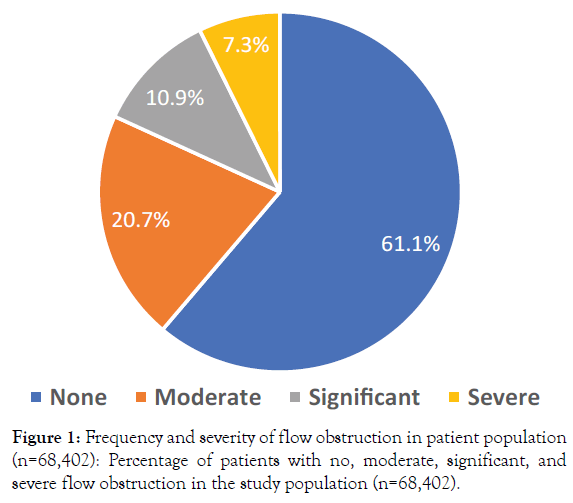
Figure 1. Frequency and severity of flow obstruction in patient population (n=68,402): Percentage of patients with no, moderate, significant, and severe flow obstruction in the study population (n=68,402).
In the study group, 35,974 patients or 52.6% of patients were hypertensive, 24,171 or 35.3% were hyperlipidemic, 19,305 or 28.2% were diabetic, and 16.1% had a history of smoking. The frequency of the risk factors that were reported for these patients is demonstrated in Table 1. It is important to note that these conditions were captured through user entry and not claims or medical record history. There are patients who may not have all conditions documented, or conversely, conditions not related to their specific health status.
| Type of Comorbidity | Number of Patients | Percent of Total |
|---|---|---|
| Hypertension | 35,974 | 52.6 |
| Hyperlipidemia | 24,171 | 35.3 |
| Diabetes | 19,305 | 28.2 |
| Smoking history | 11,046 | 16.1 |
| Claudication | 4,206 | 6.1 |
| CAD | 2,116 | 3.1 |
| Stroke+TIA | 1,884 | 2.8 |
| Non-healing wound | 758 | 1.1 |
Table 1: Frequency of Risk Factors and Comorbidities (n=68,402 patients): List of risk factors and comorbidities identified in the study cohort, with number and percentage of patients with each comorbidity. CAD=history of coronary artery disease; TIA=history of transient ischemic attack.
In addition, there were a total of 18,229 patients who had no comorbidities documented, 45,433 patients who had one to three comorbidities, and 4,730 patients who had more than three comorbidities. The frequency of flow obstruction was between 38 to 39% in the no comorbidities and one to three comorbidities groups. In the greater than three comorbidities group, the frequency of flow obstruction was 43%.
While the women over age 90 had the fewest participants, their PAD results were the highest at 64.8%. The 64 and under age group had the least at 27.2%. The age groups tested had greater PAD incidents based upon their increasing ages.
An active, 66-year-old female presented with back pain and atypical leg pains associated with walking, which were more pronounced on the right than the left side. Patient risk factors included controlled hypertension, mild hyperlipidemia, and a remote cigarette smoking history.
Without a significant past medical history, she was referred for evaluation to spine, orthopedic, and neurology specialists. Her extensive workups included MRIs and EMGs with no definitive etiology identified. She was being considered for lumbar epidural injection when she was referred for a vascular consult.
Physical examination revealed a healthy female with normal vital signs. There were no carotid bruits. Cardiac exam was regular. Her lower extremities were warm and well perfused with good capillary refill. The pedal pulses on the right were slightly less than on the left.
VPS with quantitative evaluation was performed on all four limbs. As viewed in Figure 2, the right lower extremity had severe flow obstruction and the left lower extremity had mild to moderate flow obstruction.
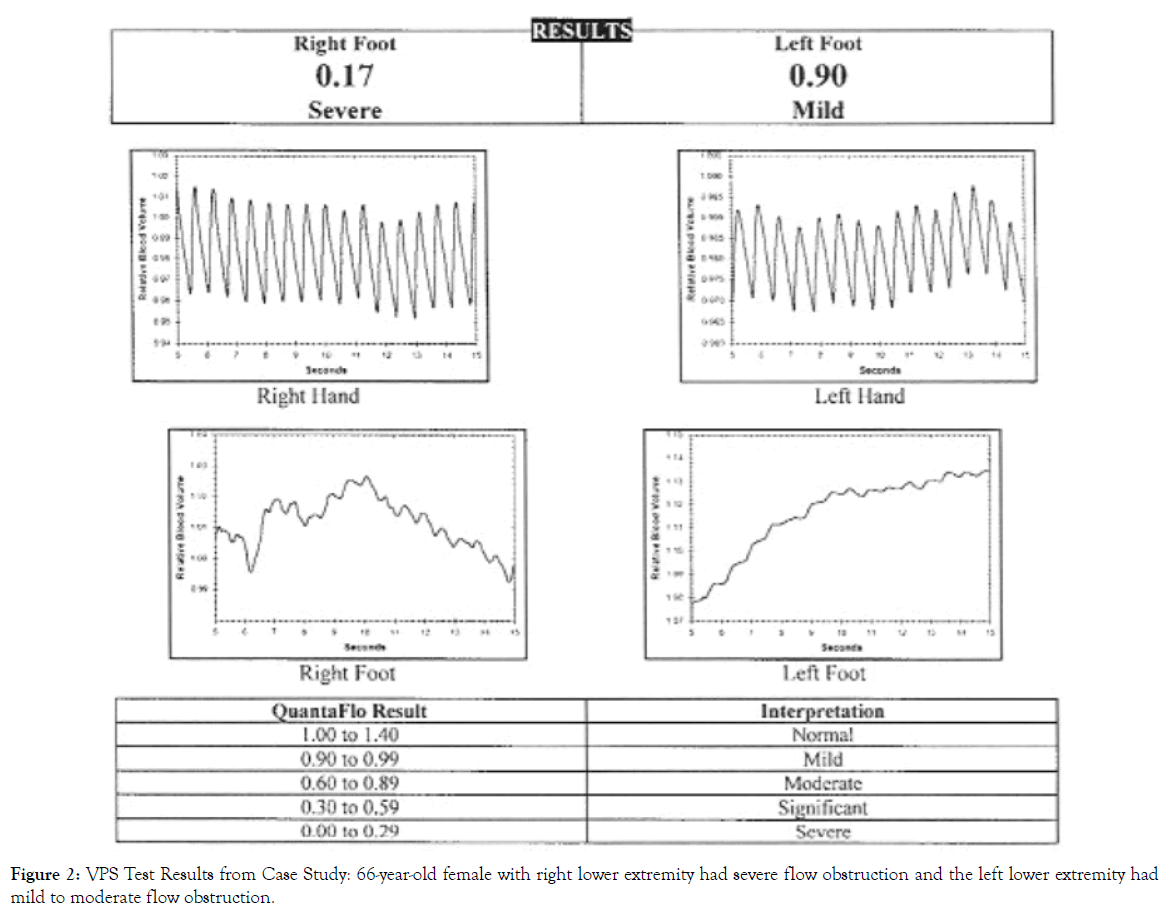
Figure 2. VPS Test Results from Case Study: 66-year-old female with right lower extremity had severe flow obstruction and the left lower extremity had mild to moderate flow obstruction.
Duplex ultrasound confirmed extensive calcific aortic disease extending into both common iliac arteries, more significant on the right than the left. There were no areas of hemodynamically significant stenosis identified in the femoral, superficial femoral, popliteal or tibial arteries bilaterally. The patient is undergoing cardiac evaluation, in anticipation of a percutaneous peripheral vascular intervention.
This was the first report on the clinical feasibility of using a VPS operated by a medical aide in the primary care setting to perform PAD testing on all female patients meeting the AHA/ACC criteria. The data showed that testing eligible patients for PAD in the primary care setting is possible and efficiently performed by routine staff [5,8].
The data demonstrated that women in the cohort had high rates of cardiovascular risk factors and comorbidities, however, the frequency of claudication was less than 10%. Of the patients tested, over one-third had flow obstructions.
Since claudication only occurs in a small portion of PAD patients, relying on a history of claudication is likely to miss many new PAD diagnoses [9]. In addition, a PAD diagnosis on the absence of a pedal pulse misses diagnoses of PAD in approximately two-thirds of cases [11]. These results suggest that VPS has critical advantages over other common methods of diagnosing PAD.
It is clinically imperative to detect PAD early to prevent vascular complications. For patients with PAD, lack of treatment is associated with increased mortality. In a 2011 five-year study involving 7,500 participants, there was a 16% mortality rate versus 4% in non-PAD subjects [12]. Among the PAD participants, the use of multiple preventive therapies was associated with a 65% lower all-cause mortality (p=0.02) [12].
Early PAD screening, diagnosis, and intervention can prevent amputations as well as cardiovascular events, particularly in the diabetic patient. In a randomized study of 2,001 participants with diabetes using screening, 128 of approximately 1,001 patients in the index group were found to be at high risk and admitted to a foot protection program [11]. At two-year follow-up, only one of 12 patients had a major amputation, while there were 12 major amputations among the approximately 1,000 patients in the control group who were not offered a foot protection program [13]. The findings of one major amputation in the index group versus 12 major amputations in the control group was significant (p<0.01) [13]. The implication for older women with PAD who are known to be more likely to undergo amputation than surgery may be profound.
The study showed the use of a specialized VPS system is an efficient means to aid in the diagnosis of PAD which may be unrecognized in vulnerable women who are currently underserved by traditional healthcare providers. In a large cohort of female patients that met the ACC/AHA criteria for PAD testing, symptoms of claudication are infrequent despite a plethora of cardiovascular risk factors. This study showed that more than one-third of the study patients have evidence of flow obstruction ranging from early vascular disease to more severe vascular disease. It is imperative that early diagnosis be afforded to women and appropriate preventive care be instituted to improve clinical outcomes.
This report was a retrospective analysis of de-identified data that was collected during routine clinical practices. The medical aides who performed the tests included clinical characteristics on the test report forms based upon patient self-reporting on questionnaires. There was no protocol to report on patients who did not meet the ACC/AHA criteria and thus did not require VPS testing. This report was limited to describing the test findings and self-reported characteristics in the population tested.
Case Presentation Contributor: John B. Long, M.D., Vascular Surgery, BASS Medical Group, 1 Daniel Burnham Court #205C, San Francisco, CA, 94109415-221-7056. The case is in addition to, and not among, the patients analyzed in the paper.
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Citation: Rashidi W (2019) Report on a Program to Improve Vascular Care for Women: Experience in 68,402 Patients Seen in Primary Care Clinics. J Women’s Health Care 8:473. doi: 10.35248/2167-0420.19.8.473.
Received: 10-Sep-2019 Accepted: 01-Oct-2019 Published: 07-Oct-2019
Copyright: © 2019 Rashidi W. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original work is properly cited.