Journal of Antivirals & Antiretrovirals
Open Access
ISSN: 1948-5964
ISSN: 1948-5964
Research Article - (2020)Volume 12, Issue 1
Background: This study aimed to investigate the relationship of T helper type (Th)1/Th2/Th17 type cytokines produced by chronically hepatitis C virus (HCV)-infected patients in response to pegylated interferon-α plus ribavirin (Peg-IFN/RBV) therapy. These finding will help us to identify the type of cytokines which participate in restoring functional antiviral T cell responses resulting in improved viral clearance.
Methods and results: Sixty genotype-4 HCV-infected patients received Peg-IFN/RBV therapy for 12 weeks. Levels of Th1 cytokines (IL-2, IL-8, IL-12, TNFα, IFNγ), Th2 cytokines (IL-4, IL-6, IL-10) and Th17 cytokines (IL-17A, IL-17F) were estimated by ELISA. In post-treatment samples, mean levels of Th1 cytokines IL-12 and IFNγ were significantly higher in patients with an early virologic response (EVR) when compared to non-EVR. In contrast, mean levels of IL-17A and IL-17F in post-treatment samples were significantly higher in non-EVR patients compared to EVR patients. Interestingly, in post-treatment samples the anti-inflammatory Th2 cytokines IL-4 and IL-6 were produced at significantly higher levels in non-EVR patients than in EVR patients. Also, after treatment, the ratios of pro-inflammatory to anti-inflammatory cytokines were higher in EVR as compared to non-EVR patients.
Conclusion: A pattern of stronger Th1- and weak Th17 reactivity in HCV-infected patients supports a favorable response to Peg-IFN/RBV therapy.
Hepatitis C virus; Pegylated interferon-α plus ribavirin therapy; Cytokines; Interleukin
The outcome of acute hepatitis C virus (HCV) infection is largely determined by complex host-viral interactions [1,2]. The overall data depict an intricate and evolving equilibrium between HCV infection and host immunity, the result of which will lead to very different clinical outcomes ranging from a resolution of the infection to chronic infection [3,4]. A combination of pegylated interferon-α (Peg-IFN) plus ribavirin (RBV) (Peg-IFN/RBV) has been the standard of care therapy for chronic HCV infection regardless of the genotype [5]. In the absence of Peg-IFN/RBV therapy, the possibility of recovery from acute HCV infection has been shown to be influenced by the existence of efficient CD4+ and CD8+ T cell responses [6-8].
The most important event during the initial immune response against viral infection is the polarization of T helper (Th) response into Th1 and Th2 cellular subsets [9]. Th1 and Th2, secrete a different range of cytokines with distinct roles in the immune responses. Each Th cellular subset stimulates certain immune responses that are active at controlling specific types of microbes but can be inactive, or even harmful if stimulated in response to other types of microbes [9]. Th17 cells are a different lineage and have been proposed to be a potentially good target for the treatment of various forms of autoimmune and inflammatory diseases [10]. Th1 cells secrete IL-2, IL-8, IL-12, TNF-α, IFN-γ cytokines, Th2 cells secrete IL-4, IL-5, IL-6, IL-10, and IL-13 cytokines while Th17 cells secrete IL-17A and IL-17F. Th1 and Th17 cytokines are generally “pro-inflammatory” cytokines, while Th2 cytokines are generally “anti-inflammatory” cytokines. Th2 cytokines encourage vigorous antibody production and are therefore associated with strong humoral immunity that is important in combating infections by extracellular organisms. Which type of reactivity, Th1 or Th2, is activated first often influences the subsequent outcome; if a particular T cell subset is stimulated preferentially in an immune response, it can suppress the development of the other subset. The overall effect is that immune responses are generally dominated by either humoral (Th2) or cell-mediated (Th1 and Th17) immunity [9,10].
Our working hypothesis is that patients who respond successfully to Peg-IFN/RBV therapy are aided by host immune responses consisting of a Th1- and Th17-biased reactivity. Contrarily, a poor response to Peg-IFN/RBV therapy may occur in a Th2-biased state. In other words, we proposed that the host cytokine profile can either dampen or augment the viral response to Peg-IFN/RBV therapy. Vigorous and appropriate host response is important in combating viral infections; thus, it stands to reason that host immune response, as reflected by cytokine production pattern, may enhance or decrease drug effectiveness.
Patient population and design
A total of 67 patients, aged 21 and above, with chronic HCV infection, were recruited for the study. All patients were followed up in the hepatology clinics at either Al-Amiri Hospital or Mubarak Al-Kabeer Hospital, Kuwait between October 2013 and September 2016. The baseline demographic and clinical data of recruited patients are shown in Table 1. A diagnosis of chronic hepatitis was made if the following criteria were satisfied: (i) anti-HCV antibodies detected in the serum; (ii) HCV RNA in blood detected by PCR; (iii) elevated serum alanine aminotransferase level (>1.5- fold of normal levels) on 2 or more occasions over a minimum period of 6 months; (iv) normal serum albumin and prothrombin time; and, (v) ultrasonography revealing an enlarged bright texture of the liver, portal tract thickening, and normal spleen. Patients who were excluded from the study comprised of those with other concurrent chronic liver diseases, cirrhosis, space-occupying lesions in the liver, thrombocytopenia (platelet count <50 × 109/L), prolonged prothrombin time (International Normalization Ratio >1.5), history of hepatotoxic drug-intake, alcohol intake, and previous antiviral, immunomodulatory or IFN therapy, or those who were co-infected with hepatitis B virus or HIV. The protocol for the study and the statement of informed consent was approved by the Ethics Committee of the Health Sciences Center at Kuwait University and the Ministry of Health, Kuwait. It conforms to the provisions of the World Medical Association’s Declaration of Helsinki in 1995 (as revised in Tokyo, 2004). All patients gave informed consent prior to inclusion in the study. All methods were carried out in accordance with the relevant guidelines and regulations mentioned above.
| Group | HC* | EVR** | Non-EVR |
|---|---|---|---|
| No. | 30 | 39 | 21 |
| Sex (male/female) | 18/12 | 23/16 | 13/8 |
| Age (years) | 29 | 30 | 32 |
| ALT† (U/l) ± SD Pretreatment At week 12 |
22 ± 7.55 ND |
78 ± 34.55 36 ± 14.76 |
68 ± 14.61 52 ± 18.40 |
| AST‡ level (U/l) ± SD Pretreatment At week 12 |
18 ± 10.5 ND |
72.5 ± 33.2 35 ± 12.1 |
79.5 ± 44.2 61 ± 29.30 |
| HCV RNA (log10 copies/ml) Pretreatment At week 12 |
ND ND |
5.94 ± 0.53 1.54 ± 0.65 |
6.24 ± 0.46 5.8 ± 0.83 |
Table 1: Baseline of demographic and clinical data of recruited subjects.
Data are shown as mean ±SD; HC*: healthy control; EVR**: early virological response; ALT†: alanine aminotransferase; AST‡: aspartate aminotransferase; ND§: not determined
The Peg-IFN/RBV therapy protocol consisted of 180 μg of pegylated IFN-α (Pegasys®, Roche, Germany) given subcutaneously once a week plus RBV 1000-1200 mg orally daily. The dose of RBV was adjusted according to body weight. Written informed consent was obtained from all participants. Blood samples were collected from all patients before the initiation of the Peg-IFN/RBV therapy (baseline) and after 12 weeks of the Peg-IFN/RBV therapy. Thirty healthy subjects, matched for sex ratio and mean age with the patient groups, were included as healthy controls (HC). Of the 67 chronically HCV-infected patients, 46 had complete early virologic response (EVR) defined as ≥2 log10 copies/ml drop in HCV RNA level at week 12 by quantitative PCR. Twenty-one were non-EVR defined as <2 log10 copies/ml drop in HCV RNA level at 12 weeks of Peg-IFN/RBV therapy [11]. As the majority of patients (n=60, 89%) were infected with HCV genotype-4, statistical analysis was done only for patients with genotype-4.
Biochemical and virological investigations
Serum biochemical investigations including bilirubin, albumin, alanine aminotransferase (ALT), aspartate aminotransferase (AST), creatine kinase, and blood urea nitrogen were measured in an automated analyzer (Hitachi 7170A; Hitachi Ltd, Tokyo, Japan). Only ALT and AST mean values were shown in Table 1 other biochemical investigations were used by the clinicians for diagnostic purposes and were not shown in the paper. Anti-HCV antibodies were determined by the Architect anti-HCV assay (Abbott Diagnostics, Wiesbaden, Germany). Serum HCV RNA was quantified by real-time PCR (Roche Molecular Systems, Inc., Branchburg, NJ). Genotyping of HCV was performed using a second-generation line probe assay (Inno-Lipa II; Innogenetics, Zwijndre, Belgium).
Isolation of peripheral blood mononuclear cells (PBMC)
PBMC were separated by Ficoll-Hypaque (Pharmacia Biotech, Sweden) density gradient centrifugation of 5 ml of peripheral blood. PBMC were washed by centrifugation at 1200 rpm for 10 minutes with RPMI-1640 medium.
Mitogen-induced proliferation of PBMC
PBMC were suspended in RPMI-1640 medium at 106 cells/ ml, aliquoted into 96 well tissue culture plates at a density of 105 cells in 100 μl per well; to this was added 100 μl (5 μg/ml) phytohemagglutinin (PHA) (Sigma Chemicals, USA) and incubated for a period of 96 hours. For each sample, a negative control was included by incubating PBMC for the same period in the absence of PHA. Supernatants from PBMC cultures were harvested after 24 hours and 96 hours.
ELISA for cytokines
Levels of Th1 cytokines (IL-2, IL-8, IL-12, TNFα, IFNγ, IL- 17A, and IL-17F) and Th2 cytokines (IL-4, IL-6, and IL-10) were measured by sandwich ELISA. Cytokines were estimated in the supernatants of cultures of PBMC from HCV-infected patients stimulated with PHA for 24 hours (for IL-2) and 96 hours (for all other cytokines). Antibody sandwich ELISA kits for estimating cytokines were obtained from Bender Medsystems (Vienna, Austria). Samples and standards were first incubated with a solid phase monoclonal antibody which captured any specific cytokine present. The solid phase-bound cytokine was then incubated with a biotinylated anti-cytokine antibody. The resulting antigen-antibody complexes were incubated with a streptavidin-enzyme conjugate after which the substrate and chromogen were added. Samples were tested in triplicate and absorbance values read using an ELISA Reader. Accurate concentrations of cytokines were determined by comparing their absorbencies with those obtained for the reference standards plotted on a standard curve.
Statistical analysis
Statistical analysis was performed using SPSS program version 11. The standard Mann-Whitney test was used for non-parametric comparisons of mean cytokine levels.
Sixty chronically HCV-infected genotype-4 patients were studied; 39 (65%) patients were with complete EVR to Peg-IFN/RBV therapy, and 21 (35%) were non-EVR. The levels of 10 pro-inflammatory and anti-inflammatory cytokines IL-2, IL-4, IL-6, IL-8, IL-10, IL- 12, IFNγ, TNFα, IL-17A, and IL-17F were analyzed by ELISA before the initiation of Peg-IFN/RBV therapy (baseline values) and post-treatment of the Peg-IFN/RBV therapy (i.e. after 12 weeks of initiation of Peg-IFN/RBV therapy). The mean levels of cytokines at baseline and after treatment are shown in Figures 1 and 2.
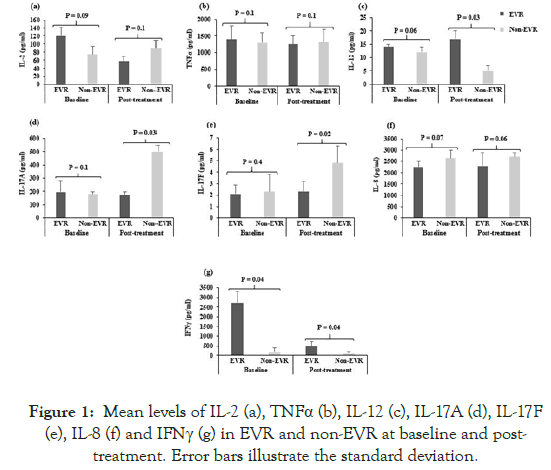
Figure 1: Mean levels of IL-2 (a), TNFα (b), IL-12 (c), IL-17A (d), IL-17F (e), IL-8 (f) and IFNγ (g) in EVR and non-EVR at baseline and posttreatment. Error bars illustrate the standard deviation.
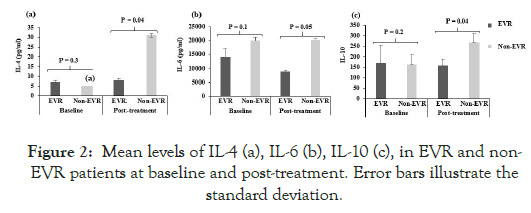
Figure 2: Mean levels of IL-4 (a), IL-6 (b), IL-10 (c), in EVR and non- EVR patients at baseline and post-treatment. Error bars illustrate the standard deviation.
The mean levels of IFNγ were significantly higher before the initiation of the Peg-IFN/RBV therapy in EVR as compared to non-EVR patients (p<0.04, Figure 1g). Levels of the other proand anti-inflammatory cytokines at baseline were not significantly different in EVR versus non-EVR (p>0.05, Figures 1 and 2).
In the post-treatment samples, the Th1-type cytokines IL-12 and IFNγ were significantly higher in EVR than in non-EVR patients (p<0.03, Figure 1c; p<0.04, Figure 1g respectively). The mean levels of IL-17A and IL-17F were significantly higher in non-EVR after treatment than in EVR patients (p<0.03, Figure 1d; p<0.02, Figure 1e respectively). Notably, the anti-inflammatory Th2-type cytokine IL-4 was produced at significantly higher levels in non-EVR patients as compared to EVR after therapy (p<0.04, Figure 2a). Similarly, IL-6 and IL-10, a Th2-type cytokine is produced at significantly higher levels in non-EVR as compared to EVR (p<0.05, Figure 2b; p<0.04, Figure 2c respectively).
Keeping in mind that the relative levels of Th1- and Th17- to Th2-type cytokines are as important, if not more important than the absolute levels of cytokines per se, we calculated the ratios of Th1:Th2 and Th17:Th2 cytokines as these ratios can indicate a dominance of one or the other cytokine pattern. As can be seen from Table 2, the majority of ratios of pro- to anti-inflammatory cytokines were higher in EVR than in non-EVR. Interestingly, while IL-2 and TNFα levels were not significantly different in EVR versus non-EVR patients, the ratios of IL-2/IL-4 and TNFα/IL-4 were higher in EVR both before and after treatment. Some of the ratios involving IL-8 and IL-12 (i.e. IL-8/IL-4 and IL-12/IL-4) were also higher in EVR patients. Ratios involving IFNγ show striking differences at baseline, and the ratios of IFN/IL-4, and IFN/IL-10 were 23-and 11-fold higher in EVR versus non-EVR subjects before treatment. Likewise, after treatment, IFN/IL-4, IFN/IL-6, and IFN/IL-10 ratios were 50-, 150- and 21-fold respectively higher in EVR patients. This suggests a stronger Th1-biased reactivity pattern in patients who respond favorably to Peg-IFN/RBV therapy.
| Baseline values | Post-treatment values | |||||
|---|---|---|---|---|---|---|
| Non-EVR | EVR | Difference‡ (fold) | Non-EVR | EVR | Difference‡ (fold) | |
| IL-2/IL-4 | 7.7 | 24 | 3.1 | 2.8 | 7 | 2.5 |
| IL-2/IL-6 | 0.004 | 0.009 | 2.3 | 0.004 | 0.006 | 1.5 |
| IL-2/IL-10 | 0.7 | 0.7 | 1 | 0.3 | 0.3 | 1 |
| TNF/IL-4 | 135 | 289 | 2 | 43 | 154 | 3.6 |
| TNF /IL-6 | 0.06 | 0.1 | 1.7 | 0.06 | 0.1 | 1.7 |
| TNF/IL-10 | 8 | 8 | 1 | 4.8 | 7.5 | 1.6 |
| IL-8/IL-4 | 266 | 444 | 1.7 | 95 | 93 | 1 |
| IL-8/IL-6 | 0.1 | 0.2 | 2 | 0.1 | 0.3 | 3 |
| IL-8/IL-10 | 0.7 | 0.7 | 1 | 0.3 | 0.3 | 1 |
| IL-12/IL-4 | 1.2 | 2.8 | 2.3 | 0.2 | 2 | 10 |
| IL-12 /IL-6 | 0.0006 | 0.001 | 1.7 | 0.0003 | 0.002 | 6.7 |
| IL-12/IL-10 | 0.9 | 0.08 | 11.3 | 0.02 | 0.1 | 5 |
| IL-17A/IL-4 | 16 | 39 | 2.4 | 15 | 21 | 1.4 |
| IL-17A/IL-6 | 0.008 | 0.01 | 1.3 | 0.02 | 0.02 | 1 |
| IL-17A/IL-10 | 0.8 | 1.1 | 1.4 | 1.7 | 1 | 1.7 |
| IL-17F/IL-4 | 0.25 | 0.37 | 1.5 | 0.17 | 0.27 | 1.6 |
| IL-17F/IL-6 | 0.0001 | 0.0001 | 1 | 0.0002 | 0.0003 | 1.5 |
| IL-17F/IL-10 | 0.01 | 0.01 | 1 | 0.018 | 0.015 | 1.2 |
| IFN/IL-4 | 24 | 560 | 23.3 | 1.3 | 65 | 50 |
| IFN/IL-6 | 0.01 | 0.2 | 20 | 0.0004 | 0.06 | 150 |
| IFN/IL-10 | 1.4 | 16 | 11.4 | 0.14 | 3 | 21.4 |
Table 2: Ratios of pro-inflammatory to anti-inflammatory cytokines in EVR and non-EVR Patients before and after treatment.
Our working hypothesis has been that patients who respond successfully to Peg-IFN/RBV therapy are aided by the host immune response consisting of a Th1-biased reactivity and that a poor response to Peg-IFN/RBV therapy occurs in a Th2-biased state. In other words, we proposed that the host cytokine profile can either aid or dampen the viral response to Peg-IFN/RBV therapy.
This study shows that IFNγ is produced at significantly higher levels in patients with EVR than in non-EVR patients after Peg- IFN/RBV therapy. IFNγ is a classical Th1 cytokine, with critical roles in anti-viral responses [12,13]. The increased production of this Th1-type pro-inflammatory cytokine supports the possibility that a Th1-biased reactivity may promote the development of an anti-viral state triggered by Peg-IFN/RBV therapy. IFNγ produced by Th1 cells induces an antiviral state in cells; it also activates Natural Killer (NK) cells which play critical roles in viral infections [14]. Zhang et al. demonstrated that while there was no difference between responder and non-responder patients to IFNα therapy before the start of therapy, IFNγ levels increased after IFNα therapy in EVR patients [15]. This is in line with our observation that IFNα therapy resulted in greater production of IFNγ in EVR patients.
In a study aimed at evaluating the association of IFNγ and chronic HCV infection among patients treated with Peg-IFN/RBV, Sadeq and coworkers found an increase in IFNγ after 12 weeks of treatment with combination therapy; thus, our observations on the possible association of IFNγ production with the responsiveness of EVR patients to IFNα/RBV therapy is significant [16].
The production of the pro-inflammatory cytokines TNFα and IL-2 were not significantly different in EVR versus non-EVR patients, but ratios of TNFα/IL-4 and IL-2/IL-4 were higher in EVR patients; also, levels of IL-12, a Th1-inducing cytokine, were higher in EVR than in non-EVR patients, supporting the notion of a stronger bias towards pro-inflammatory (Th1) cytokines in patients who responded to drug treatment (Table 2).
We also observed interesting differences in the levels of IL-17A and IL-17F produced. The levels of both these cytokines were significantly higher in non-EVR as compared to EVR patients. These observations support the recent study by Hammad et al. which showed a positive correlation between HCV viral load and IL-17 levels, with progressive exacerbation of the initial HCV-induced liver damage [17]. Serum levels of IL-17 have been shown to be higher in chronically HCV-infected patients [18,19]. Patients with chronic HCV infection were shown to have increased levels of both circulating and liver-infiltrating Th17 cells compared to healthy individuals, and Th17 levels were correlated with the severity of liver inflammation [20,21]. Contrary to these observations, however, there are studies that suggest a protective role for IL-17 in chronically HCV-infected patients [22]. Jimenez- Sousa et al. reported that Peg-IFN/RBV therapy down-regulates IL-17 levels and that this down-regulation is greater in responder (EVR) than in non-responder (non-EVR) patients, which is in contrast to our observations. No recent studies were published to support these results [23]. Recently, Liu et al. demonstrated that the frequencies of T follicular helper (Tfh) 17 cells were not increased in chronically HCV-infected patients treated with sofosbuvir/RBV or Peg-IFN/RBV therapies [24]. Clearly, the relevance and roles of Th17 reactivity in HCV infection merit further investigation.
Levels of Th2 cytokines also showed an interesting trend. Post-treatment levels of IL-4, a classical anti-inflammatory Th2 cytokine, were higher in non-EVR compared to EVR patients. IL-6 is also produced at higher levels during treatment in EVR patients. Par et al. have recently demonstrated that patients who had a rapid virological response to Peg-IFN/RBV therapy had decreased levels of both IL-4 and IL-10 as compared to non-EVR patients [25]. In contrast Liu et al., recently reported increased frequencies of T follicular helper (Tfh) 4 cells in chronically HCV-infected patients treated with sofosbuvir/RBV or Peg-IFN/RBV therapies [24]. While IL-10 showed a similar trend in the present study, the differences between the two groups were not significantly different. An earlier report has suggested that low IL-10 levels may actually help predict SVR to Peg-IFN/RBV therapy [26]. Recently, Saraiva et al. reported significantly low IL-10 levels after direct-acting antiviral therapy, reaching values similar to non-infected controls [27]. Our observation on IL-6 levels resonates with the study by Guzman-Fulgencio et al. which reported an association between high plasma levels of IL-6 in HIV co-infected HCV patients and failure to IFN-α therapy [28]. Also recently, Winckler et al. described that non-SVR patients were presented with higher IL-6 levels than those in the SVR group [29].
Keeping in mind the notion that the ratios of Th1 to Th2 cytokines are more indicative of an overall bias in T cell reactivity than the levels of cytokines alone, we calculated the ratios of Th1 to Th2 cytokines in different combinations (Table 2). IFN/IL-4 and IFN/IL-10 ratios are higher in EVR than in non-EVR patients; this indicates that patients who responded to drug treatment had a stronger bias towards Th1- type reactivity, and supports the hypothesis that strong Th1-oriented reactivity aids responses to treatment. In a murine model, Brenndorfer and coworkers recently showed that HCV infection engenders an intra-hepatic shift from Th1 to Th2 and that this effect is reversed by RBV, leading the authors to propose that RBV complements the anti-viral activity in an immunomodulatory manner [30]. In a study aimed at exploring whether and how RBV polarizes the Th1/Th2 balance toward Th1 dominance, Atsukawa et al. showed that RBV down modulates the expression of inducible costimulator (ICOS), a ligand for the costimulator B7-H2 on dendritic cells; these researchers suggest that this could be the mechanism by which Th2 reactivity is down-regulated [31]. In two elegant studies, Flynn et al. and Alhetheel et al. found that the clearance of HCV after interferon-based therapy seems to be associated with skewing the Th2 immune response to Th1 response [32,33].
The current study on pre-treatment and post-treatment profiles of cytokines could be a useful contribution to the understanding of immune modulation of the host response to Peg-IFN/RBV therapy. Most of the previous studies were based on the measurement of cytokine levels in the serum which is not as informative as cytokine levels secreted by blood lymphocytes to elucidate cytokine production patterns. We may also infer from this data that IFNγ levels and IFNγ/ IL-4 ratios seem to be predictors of viral responsiveness to drug treatment. Our data support the association between a stronger proinflammatory/ Th1 cytokine biases in responders to drug therapy.
It is also important to note some potential limitations of this study. Firstly, PBMC were used to determine and associate the level of Th1 and Th2 cytokine responses in EVR and non-EVR patients. It would be more pertinent to measure cytokine production in the local milieu, i.e. in the liver. Secondly, the majority of participants were genotype 4; the availability of other genotypes would have allowed a better definition of Th1 and Th2 cytokine responses. Thirdly, the correlation of cytokine profiles with post-treatment relapse was not studied.
We proposed that patients who respond successfully to treatment with Peg-IFN/RBV are aided by a host immune response consisting of Th1-biased reactivity and that a poor response to Peg-IFN/RBV therapy may occur in a Th2-biased state. Based on our data, and observations from other laboratories, we suggest that host immune responses, as reflected by cytokine production patterns, appear to be associated with the effectiveness of Peg-IFN/RBV therapy. The findings of this study could offer new possibilities for immunebased interventions with the aim of restoring functional antiviral T cell responses resulting in improved viral clearance.
This study was funded by the Kuwait University Research Sector Grant No. MI 01/12.
This manuscript is not under consideration for publication elsewhere. The authors declare no conflict of interest, no libelous statements and no competing financial interests exist.
S.E. and R.R. planned, designed, performed experiments, analyzed data and wrote the paper. I. S. recruited patients, collected clinical data, followed patients and revised the paper. S.E, I.S., W.A., R.R. discussed the results, contributed ideas to the project and to the final manuscript.
Citation: Essa S, Siddiqque I, Al-Nakib W, Raghupathy R (2020) Relevance of T helper Type 1, 2 and 17 Cytokine Profiles to the Outcome of Therapy in Hepatitis C Patients. J Antivir Antiretrovir. 12:193. DOI: 10.35248/1948-5964.20.12.193
Received: 09-Jan-2020 Accepted: 23-Jan-2020 Published: 31-Jan-2020 , DOI: 10.35248/1948-5964.20.12.193
Copyright: 2020 Essa S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Sources of funding : This study was funded by the Kuwait University Research Sector Grant No. MI 01/12.