Journal of Physical Chemistry & Biophysics
Open Access
ISSN: 2161-0398
ISSN: 2161-0398
Review Article - (2021)Volume 11, Issue 3
The adverse health effects of exposure to radon are caused primarily by damage due to alpha-particles. The possible effects will depend on exposure level. The main danger from high radon exposure is an increased risk of lung cancer. Radon as a noble gas is rapidly exhaled after being breathed in; however, radon progeny combine with other molecules in the air and with particles of dust, aerosols or smoke, and readily deposit in the airways of the lung. While lodging there, the progeny emit ionizing radiation in the form of alpha particles, which can damage the cells lining the airways. Experiments have confirmed that ionizing radiation affecting bronchial epithelial cells could cause cancer.
Radon gas; Radioactive gas; Track etching; Cancer
Radon is a natural radioactive gas discovered in the 1900 by Dorn, who called it Radium emanation. Since 1923, it has been called radon. The atomic number of radon is 86, and the atomic weight is 222 [1]. Radon is a naturally occurring, colourless, odourless gas that is soluble in water. It is radioactive, which means that it breaks down or "decays" to form other elements. Radon occurs as a product of uranium decay. Uranium is a natural radioactive material found in varying amounts in all rocks, soil, concrete and bricks. It occurs everywhere on earth, especially in rocky and mountainous areas, radon occurs as three natural isotopes [2]:
• Radon 222Rn: This is the isotope belongs to a series 238U an isotope the longest age of radon isotopes, with a half-life of (3.825) days and this age gives it the ability to propagate only limited distances in the atmosphere and is an emitter of alpha particles with energy (5.4 MeV).
• Thorn 220Rn: This is the isotope belongs to a series 232Th a radon isotope the longest age of radon isotopes, with a half-life of 55.6 second.
• Action 219Rn: emanates from actinium, and it has a half-life of 3.96 second and is also an alpha emitter. However, our concern in the present work is to measure radon (222Rn) concentration in soil.
Figure 1 shows that more than eighty percent (84%) of human exposure comes from natural sources, radon gas, the human body outer space, rocks and soil. The remaining per cent (16%) comes from man-made radiation sources, primarily medical x-rays. Manmade radiation is more harmful because it is more concentrated natural radiation is more spread out and less concentrated, and is therefore less harmful [3]. The largest fraction of natural radiation exposure comes from Radon, a radioactive gas, due to decay of radium contained in rocks and soil as part of the uranium radionuclide chain.
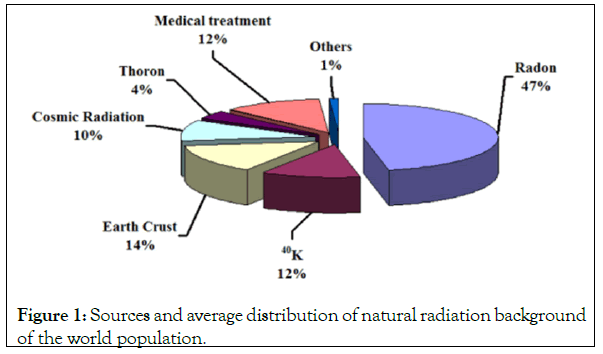
Figure 1: Sources and average distribution of natural radiation background of the world population.
Thorium and uranium are both sources of radon and they are common naturally-occurring elements that are found in low concentrations in rock and soil. Radon is produced from the radioactive decay of the element radium, which is itself a decay product of either uranium or thorium, so a source of radon and its decay products in the environment is the radium present in various materials such as soil, water, and building materials [4]. It is estimated that every square mile of soil to a depth of 6 inches contains about one gram of radium, which release radon in tiny amount in the atmosphere. There are four radon short living daughters 218Po, 214Pb, 214Bi and 214Po. Two of the radon daughters are alpha emitters (218Po and 214Po) and the others are beta emitters (214Pb and 214Bi) shown in Figure 2. When 222Rn decays, its progeny undergoes a series of transformations within hours, consisting of two alpha decays and two beta decays, finally resulting in relatively stable lead-210 [5]. While the beta emissions of this decay sequence can damage living cells by ionization, it is the alpha particles generally 20 times more damaging than beta particles, that contribute, the most risk to exposed, regenerative cells. The primary concern about indoor radon gas is the increased risk of lung cancer that exists from breathing radon and its products. It is worth remembering that radon produced in any material at a steady rate that is independent of any external factor [6].
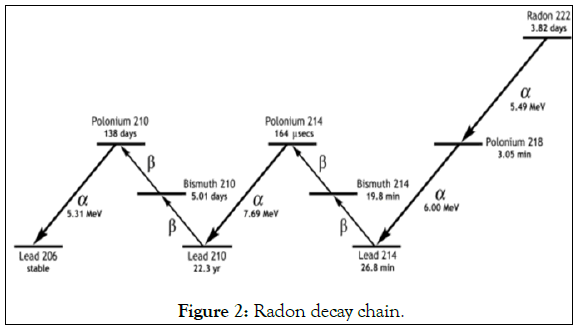
Figure 2: Radon decay chain.
Radon (222Rn) is naturally occurring radioactive gas. It is produced and decays by alpha emission to a polonium isotope (218po) which further decays is an isotope of lead, bismuth and polonium ends with a stable isotope of lead (206pb), Figure 2 shows alpha decay properties of radon which short-lived decay products [7]. Radon is a chemically inert noble gas with the slightest ability to form compounds under laboratory condition. It occurs in almost all materials and for the most part (90%) is trapped in the solids carrying its precursors 226Ra. At ordinary temperature radon is a colourless gas shown in Table 1 when cooled below the freezing point, radon exhibits a brilliance phosphoresce which becomes yellow as the temperature is lowered and orange-red at the temperature of liquid air. Some of radon may diffuse into other media such as the surrounding water or air and frequently the concentration of radon is higher than the concentration of 226Ra [6].
| Property | Value |
|---|---|
| Volume at 1 Bq of Rn-222 at NTP | 1.6 × 102 m3 |
| Boiling point | -61.8°C |
| Melting point | -71.0°C |
| Vapor pressure at -144°C | 0.13 Pa |
| Density at NTP | 9.96 kg/m3 |
Table 1: Some properties of radon.
Soil as a radon source
The major source of radon in the atmosphere at least 80% is from emanations from soil that is derived from rocks. These rocks contain some uranium, where the decay of 238U through 226Ra gives radon. Certain types of rock, including granites, dark shale, light-colored volcanic rocks, sedimentary rocks containing phosphate and metamorphic rocks derived from these rocks have higher average uranium contents [8]. Because radon is a gas, it has much greater mobility than uranium and radium, which are fixed in the solid matter in rocks and soils. Radon can more easily leave the rocks and soils by escaping into fractures and openings in rocks and into the pore spaces between grains of soil as shown in Figure 2 [9].
Sources of radon in groundwater
It may be supposed that radon in groundwater can be derived from two different sources [10].
• Radioactive decay of dissolved radium.
• Direct release of radon from the uranium and thorium decay series.
Radon is formed in radium-bearing materials; some of it leaves the grains to the pore space. This fraction is relatively free to move between the pores as shown in Figure 3. Radon can therefore reach the air or water to which humans have access to provided that transport is sufficiently rapid to be completed before the radon decays [11].
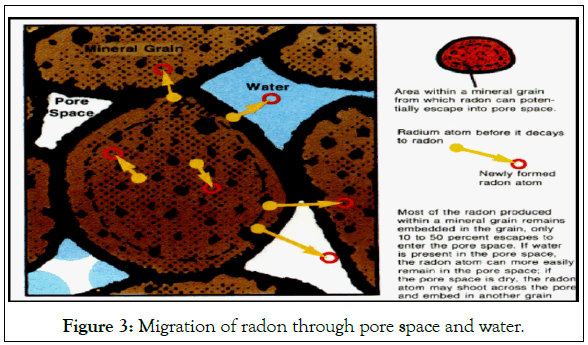
Figure 3: Migration of radon through pore space and water.
Radon entry into buildings
Man is continuously exposed to ionizing radiation from Naturally Occurring Radioactive Materials (NORM). The origin of these materials is the earth's crust, but they find their way into building materials, air, water, food and the human body itself. The worldwide average indoor effective dose due to gamma rays from building materials is estimated to be about 0.4 mSv per year. In many parts of the world, building materials containing radioactive material have been used for generations. As individuals spend more than 80% of their time indoors, the internal and external radiation exposure from building materials creates prolonged exposure situations. Building materials are the main source of indoor gamma radiation, beside terrestrial and cosmic radiation [12]. All stone based building materials contain radioactive nuclides, 226Ra, 232Th, their progenies, and 40K, are the most important. 226Ra in construction materials may be in some cases the predominant source. Much of the radon is released from the radium trapped in the mineral grains in building materials. The gas then escapes into the air because the radon diffusion length is comparable to the material thickness. This contribution depends on radium concentration, which is generally low in materials of normal activity (40 Bq.kg-1). The radon diffusion length in soil is estimated to range from 1 cm in moist clay soil to 2 m in well drained gravel soils shown in Figure 4 [13].
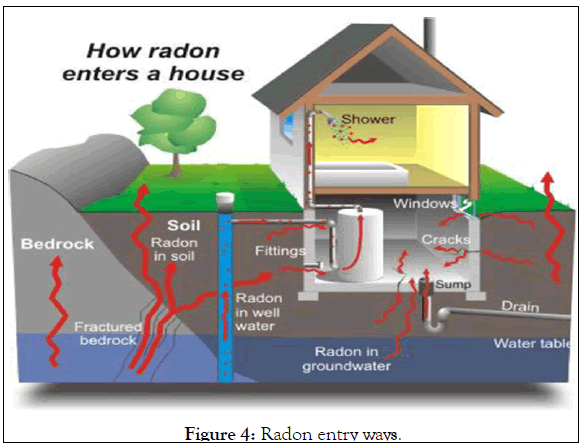
Figure 4: Radon entry ways.
The recognition that radon levels in some homes approximated those in mines led to epidemiologic studies of residential radon and lung cancer. These established radon as a cause of lung cancer in the general population, second in importance only to smoking, where it accounts for approximately 21,000 deaths per year in the US. On the inhalation of radon, alpha particles are released through the short-lived decay products of radon (Po-218 and Po- 214) and these alpha particles interact with lung tissues and can cause the DNA damage. It is supposed that cancer can occur even mutation in one cell which can act as a pool of developing cancer cells by damaging the DNA shown in Figure 5. Hence, a single alpha particle can cause the DNA damage at any level of exposure. Radon is inhaled and ingested when a human being is exposed to it and due to the harmful effects of radon on human; its monitoring is going to increase worldwide. During the regular activities, such as flushing toilets, washing clothes, radon is released, which increases the concentration of indoor radon by mixing. The radon released from water also contributes to inhalation risk of radon to indoor air. Therefore, natural sources play the significant role in increasing the radiation that human receives [14].
Figure 5: Doses of a particle radiation protected and incidence of lung cancer.
There are many types of techniques and detectors that are used to detect radiation, such as ionizing chamber, RAD7. Muller Counters Scintillation and Solid State Nuclear Track Detectors (SSNTDs) CR-39. The most common device for the measurement of radon concentration is the passive diffusion radon dosimeter containing solid state nuclear track detector CR-39. This type of detector is used throughout the present work.
Solid State Nuclear Track Detectors (SSNTDs)
Solid State Nuclear Track Detectors (SSNTDs) are a passive technique which has several advantages; low cost, cheap and can be easily obtained, long term method, most widely used for measuring radon and can be used for site assessment both indoor and outdoor [15]. SSNTDs are sensitive to alpha particles in the energy range of the particles emitted by radon. SSNTDs are largely insensitive to beta and gamma rays. SSNTDs also have the advantage to be mostly unaffected by humidity, low temperatures, moderate heating and light. They do not require an energy source to be operated from their detecting property is an intrinsic quality of the material they are made. There are three types of commercially available SSNTDs [16]:
• Poly Allyl Diglycol Carbonate (C12H18O7) was known as CR-39.
• Cellulose Nitrate (C6H8O8N2) was known as CN-85.
• Plastic track detector known as LR-115
CR-39 a better detector as compared to other detectors used for radon concentration measurement.
Characteristics of SSNTDs
These types of detectors has special characteristics and are being used in various fields, such as nuclear physics, elementary particle physics, cosmic rays biology, medicine, chemistry, geochemistry, reactors and neutron dosimetry. Some of their characteristics are [10,11]:
• They are tough and easy to use, so they can be used at high altitude for cosmic ray spectroscopy.
• The different types of SSNTDs have different sensitivities some of them (especially CR-39) are sensitive to alpha particles in the energy range of the particles emitted by radon.
• They are low-cost, simple to process, insensitive to beta and gamma rays unaffected by wetness, low temperatures.
• The etched detectors can usually be stored for longer periods of time under various environmental conditions without changing the track number or its shape.
• They have better charge and energy resolutions.
• In these detectors the detailed information is available from individual particles the size and shape of these tracks yield information about the mass, charge, energy and direction of motion of the bombarding particles.
• Their high melting point allows them to be exposed directly to heavy charged particles such as fission fragments. These fragments can be recorded and distinguished from a very high background of lighter charged particles like α, neutrons and beta particles, x-ray and gamma rays [17].
• The detectors can be placed in direct contact with fission fragment and other, sources giving very high efficiency and sensitivity.
Like any other detectors put them on because it is a new paragraph has few disadvantages. For example: they require a long measurement period. Also, these detection devices are not always precise for measurements of low radon concentration [18]. They are also laborious and time consuming when the numbers of the registered tracks are manually counted using an optical microscope, but this is disgraced, if an automatic scanning technique is used. For the detectors advantages they are regarded as a good choice to be used as radon measurements. CR-39 (Columbia Resin) that is used in this study is made of Polyallyl Diglycol Carbonate (PADC) with the chemical formula (C12H18O7) [19].
The chemical stricture monomer (Figure 6) also known as test track, CR-39. It was synthesized in 1978. It is a clear, colorless, rigid thermoset plastic, totally amorphous, resistant to all solvents, and very sensitive to heavy-ion damage. It has been found to be the most sensitive and efficient detector among all the plastics, to detect all ions including low energy protons and high energy charged particles. In addition to this it can discriminate the different energies of alpha particle [20].
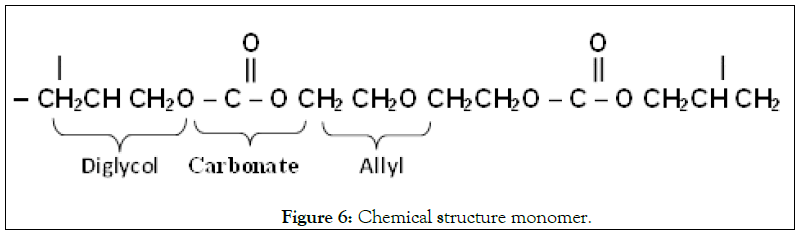
Figure 6: Chemical structure monomer.
Principles of track formation
Any detailed theory of how tracks are formed must fit the fact that the passage of heavily ionizing nuclear particles through most insulating solids creates stable and narrow paths of intense damage on the atomic scale. These damages are chemically reactive centers of strain that are composed mainly of displaced atoms rather than of electronic defects [21]. The most damage is produced at Bragg peak and therefore the latent track is most resistant at that position. The diameter of the latent tracks is of the order of tens of nm (Figure 7) [10]. The chemistry of these detectors is as follows: as seen in Figure 6, the film structure is composed of atoms of carbon, hydrogen and oxygen.
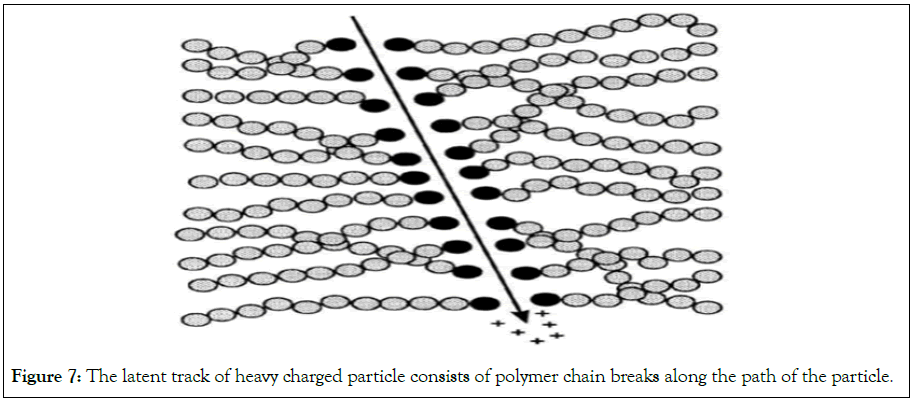
Figure 7: The latent track of heavy charged particle consists of polymer chain breaks along the path of the particle.
Oxygen has the largest atomic radius and more electrons in its orbits the alpha particles emitted from the decay of radon interact with the oxygen atoms. When an alpha passes through the film, it collides with the electrons of oxygen atoms and losses almost all of their energy, as this process leads to the positive ionization of oxygen atoms in detector film [22]. Monomer positive ionization of oxygen atoms in detector film as mentioned below:
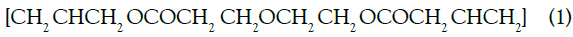
Track etching: The density of latent tracks is proportional to the exposure to heavy charged particles, for instance to radon and its progenies. The latent tracks are visible only by transmission electron microscopy and they are not easy to count and to estimate their density. Therefore, they are etched by a suitable etchant, in the case of plastics the enchants are alkali NaOH or KOH [22]. In the present work, we have chosen (NaOH) as an aqueous solution for chemical treatment. In practice, the most important parameters for control of the etching speed of the detectors are temperature, concentration of the etching solution and time etching, as indicated in Figures 8 and 9. The etching enlarges the latent tracks, making them visible by microscope, but also fix them making them stable and permanent (Figure 9), the process of the track etching.
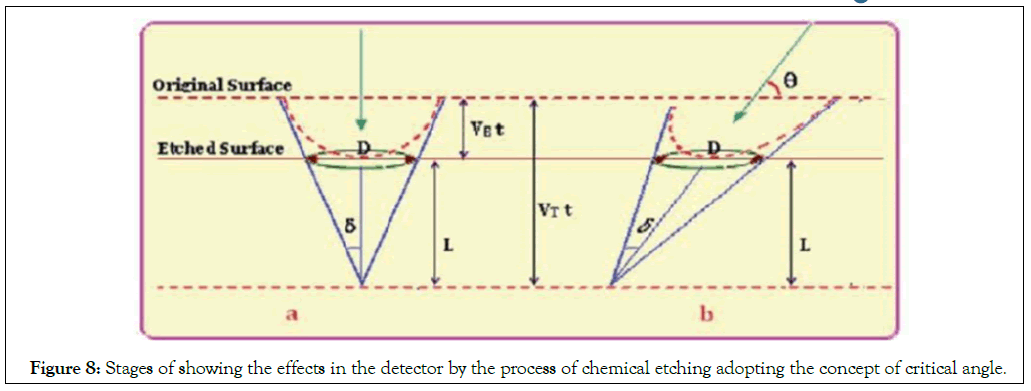
Figure 8: Stages of showing the effects in the detector by the process of chemical etching adopting the concept of critical angle.
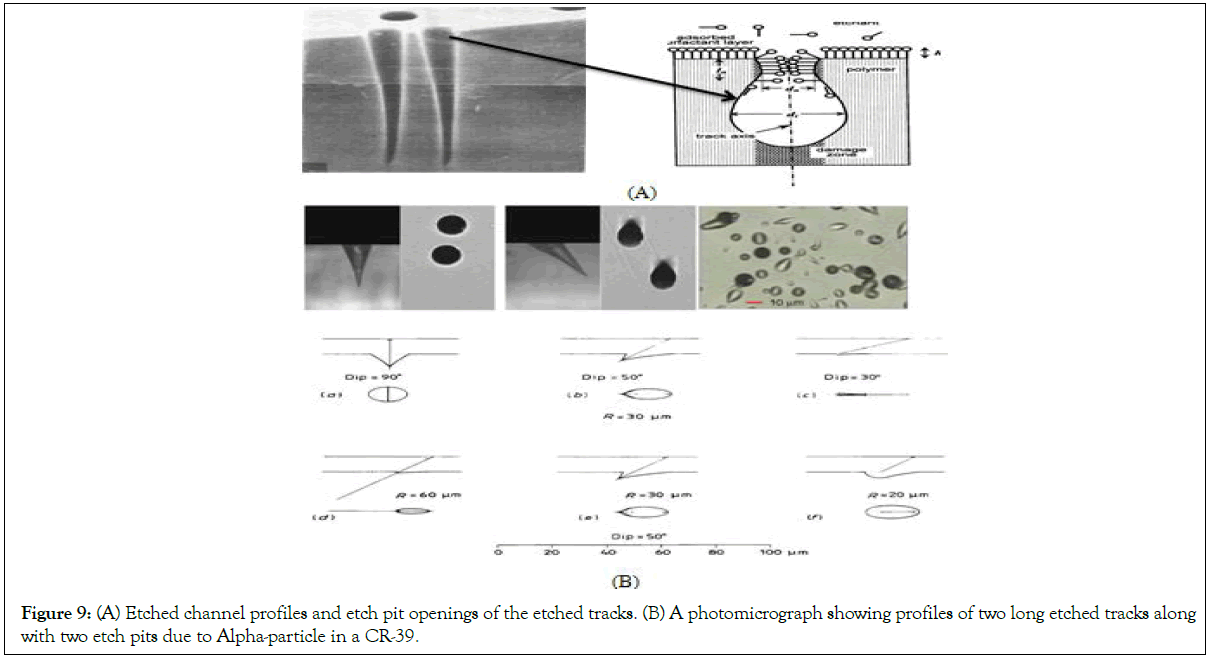
Figure 9: (A) Etched channel profiles and etch pit openings of the etched tracks. (B) A photomicrograph showing profiles of two long etched tracks along with two etch pits due to Alpha-particle in a CR-39.
There is a minimum density of damage along the track that is required before the etching rate is sufficient to create a pit. Because the density of damage correlates with the stopping power (-De/ dx) of the particle in the material, it is high for the heavy charged particles. In any given material, a certain minimum value for the stopping power (-dE/dx) is required before tracks will develop. For example, in the mineral mica, tracks are observed only from energetic heavy ions whose mass is ten or twenty atomic mass units or greater. Many common plastic materials are more sensitive and will develop etch [23].
Track geometry: When an irradiated polymer is subjected to an
etchant, the surface of the polymer is removed with a velocity called
general etching velocity VG, which is perpendicular to the surface.
The damaged region is also etched, but with a different etching
velocity known as the track etching rate VT, as shown in Figure 9 [24]. A track will be enlarged by etching only if the rate of etching
along the track VT exceeds the general etching rate VG. If the track
is not perpendicular to the surface, then the component of (VT.t)
perpendicular to the surface must exceed (VG.t) for registration to occur, i.e., since the distance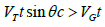 this gives
this gives 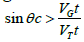 Therefore [25]:
Therefore [25]:

Hence there exists a critical angle of registration given by θc, under which the damage trail is not developed into a track. This probability of observation is usually referred to as the etching efficiency, where it is defined as the fraction of tracks intersecting a given surface that are etched on the surface under specified etching conditions [6], the shape of the etch pit and the diameter (or the major/minor axis) of the etch pit opening depend basically on two parameters [26]:
• The etching characteristics of the given medium, signified by its general (bulk) etching velocity VG for the etchant used under the given etching conditions such as the type, molarity, time of etching and the temperature of the etchant.
• The characteristics of the charged particle such as the mass, charge and energy of the particle interacting with the medium, signified by the track etching velocity VT along the trajectory of the particle. Consider the simplest case of normal incidence. (Equation 4.10) how the surface material is removed at the same etching time t that the etched track is developing, so that all of the directly observable quantities, the track diameter d and the visible track length L, are the result of the competition between the effects of VG and VT.
The track length L is given by [23]:

To find the diameter d, the angle δ can be defined from the triangle (ABO) therefore
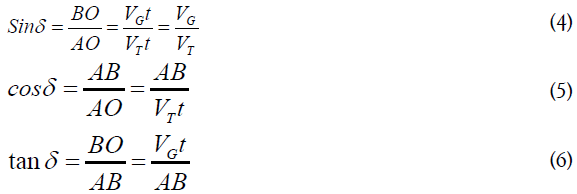
To determine AB, from the triangle (ABO)
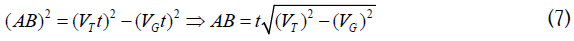
After substituting equation (4.6) in (4.5), one can get:
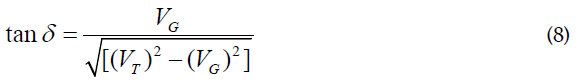
The angle δ can be defined from the triangle (ACD), where:
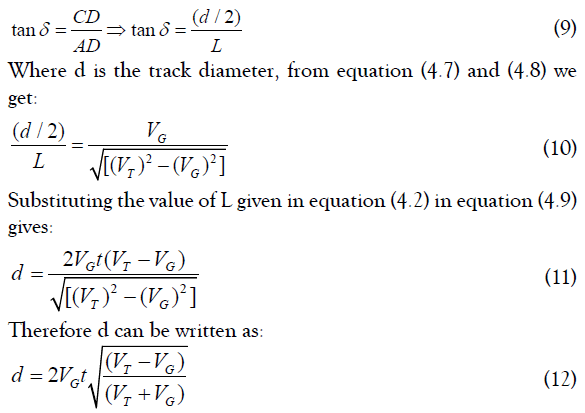
The above expression can be used to find the diameter of the track (d) at any stage.
The link between lung cancer and radon exposure remains apparent in light of present knowledge. Experiments have shown that ionising radiation can cause cancer in bronchial epithelial cells. Our work is part of a series on the nature of radon gas and the dangers of rising concentrations. This experiment also highlighted the issue of people misinterpreting the issue of radon exposure and mistaking the symptoms of radon exposure for symptoms of carbon monoxide poisoning.
Citation: Salman EF, Murad AK (2021) Radioactivity: Radon Gas, its Properties and the Risks of Increasing its Concentration. J Phys Chem Biophys. 11:294.
Received: 02-Jun-2021 Accepted: 16-Jun-2021 Published: 23-Jun-2021 , DOI: 10.35248/2161-0398.21.11.294
Copyright: © 2021 Salman EF, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.