
Biochemistry & Pharmacology: Open Access
Open Access
ISSN: 2167-0501

ISSN: 2167-0501
Research Article - (2017) Volume 6, Issue 3
We examined monocarboxylic and dicarboxylic acids and their partition coefficients for osmotic fragility (OF) in isolated red blood cells (RBCs) in rats. The dense packed RBC was incubated in a phosphate–NaCl buffer solution containing each carboxylic acid at 0 to 100 mM at 37ºC for 1 h. The RBC suspensions were transferred into the OF test tubes containing NaCl from 0.2 to 0.9%. The hemoglobin concentration was determined and NaCl concentration inducing 50% hemolysis was calculated as OF value. The OF in RBCs was dose-dependently increased by exposure to some of monocarboxylic acids possessing certain length of hydrocarbons with more than 4 carbons. A positive and statistically significant correlation was obtained between the partition coefficients and the degree of change in OF for monocarboxylic acids. Dicarboxylic acids corresponded with the monocarboxylic acids had either no effect or rather decreased OF, and there was no correlation between the partition coefficients and change in OF for these acids. The partition coefficients of the monocarboxylic acids were higher than those for the corresponding dicarboxylic acids. Whereas monocarboxylic acids are thought to act on the hydrophobic acyl-chain of phospholipids, which exists in a deeper region, dicarboxylic acids act on the interface region, which is hydrophilic and in a shallower area of the RBC membrane. Both carboxylic acids are speculated to cause physicochemical changes in the deep or swallow portion of phospholipid layer through different mechanisms and result in changes in the resistance to osmotic pressure in the rat RBC membrane.
<Keywords: Carboxylic acid; Erythrocyte; Partition coefficient; Membrane; Osmotic fragility; Hemolysis; Rat
In our previous reports, we have shown that the application of monocarboxylic acids with a certain number of hydrocarbons has the potential to weaken the cell membrane to osmotic pressure and increase osmotic fragility (OF), inducing hemolysis in red blood cells (RBCs) in rats in vitro [1-3]. On the other hand, in an experiment using the same method, the corresponding dicarboxylic acids either had no effect or increased membrane resistance to osmotic pressure and decreased OF in rat RBCs [3]. As preliminary treatment on rat RBCs with trypsin did not change the OF response to carboxylic acids, we assumed that the outer protein on the RBC membrane was less involved in the effects of carboxylic acids on changes in OF, and thus the phospholipid layer of the cell membrane was much more involved in this phenomenon [1].
Monocarboxylic acids are composed of one hydrophilic carboxylic group and a hydrophobic hydrocarbon chain of various lengths, with the whole molecule being amphiphilic in physicochemical terms. Although there is some debate over whether monocarboxylic acids possessing a small number of hydrocarbons (<10) should be categorized as surfactant [4] or not [5], they could be recognized as a kind of surfactant-like substance due to their chemical structure and amphiphilic characteristic.
Surface-active substances have been known to permeate the cell membrane and produce mixed surfactant/lipid bilayers, which is generally called mixed micelle formation, before saturation of the surfactant: phospholipid ratio [6-8]. The concentrations of each substance required to permeate the lipid layer induce solubilization of the membrane is defined as the critical micellar concentration (CMC) and is measured by various physicochemical methods [9-11]. Hemolysis induced by solubilization of membrane usually occurs above the CMC, and surfactants with a low CMC are more hemolytic [12-15].
The partition coefficient is one of the physicochemical parameters indicating the hydrophobic/hydrophilic balance of chemical substances and is defined as the ratio of the concentrations of a substance between two solvents [16]. There have been a number of reports on the relationship between the partition coefficient (solvent/water) and permeation coefficient of small molecules into the membrane [17-20]. The determination of these parameters for carboxylic acids was performed using artificial [21-24] and RBC membranes [25], or RBCs themselves [26].
The partition coefficient for octanol/water is widely used as an indicator of the distribution of hydrophobic drugs in cells, tissues and the body in general [27-29], as n-octanol is closest in nature to the phospholipid membrane among the non-polar solvents [30]. The logarithm of the partition ratio of chemicals using octanol and water was evaluated and the log P values of each substance used [31]. The log P value for various chemical substances are commonly provided on a website of the PubChem [32]. It is assumed that the partition coefficient value is a valuable indicator for explaining differences between carboxylic acids and their actions on the cell membrane, including the induction of changes in OF in rat RBCs.
In the present report, based on data from our previous experiment [3], we evaluated the relationship between the partition coefficients of both monocarboxylic and dicarboxylic acids and the degree of change in OF in rat RBCs exposed to those carboxylic acids. We speculated that this approach could clarify whether the partition coefficient can be used to explain the actions of both monocarboxylic and dicarboxylic acids on the rat RBC membrane, particularly the interaction between the carboxylic acids and the phospholipid layer. In addition, we sought to elucidate whether the partition coefficient could explain the opposite actions observed for monocarboxylic and dicarboxylic acids on the RBC membrane.
Reagents
All reagents were of biochemical grade and used as reported previously [3]. The following carboxylic acids were purchased from Tokyo Kasei Kogyo Co., Ltd. (Tokyo, Japan) or Wako Pure chemical Co., Ltd. (Osaka, Japan): formic acid, acetic acid, propionic acid, n-butyric acid, n-valeric acid, n-caproic acid, n-enanthic acid, n-caprylic acid, oxalic acid, malonic acid, succinic acid, glutaric acid, adipic acid, pimelic acid, suberic acid, azelaic acid, benzoic acid, phthalic acid, isophthalic acid and terephthalic acid. The chemical structures of these carboxylic acids are shown in Tables 1-3 in the Results section.
Animals and treatment of blood
The animals used and preparation of rat RBCs were as reported previously [3]. Briefly, blood samples were taken from male Sprague- Dawley rats (386 ± 47 g, n=24) between 9 and 11 weeks old (10 ± 1 weeks). On the day of the experiment, the rats were anesthetized with pentobarbital sodium (30 mg/kg) and blood (12-15 ml) was collected from the abdominal aorta into heparinized test tubes. The RBCs were separated from the plasma and leucocytes by centrifugation at 2000 g for 15 min at 4ºC (Himac R22, Hitachi Inc., Tokyo, Japan) and the crude RBCs (6 ml) were then washed three times with 12 ml of cold 0.9% NaCl solution. A dense-packed cell suspension was obtained and thereafter kept in ice-cold water until subsequent treatment.
The procedure for the evaluation of the OF value in the rat RBCs was also described in a previous report [3]. In brief, the dense-packed RBCs (40 μl) were transferred into 0.8 ml of a phosphate-NaCl buffer solution (pH 7.4) containing carboxylic acids at 0, 0.1, 0.25, 0.5, 1, 2.5, 5, 10, 25, 50 or 100 mM in 1.5 ml micro test tubes (Nichiryo Co., Ltd., Tokyo, Japan). The osmolarity was regulated by the amount of NaCl added to the buffer solution when each substance was prepared. The RBC suspensions were treated with carboxylic acids by shaking (1 stroke/sec) at 37ºC for 1 h (Shaking Bath TBK202DA. Advantec Co., Ltd., Tokyo, Japan) and then 50 μl of each suspension was transferred into a 96 well deep-well microplate (2 ml volume, Whatman Inc., Piscataway, NJ, USA) containing 1 ml of a 0.2-0.9% NaCl solution. This plate was immediately centrifuged at 1300 g (Plate Spin II, Kubota Inc., Tokyo, Japan) for 10 min, inducing hemolysis of the RBCs. The supernatants (200 μl) containing hemoglobin were transferred into another 96-well microplate (300 μl volume, Whatman Inc., Piscataway, NJ, USA) and determined colorimetrically at 540 nm (Microplate reader Model 680, Bio-Rad Laboratories, Tokyo, Japan).
In the present report, we used the values calculated from the data obtained in our previous study [3]. Complete hemolysis of the RBC suspension occurred in the 0.2% NaCl solution, for which the hemoglobin concentration was defined as 100%. Hemolysis of the RBCs did not occur in the 0.9% NaCl solution, for which the hemoglobin concentration was defined as 0%. The NaCl concentration causing 50% hemolysis (EC50) of the RBCs exposed to carboxylic acid was calculated from the hemolysis curve by using a straightline equation between the points immediately adjacent to 50%. The difference in OF between that at 0 (control) and each 10, 25, 50 and 100 mM of carboxylic acid was obtained and expressed as the ΔEC50. All values are expressed as means ± SD. We used the previous data about the significance of differences between the control (0 mM) and subsequent concentrations (0.1-100 mM) determined by Dunnett’s test following one-way ANOVA. The partition coefficients of each substance examined in previous experiment [3] were quoted in general from a website (Pub Chem) for chemical and physical properties [32]. Regression analysis was used to confirm the relationship between the partition coefficient of each carboxylic acid and ΔEC50 of the rat RBCs. Statistical analyses were performed using Excel Tokei for Windows 2012 (SSRI Co., Ltd., Tokyo, Japan). A difference with P <0.05 was considered statistically significant.
Figure 1 shows typical hemolytic curves for rat RBCs treated with monocarboxylic and dicarboxylic acids. Values are the mean ± SD (n=7). Curves were expressed for n-caproic acid, which does a monocarboxylic acid possessing a straight 5-carbon hydrocarbon chain, and terephthalic acid, which is a dicarboxylic acid possess a benzene nucleus, at concentration of 100 mM. The hemolysis curve for n-caproic acid or terephthalic acid was shifted to the right or the left, respectively. The ΔEC50 value was calculated as the difference between the control value at 0 mM and those induced by the application of chemicals at 10, 25, 50 and 100 mM as shown in Tables 1-3.
| No of carbon | Monocarbixylic acid | Partition coefficient | Dose (mM) | Change in OF ⊿EC50 (NaCl %) | |
|---|---|---|---|---|---|
| 0 | Formic acid H-COOH | -0.54 | 10 | -0.004 ± 0.027 | |
| 25 | -0.002 ± 0.023 | ||||
| 50 | 0.000 ± 0.027 | ||||
| 100 | 0.014 ± 0.030 | ||||
| 1 | Acetic acid CH3-COOH | -0.17 | 10 | 0.000 ± 0.017 | |
| 25 | 0.004 ± 0.019 | ||||
| 50 | 0.004 ± 0.008 | ||||
| 100 | 0.025 ± 0.019 | ||||
| 2 | Propionic acid CH3-CH2-COOH | 0.33 | 10 | 0.009 ± 0.010 | |
| 25 | 0.018 ± 0.012 | ||||
| 50 | 0.023 ± 0.013 | ||||
| 100 | 0.049 ± 0.013 | ** | |||
| 3 | n -Butyric acid CH3-(CH2)2- COOH | 0.79 | 10 | 0.026 ± 0.021 | |
| 25 | 0.036 ± 0.023 | * | |||
| 50 | 0.046 ± 0.013 | ** | |||
| 100 | 0.067 ± 0.024 | ** | |||
| 4 | n -Valeric acid CH3-(CH2)3- COOH | 1.39 | 10 | 0.045 ± 0.019 | ** |
| 25 | 0.070 ± 0.023 | ** | |||
| 50 | 0.102 ± 0.027 | ** | |||
| 100 | 0.157 ± 0.020 | ** | |||
| 5 | n -Caproic acid CH3-(CH2)4- COOH | 1.92 | 10 | 0.070 ± 0.019 | ** |
| 25 | 0.112 ± 0.013 | ** | |||
| 50 | 0.144 ± 0.028 | ** | |||
| 100 | 0.176 ± 0.035 | ** | |||
| 6 | n -Enanthic acid CH3-(CH2)5- COOH | 2.42 | 10 | 0.131 ± 0.038 | ** |
| 25 | 0.166 ± 0.029 | ** | |||
| 50 | 0.190 ± 0.028 | ** | |||
| 100 | 0.217 ± 0.030 | ** | |||
| 7 | n -Caprylic acid CH3-(CH2)6- COOH | 3.05 | 10 | 0.109 ± 0.008 | ** |
| 25 | 0.150 ± 0.029 | ** | |||
| 50 | 0.245 ± 0.039 | ** | |||
| 100 | No data | ||||
Table 1:Monocarboxylic acids possessing straight hydrocarbon chains, their chemical structure, partition coefficients and effect on the OF in rat RBCs in vitro. Values are means ± SD (n=7). Asterisks (* and **) indicate that there was a significant difference (P < 0.05 and P < 0.01) between the control (0 mM) and subsequent concentration (0.1-100 mM) on the basis of Dunnett’s test [3]. The partition coefficients were obtained from a website of PubChem [32]. As there were no significant changes for exposure to 0.1-5 mM of all tested monocarboxylic and dicarboxylic acids, the EC50 value at those doses are omitted and the data for 10, 25, 50 and 100 mM are presented.
| No of carbon | Dicarbixylic acid | Partition coefficient | Dose (mM) | Change in OF ⊿EC50 (NaCl %) | |
|---|---|---|---|---|---|
| 0 | Oxalic acid HOOC-COOH | -0.81 | 10 | -0.004 ± 0.022 | |
| 25 | -0.005 ± 0.011 | ||||
| 50 | 0.002 ± 0.025 | ||||
| 100 | -0.069 ± 0.065 | ** | |||
| 1 | Malonic acid HOOC-CH2-COOH | -0.81 | 10 | -0.011 ± 0.013 | |
| 25 | -0.039 ± 0.013 | ||||
| 50 | -0.064 ± 0.011 | ** | |||
| 100 | -0.075 ± 0.023 | ** | |||
| 2 | Succinic acid HOOC-(CH2)2-COOH | -0.59 | 10 | -0.009 ± 0.012 | |
| 25 | -0.037 ± 0.013 | ||||
| 50 | -0.047 ± 0.018 | ** | |||
| 100 | -0.059 ± 0.017 | ** | |||
| 3 | Glutaric acid HOOC-(CH2)3-COOH | -0.47 | 10 | -0.003 ± 0.012 | |
| 25 | -0.019 ± 0.015 | ||||
| 50 | -0.036 ± 0.018 | ||||
| 100 | -0.054 ± 0.010 | ** | |||
| 4 | Adipic acid HOOC-(CH2)4-COOH | -0.29 | 10 | -0.007 ± 0.014 | |
| 25 | -0.010 ± 0.011 | ||||
| 50 | -0.030 ± 0.016 | ||||
| 100 | -0.035 ± 0.017 | * | |||
| 5 | Pimelic acid HOOC-(CH2)5-COOH | 0.61 | 10 | -0.006 ± 0.012 | |
| 25 | -0.014 ± 0.016 | ||||
| 50 | -0.020 ± 0.025 | ||||
| 100 | -0.036 ± 0.024 | ||||
| 6 | Suberic acid HOOC-(CH2)6-COOH | 0.8 | 10 | -0.000 ± 0.014 | |
| 25 | -0.008 ± 0.016 | ||||
| 50 | -0.014 ± 0.022 | ||||
| 100 | 0.032 ± 0.031 | ||||
| 7 | Azelaic acid HOOC-(CH2)7-COOH | 1.57 | 10 | -0.004 ± 0.011 | |
| 25 | -0.005 ± 0.018 | ||||
| 50 | 0.018 ± 0.026 | ||||
| 100 | 0.059 ± 0.029 | ** |
Table 2: Dicarboxylic acids possessing straight hydrocarbon chains, their chemical structure, partition coefficients and effect on the OF in rat RBCs in vitro. Values are means ± SD (n=7). Asterisks (* and **) indicate that there was a significant difference (P < 0.05 and P < 0.01) between the control (0 mM) and subsequent concentration (0.1-100 mM) on the basis of Dunnett’s test [3]. The partition coefficients were obtained from a website of PubChem [32]. As there were no significant changes for exposure to 0.1-5 mM of all tested monocarboxylic and dicarboxylic acids, the EC50 value at those doses are omitted and the data for 10, 25, 50 and 100 mM are presented.
| No of carbon | Carbixylic acids with benzene ring | Partition coefficient | Dose (mM) | Change in OF ⊿EC50 (NaCl %) | |
|---|---|---|---|---|---|
| 6 | Benzoic acid |
1.87 | 10 | 0.025 ± 0.013 | |
| 25 | 0.014 ± 0.018 | * | |||
| 50 | 0.065 ± 0.030 | * | |||
| 100 | 0.109 ± 0.026 | * | |||
| 6 | Phthalic acid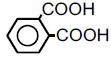 |
0.73 | 10 | 0.013 ± 0.010 | |
| 25 | 0.011 ± 0.015 | ||||
| 50 | 0.011 ± 0.019 | ||||
| 100 | 0.023 ± 0.020 | ||||
| 6 | Isophthalic acid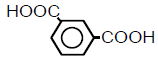 |
1.66 | 10 | -0.009 ± 0.021 | |
| 25 | -0.021 ± 0.023 | ||||
| 50 | -0.063 ± 0.026 | * | |||
| 100 | -0.078 ± 0.024 | * | |||
| 6 | Terephthalic acid |
2 | 10 | -0.004 ± 0.016 | |
| 25 | -0.023 ± 0.031 | ||||
| 50 | -0.043 ± 0.024 | ||||
| 100 | -0.058 ± 0.022 | * | |||
Table 3: Monocarboxylic and dicarboxylic acids possessing a benzene ring, their chemical structure, partition coefficients and effect on the OF in rat RBCs in vitro.
Values are means ± SD (n=7). Asterisks (* and **) indicate that there was a significant difference (P < 0.05 and P < 0.01) between the control (0 mM) and subsequent concentration (0.1-100 mM) on the basis of Dunnett’s test [3]. The partition coefficients were obtained from a website of PubChem [32]. As there were no significant changes for exposure to 0.1-5 mM of all tested monocarboxylic and dicarboxylic acids, the EC50 values at those doses are omitted and the data for 10, 25, 50 and 100 mM are presented.
Figure 1: Typical hemolytic curves for rat RBCs exposed to a monocarboxylic acid and dicarboxylic acid. Values are means ± SD (n=7). Hemolytic curves were determined after exposure to n-caproic acid (A) and terephthalic acid (B) at 0 (control) and 100 mM for 1 h. The EC50 value for hemolysis (% of NaCl concentration) was obtained by using a straight-line equation between the points immediately above and below 50%. The calculated value was used as a measure of OF.
Table 1 shows the chemical structures of the monocarboxylic acids possessing straight hydrocarbon chains, their partition coefficients and their effects on the ΔEC50 values in rat RBCs. Partition coefficients of the monocarboxylic acids were increased with increases in the numbers of carbons in the hydrocarbon chain in their moiety. The OF value was not changed by the application of formic or acetic acid. An increase in OF was induced by propionic acid at 100 mM, but not at concentration from 10 to 50 mM. The OF was also increased by n-butyric acid to n-caprylic acid at 10 to 100 mM. These increases in OF occurred in a dose-dependent manner and were also dependent on the number of carbons in the hydrocarbon chains. The application of n-caprylic acid also increased OF dose-dependently, but 100 mM of n-caprylic acid resulted in the rat RBCs bursting so that OF values could not be obtained.
Table 2 shows the chemical structures of dicarboxylic acids possessing straight hydrocarbon chains, their partition coefficients and their effects on ΔEC50 values in the rat RBCs. The partition coefficients of the dicarboxylic acids were also increased with increases in the number of carbons in the hydrocarbon chain in their moiety. The partition coefficient values of the dicarboxylic acids were lower than those for the corresponding monocarboxylic acids possessing same number of carbons (Table 1). Although dicarboxylic acids form oxalic acid to adipic acid decreased the OF, pimeric and suberic acids did not affect the OF and azelaic acid tended to increase OF at 100 mM. The decrease in OF occurred dose-dependently for each of the dicarboxylic acids. Those changes in OF were, however, not dependent on the number of carbon atoms in the respective dicarboxylic acids.
Table 3 shows the chemical structures of benzoic acid and three isomers of phthalic acid possessing a benzene nucleus, their partition coefficients and their effects on the ΔEC50 value in the rat RBCs. The partition coefficients varied markedly among the 4 substances, particularly for phthalic acid and its isomers, depending on the position of second carboxylic group on the benzene ring. The OF was increased by benzoic acid and decreased by isophthalic and terephthalic acids, but remained unchanged in the presence of phthalic acid. These changes in OF occurred in a dose-dependent manner for each substance.
Correlations between the partition coefficients of the carboxylic acids and their effects on cell membrane, as represented by OF, in the rat RBCs are shown in Table 4 and Figure 2. For this regression analysis, we used 2 different sets of parameters based on the exclusion or inclusion of benzoic acid and the phthalic acid isomers. Among the monocarboxylic acids examined in this study, those possessing straight hydrocarbon chains (with 0-7 carbons) demonstrated a statistically significant positive correlation between their partition coefficients and hemolytic effects as indicated by the ΔEC50 value (Table 4). A statistically significant positive correlation was also obtained between the partition coefficients of monocarboxylic acids including benzoic acid and the ΔEC50 value (P < 0.05) (Figure 2 and Table 4). On the other hand, dicarboxylic acids with a straight hydrocarbon chain (with 0-7 carbons) or a benzene ring (phthalic acid and its isomers) between two carboxylic groups showed no statistically significant linear relationship between the partition coefficient and the ΔEC50 value.
| Substances | Dose (mM) | r value | P value |
|---|---|---|---|
| Monocarboxylic acids (- benzoic acid ) | 10 | 0.9489 | < 0.001 |
| 25 | 0.9683 | < 0.001 | |
| 50 | 0.9851 | < 0.001 | |
| 100 | 0.9805 | < 0.001 | |
| Dicarboxylic acids (- isomors of phthalic acid ) | 10 | 0.523 | 0.1835 |
| 25 | 0.5561 | 0.1523 | |
| 50 | 0.669 | 0.0697 | |
| 100 | 0.6007 | 0.1153 | |
| Monocarboxylic acids (+ benzoic acid ) | 10 | 0.8854 | < 0.005 |
| 25 | 0.9103 | < 0.001 | |
| 50 | 0.9369 | < 0.001 | |
| 100 | 0.9385 | < 0.005 | |
| Dicarboxylic acids (+ isomors of phthalic acid ) | 10 | 0.2059 | 0.5435 |
| 25 | 0.2785 | 0.407 | |
| 50 | 0.1897 | 0.5764 | |
| 100 | 0.0728 | 0.8316 |
Table 4: Correlation between the partition coefficients of carboxylic acids and change in EC50 during hemolysis in rat RBCs. (Values were calculated by regression analysis (mean value of each carboxylic acid; n=7) between the partition coefficients and changes in EC50 during hemolysis induced by each dose of the monocarboxylic and dicarboxylic acids, with benzoic acids, phthalic acid and its isomers included or not. Correlation efficiency “r" and significant values “P“ are shown. P < 0.05 is defined as statistically significant in the present study.)
Figure 2: Relationship between the partition coefficient and degree of change in the OF obtained by exposure to monocarboxylic or dicarboxylic acids. Values are means ± SD (n=7). The partition coefficients were obtained from a website of PubChem. The OF value was obtained from the changes in ED50 induced by the application of monocarboxylic acids including benzoic acid (A) or dicarboxylic acids including phthalic acid and its isomers (B) at 50 and 100 mM.
In the present study, we sought to clarify the cause of the difference in the effect of the two groups of carboxylic acids on the RBC membrane through an evaluation of the relationship between the changes in OF value and the partition coefficients of each group of carboxylic acids in rat RBCs in vitro. With regard to the mechanism underlying the effects observed for the carboxylic acids, we applied a regression analysis using the data for changes in OF value induced by monocarboxylic and dicarboxylic acids obtained in a previous report [3] and the partition coefficients of those carboxylic acids obtained from a website of the PubChem [32].
The partition coefficients of the monocarboxylic acids used in this experiment increase with increases in the number of carbons in hydrocarbon chain bound to the carboxylic group. For acids with more than 4 carbons in the chain, the OF value rose in accordance with both the monocarboxylic acid concentration and the number of carbons in the hydrocarbon chain in their moiety. Significant positive correlations were found between the partition coefficients and the degree of changes in the OF induced by monocarboxylic acids possessing straight hydrocarbon chains. When benzoic acid was added to those monocarboxylic acids, significant positive correlations were also obtained. On the other hand, for the dicarboxylic acids examined in this experiment, whether phthalic acid and its isomers were included or not, no statistically significant correlation was obtained between the partition coefficients and the changes in the OF in rat RBCs.
There are some reviews of the action of surfactant compounds on the membrane in physicochemical and pharmacological terms [6-8]. One report discussed the detailed mechanism for the changes in the RBC membrane and subsequent hemolysis by surfactants [4]. The first step in the surfactant effect on the cell or tissue is permeation or partition of compounds into the cell membrane. The permeability of substances, particularly when those substances are small simple molecules, generally demonstrates a positive correlation with the partition coefficients. In amphiphilic substances such as alcohol, the degree of action on the membrane is reported to depend on the length and structure of the hydrocarbons in their moieties [33,34]. Monocarboxylic acids possessing a short straight hydrocarbon chain can permeate RBCs [26], isolated RBC membranes [25] and artificial membrane models [21-24], although the rate of permeation of those fatty acids in each membrane is generally increased with the number of carbons in the hydrocarbon chain. The permeation rate of formic acid, which has no CH3 is, however, equal to or higher than that of acetic acid and is nearly the same as that of propionic acid in RBCs [26] or a membrane model [21].
It has been reported that, on entering the cell membrane, the surfactant first increases the transmembrane motionrate of phospholipids in the lipid bilayer [35]. Small amphiphilic compounds, including n-caprylic acid, also show phospholipid acceleration of in human RBCs [36]. Following these phenomena, micelle formation in RBC membrane also occurs by interaction between the phospholipids and surfactants, resulting in changes in the membrane fluidity of the RBCs [37]. These consecutive steps are expected to induce changes in the normal structure and physicochemical strength of the RBC membrane, leading to hemolysis.
We speculated that the hydrophobic hydrocarbon element of monocarboxylic acids enters the hydrophobic layer of the RBC membrane and interacts with the acyl-chains in the phospholipids (Figure 3). The monocarboxylic acids possessing longer hydrocarbon chains, for which the partition coefficients are larger than for those possessing shorter chains, have stronger affinity to the acyl-chains of the phospholipid than that of monocarboxylic acids possessing shorter hydrocarbon chains. Thus the hydrocarbon chain can reach a much deeper portion of the membrane and increase the transmembrane motion rate of the phospholipid layer as mentioned above [35,36], weakening the resistance of the cell membrane to osmotic pressure [37].
Figure 3: Schematic representation illustrating distribution of monocarboxylic acids and dicarboxylic acids in the RBC membrane. Monocarboxylic acids with high partition coefficient permeate deeply into phospholipid layer and have a surfactant-like effect on the RBC membrane. Monocarboxylic acids with low partition coefficient locate close to water-lipid interface and do not have a surfactant effect. Dicarboxylic acids also locate the water-lipid interface and fill the space composed of heads and roots of acyl-chains of the phospholipids in the RBC membrane. We proposed that the effect of dicarboxylic acids, including isomers of phthalic acids, on the RBC membrane can be regarded as a “wedge-like effect” in our previous report [3].
Unlike monocarboxylic acids, dicarboxylic acids did not increase the OF, with some of those possessing a small numbers of hydrocarbons (0-3) actually decreasing the OF in rat RBCs. Partition coefficient of each dicarboxylic acid was lower than that for the corresponding monocarboxylic acid with the same number of hydrocarbons in the moiety. This finding suggests that the permeability of dicarboxylic acids into the RBC membrane phospholipid layer is smaller than that of monocarboxylic acids. The partition coefficients of dicarboxylic acids also increased in accordance with the length of the hydrocarbon chain between the two carboxylic groups located at each end of the molecules. However, there was no obvious correlation between the partition coefficient of the dicarboxylic acids, whether the phthalic acid isomers were included or not, and their effect on the OF in the rat RBCs.
Dicarboxylic acids have a hydrocarbon chain of various lengths between two carboxylic groups. It is difficult to assume, judging from their chemical structure and partition coefficients, that dicarboxylic acids enter the phospholipid bilayer deeply enough to form micelles with the phospholipids in the RBC membrane as is observed for monocarboxylic acids. We speculated that dicarboxylic acids may locate on the water-lipid interface of the cell membrane and interact with the heads and upper part of the acyl-chains in the phospholipids, resulting in stabilization of the RBC membrane (Figure 3). When these substances act on the RBC membrane, the hydrocarbon chain is expected to take a U- or V-shaped conformation between the two carboxylic groups. We also proposed that the stabilizing effect of dicarboxylic acids on the RBC membrane and subsequent increase of the OF value to osmotic pressure be referred to as a “wedge-like effect” [3]. The concept of wedge-like effect in dicarboxylic acids is probably used for a new tool increasing strength of the membrane in RBCs or other types of the cells. In addition, it may have a possibility that the functions of target cells are affected by this mechanism.
Although the value of the octanol/water partition coefficient has been widely used as an indicator for the distribution of hydrophobic drugs in cells, tissues and the body in general [27-29], it has been reported that, in many cases, chemicals and their actions on biological or artificial phospholipid membranes did not correspond to the partition coefficient of each substance [38-40]. This is thought to be due to not only the chemical properties, such as shape, dimension and ionization of the interacting chemicals, but also those of the membrane, such as form and length of the acyl-chain, type of phospholipid and amount of cholesterol contained in the membrane [41-46].
In conclusion, we found a significant positive correlation between the partition coefficient of monocarboxylic acids and their effect on the OF in rat RBCs. This finding indicated that the interaction between the hydrocarbon chain in the monocarboxylic acid and the acyl-chain in the phospholipids increases the trans-bilayer motion of phospholipids and micelle formation in the RBC membrane, resulting in an increase in OF in the rat RBCs. Further investigation by using various types of chemicals, including carboxylic acids with a more complex chemical structure such as branched or cyclic hydrocarbons, is needed to extend these findings. On the other hand, dicarboxylic acids, which have two carboxylic groups and a hydrocarbon chain of various lengths increased the membrane resistance to osmolarity and decreased the OF in the rat RBCs. Partition coefficient was found not to be an indicator of the effects of these substances on the RBC membrane. The mechanism of the membrane stabilizing effect produced by the dicarboxylic acids is a matter of interest that also needs to be clarified.
I thank Miss N. Amita, M. Kawawake and A. Higuchi of Hokkaido Bunkyo University, for their excellent technical assistance.