Andrology-Open Access
Open Access
ISSN: 2167-0250
ISSN: 2167-0250
Research Article - (2022)
Gonadotropins, as follicle-stimulating hormone (FSH) and luteinizing hormone (LH), belong to a family of glycoprotein hormones along with human placental chorionic gonadotropin (hCG), and thyroid-stimulating hormone (TSH). LH and FSH are key regulators of reproduction, and they act in an endocrine manner to regulate steroidogenesis and gametogenesis in the ovary and testis. Nowadays, highly purified and recombinant formulations of the gonadotropins are commonly used in the treatment of hypogonadism and infertility. Therefore, the accurate measurement and characterization of serum gonadotropins is essential to monitor the hormonal response of patient treatment, but their absolute quantification is a great challenge due to their extensive heterogeneity, and their very low concentrations in serum. The reference method to quantify circulating gonadotropins, the enzyme-linked immunosorbent assay (ELISA), is limited by the availability of high-quality antibodies for each biomarker candidate and to the specificity of the antigen-antibody-affinity.
The aim of this study was to set-up a valid alternative to the common immunoassays, based on tandem mass spectrometry in multiple reaction monitoring (MRM) ion mode, to quantify gonadotropins (LH and FSH) and TSH in serum. The developed method allows the identification and quantification of target proteins by monitoring 3 specific prototypic peptides (precursor ion) and 3 to 5 best fragments (product ion) for each hormone, and it was successfully applied to the analysis of sera from a small cohort of women. Results show comparable sensitivity to ELISA assays, with the advantage of being faster and more selective. Furthermore, this method could be easily implemented with other serum protein of interest saving time and cost in routine analysis.
Glycoprotein hormones; Bio-fluid; Mass spectrometry; LC-MRM/MS
Gonadotropins are a family of glycohormones that regulate ovarian and testicular function and are crucial for normal growth, sexual development and reproduction. The human gonadotropins include follicle-stimulating hormone (hFSH) and luteinizing hormone (hLH), which are synthesized and secreted by the gonadotropic cells of the anterior pituitary gland, and chorionic gonadotropin (hCG), which is produced by trophoblast cells of the developing embryo. Such gonadotropins are heterodimeric proteins that consist of two peptide chains, the alpha chain common to all mentioned proteins, whereas the beta chain is unique and determines the receptor specificity and function [1].
The amount of all gonadotropins and secretion rate varies widely through life and during the various phases of female menstrual cycle. Altered levels of these hormones in the course of life for women and men could indicate the onset of many diseases or problems on the reproductive system [2]. Due to their essential role in reproductive system, highly purified and recombinant formulations of the gonadotropins have been developed and used in the treatment of hypogonadism and infertility. Actually, hormonal stimulation is an essential part of modern assisted reproductive technology (ART) [3]. By stimulating folliculogenesis, these hormones increase the number of oocytes or embryos to be used for ART. The determination of serum gonadotropin values is fundamental both to evaluate the functional state of the hypothalamic-ovarian axis in women, and a gonadotropin deficiency in men. Numerous studies have been conducted on female and male infertility [4,5] defined by decreasing levels of gonadotropins [2,6]. The evaluation of the various causes of anovulation can be complex and the monitoring of body weight and height, serum FSH, LH, prolactin, thyroidstimulating hormone (TSH), and androgens can be of help in the diagnostic process. The progestin dosage may be helpful to evaluate the degree of hypogonadism and may help to guide treatment choices. TSH is another important glycohormone produced by pituitary gland, invoved in hormone production and metabolism.
The monitoring of gonadotropins and TSH levels in sera samples is a common clinic practice, but their absolute quantification is a great challenge due to their extensive heterogeneity, and their very low concentrations in serum. In fact, the secretion of FSH and LH is very low before puberty; following puberty, more gonadotropins are secreted; during the menstrual cycle there is a dramatic increase of FSH and LH serum concentrations in ovulation phase, and the secretion of these hormones increases 10- to 15-fold in postmenopausal women (Table 1) [1]. Also, the TSH can vary widely based on age, sex, and stage of life. The typical range of reference for TSH levels is between 0.45 to 4.12 milliunits per liter (mU/L) for both men and women [7-10].
| Sex | Time of Life | FSH normal levels (mIU/mL) | LH normal levels (mIU/mL) |
|---|---|---|---|
| Male | Prepubertal | 0-5 | 0,3-6 |
| Pubertal | 0,3-10 | - | |
| Adult | 1,5-12,4 | 1,8-12 | |
| Female | Prepubertal | 0-4 | 0-4 |
| Pubertal | 0,3-10 | 0,3-31 | |
| Adult (follicular phase) | 1-11 | 01-18 | |
| Adult (Mid-Cycle) | 1-26 | 20-105 | |
| Adult (luteal phase) | 5-15 | 0.4-20 | |
| Menopausal | 25,8-134,8 | 15-62 |
Table 1: Normal levels of FSH, follicle-stimulating hormone, and LH luteinizing hormone.
Since exogenous FSH and LH are used clinically both to induce ovulation in women as part of ART treatments and to treat infertility due to gonadotropins deficiency in men, the accurate measurement and characterization of serum gonadotropins is essential to monitor the hormonal response of patient to the performed stimulation and to get a clear diagnosis. Nowadays, enzyme-linked immunosorbent assay (ELISA) is the reference method to quantify circulating gonadotropins. Although ELISA tests are successfully used in diagnostics for the detection of many proteins, this method is limited by the availability of high-quality antibodies for each biomarker candidate and to the specificity of the antigen-antibody-affinity [11]. An alternative to ELISA is mass spectrometry (MS)-based targeted protein assays, providing the use of liquid chromatography (LC)-MS/MS tandem in multiple reaction monitoring (MS-MRM) ion mode. Previous studies have demonstrated the robustness, accuracy and precision of MRMMS based analytical methodology for protein quantification [12-15]. Furthermore, the MRM-MS approach has the advantage of identifying and quantifying multiple proteins in a single run, saving time and costs. MRM-MS improves the sensitivity performances of tandem mass spectrometry by monitoring predetermined m/z precursor ions and m/z of specific fragment ions, allowing the detection of low abundant proteins in a very complex matrix; the detection limit of MRM ion mode for peptides decreases up to 100-fold (compared to full scan MS/MS analysis) due to rapid and continuous monitoring of specific ions of interest. The goal of this study was to set-up an analytical method based on tandem mass spectrometry in MRM ion mode, by using the external standard method to quantify circulating target proteins, e.g. FSH, LH and TSH hormones in 14 serum samples.
Chemicals and reagents
FSH, LH and TSH standards were purchased from Sigma-Aldrich. Dithiothreitol (DTT), ethylene diamine tetra acetate (EDTA), trypsin, iodoacetamide (IAM) and ammonium bicarbonate (AMBIC) were purchased from Sigma-Aldrich. Formic acid (HCOOH), methanol and acetonitrile (ACN) are from J.T. Baker. Water (H2O) and Stage Tip C18 47 mm were purchased from Sigma-Aldrich (Saint Louis, USA). 14 serum samples were supplied by Istituto Nazionale Tumori (INT) “Fondazione G. Pascale” of Naples.
MRM-MS method development
Sample treatment: Standard proteins (10 μg) were subjected to in solution hydrolysis protocol by using trypsin as hydrolytic enzyme. Reduction and alkylation were carried out before tryptic hydrolysis by using 10 μl of DTT (100mM) and incubated at 60°C for 1 h; then, 10 μL of IAM (100 mM) was added to perform carboamidomethylation. The mixture was then incubated in the dark at room temperature for 1 h. Protein digestion was carried out using trypsin at a 50:1 (protein: enzyme) mass ratio in 10 mM AMBIC. The sample was incubated at 37°C for 16 h. The sample clean-up was performed by using homemade C18 reverse phase chromatography. Samples were dried under vacuum and suspended in 50 μL of 98% H2O/2% ACN/0.1% HCOOH to perform the MRM-MS analysis. Standard FSH, LH and TSH tryptic peptides solutions were prepared by 8 serial dilutions from stock solutions and used for calibration curves (0.002, 0.02, 0.2, 1, 2, 10, 20, 40 ng/μL). 10 μL of each serum sample was used for the same protocol.
LC-MRM/MS analysis: Skyline software (3.7, 64-bit version MacCoss Lab Software, University of Washington, USA) was used for the in silico selection of peptides with unique sequence for each target protein. Prototypic fully tryptic peptides, with no missed cleavages, with a length ranged from 5 to 30 amino acids and devoid of methionine and cysteine residues, were chosen for MRM assays development. In addition, sequences that may cause incomplete digestion, such as continuous sequences of arginine (R) or lysine (K) and a proline (P) at the C-terminal side of R or K were also excluded since partial tryptic hydrolysis at the peptide bond is often observed in MS/MS. For each peptide, m/z precursor ion, m/z product ions (related to the top 3 or 5 transitions) and relative collision energy were provided by Skyline. Peptide mixture was analysed by LC-MS/MS analysis using a Xevo TQ-S (Waters) equipped with an Ion-Key UPLC Micro flow Source coupled to an UPLC Acquity System (Waters).
LC-MRM/MS method: 1 μL peptide mixture was injected and separated on an iKey Peptide BEH C18 Separation Device, 130 Å, 1.7 μm, 150 μm × 50 mm (Waters, Milford, MA, USA) at 45°C with flow rate of 3 μl/min using 0.1% HCOOH in water (LCMS grade) as eluent A and 0.1% HCOOH in ACN as eluent B. Peptides were eluted (starting 1 min after injection) with a linear gradient of eluent B in A from 7% to 95% in 55 min. The column was re-equilibrated at initial conditions for 4 min. The MRM mass spectrometric analyses were performed in positive ion mode; the duty cycle was set to automatic and dwell times were minimal 5 ms. Cone voltage was set to 35 V.
Method validation limit of detection and quantitation: The limits of detection (LODs) were defined as the lower limit of concentration below which the sample could not be revealed and were determined by making 5 replicate measurements of blank samples. Calculations were made according to the following formula:
LOD=3 × SD
Where SD was the standard deviation of the Blank.
The limit of quantification (LOQ) is the lowest concentration at which the analyte can not only be reliably detected but also quantitated. The LOQ may be equivalent to the LOD or higher. LOQ was determined as the analyte concentration giving a signal to noise ratio (S/N) of 3 times higher than the LOD. Possible matrix effects were evaluated by comparing standard and matrix matched calibration curves for each analyte. Standard solutions were prepared as described before. The calibration curves were repeated three times. Matrix effects were evaluated by comparing five points’ standard and matrix-matched calibration curves.
Serum FSH, LH and TSH absolute quantification by MRM/MS based method
The aim of this work was to setup an analytical method based on tandem mass spectrometry in MRM ion mode, to quantify in a single analysis FSH, LH and TSH in sera samples. Since gonadotropins and thyrotropin have an identical α-polypeptide chain, for the development of the MRM method, only peptides belonging to the β chain for each hormone were selected. Thanks to skyline software, the best precursor ion-product ion transitions and the relative collision energies were selected for each prototypic peptide in order to develop the MRM method (Table 2).
| Protein | Peptide | Peptide β- Chain values | Precursor Ion (m/z) | Product Ion (m/z) | CE |
|---|---|---|---|---|---|
| β-LH | R.LPGCPR.G | 83,88 | 350.18++ | 586.27+ | |
| 489.22+ | 12 | ||||
| 432.20+ | |||||
| R.STSDCGGPK.D | 110,118 | 454.69++ | 821.34+ | 16 | |
| 720.29+ | |||||
| 633.26+ | |||||
| 518.24+ | |||||
| β-TSH | K.YALSQDVCTYR.D | 64,74 | 688.31++ | 1028.44+ | 24 |
| 941.41+ | |||||
| 813.35+ | |||||
| 698.33+ | |||||
| 599.26+ | |||||
| K.LFLPK.Y | 59,63 | 309.20++ | 504.31+ | 10 | |
| 357.24+ | |||||
| 244.16+ | |||||
| β-FSH | R.DLVYK.D | 53-57 | 319.18++ | 552.32+ | 11 |
| 409.24+ | |||||
| 310.17+ | |||||
| 491.25+ | |||||
| K.DPARPK.I | 58-63 | 342.19++ | 568.35+ | 12 | |
| 471.30+ | |||||
| 400.27+ | |||||
| K.ELVYETVR.V | 72-79 | 504.77++ | 879.49+ | 18 | |
| 766.40+ | |||||
| 667.34+ |
Note: ++: Precursor Ion,+: Product Ion
Abbrevations: FSH: Follicle-Stimulating Hormone, LH: Luteinizing Hormone, TSH: Thyroid-Stimulating Hormone,
Table 2: Best precursor ion (m/z) → product ion (m/z) transitions and the relative collision energy (CE) selected for each prototypic peptide by Skyline software.
Quantitative analysis: External standard method
The quantitative analysis has been performed by the external standard method by using a mixture of protein standards. The standard protein was treated as reported in previous paragraph (sample treatment). Solution at known concentration of peptide mixture were analysed in triplicate to build up the calibration curves. The analysis was performed in triplicate for each point of the calibration curves to evaluate the reproducibility of the developed method. The coefficients of determination (R2) were greater than 0.99, and the calculated CV values were under the 10%. The analytical parameters (LOD, LOQ and recovery values) for the target protein were evaluated by y-intercept method as reported by Pinto, et al. and reported in Table 3 [16]. Moreover, precision was expressed as RSD% for each point of the calibration curve (Table 4).
| Linearity Range | LOD (pg/µL) |
LOQ ng/μL |
m | q | R2 | |
|---|---|---|---|---|---|---|
| FSH | 20 pg/μL-40 ng/μL | 20 | 60 | 4000000 | 504744 | 0.9964 |
| LH | 20 pg/μL-40 ng/μL | 20 | 60 | 10688 | -381767 | 0.9975 |
| TSH | 100 pg/μL-10 ng/μL | 100 | 300 | 6608 | -197594 | 0.998 |
Abbrevations: FSH: Follicle-Stimulating Hormone, LH: Luteinizing Hormone, LOD: Limit Of Detection, LOQ: Limit Of Quantitation, TSH: Thyroid-Stimulating Hormone
Table 3: Analytical parameters.
| Concentration ng/mL | RSD% | ||
|---|---|---|---|
| FSH | LH | TSH | |
| 2 | 22.61 | - | - |
| 20 | 0.41 | 15.20 | - |
| 100 | - | - | 12.01 |
| 200 | 1.17 | 0.33 | 0.67 |
| 500 | - | - | 1.12 |
| 1000 | 1.55 | 0.76 | 1.55 |
| 2000 | 0.05 | 0.88 | - |
| 5000 | - | - | 1.23 |
| 10000 | 4.35 | 0.60 | 1.01 |
| 20000 | 1.70 | 1.10 | - |
| 40000 | 1.76 | 1.56 | - |
Abbrevations: FSH: Follicle-Stimulating Hormone, LH: Luteinizing Hormone, RSD: Relative Standard Deviation, TSH: Thyroid-Stimulating Hormone.
Table 4: RSD% calculated for each calibration curve point.
This method showed detection and quantification limits comparable to ELISA assays [17]. Moreover, to define the recovery in matrix, a standard solution of each target protein was prepared by spiking known amount of standard in mice pooled sera. Recovery value results ranged between 75%-90%, hence this strategy has demonstrated high reproducibility and linearity.
Application to real samples
In order to reduce the biological variability and evaluate the instrumental response, five pools of sera were analysed. Once the instrumental response and biological variability were assessed, 14 sera were treated with the same protocol and subjected to LCMRM/ MS analysis. The Table 5 showed the hormonal values obtained for each sample analysed. The variability settles around 10% in the determination of all proteins in serum and for most of the samples the LH values proved to be below the LOD. Although the clinical history of patients was not known, at least in two samples (serum #21 and #44) elevated value of TSH and low values of LH and FSH were observed, indicating a hypothetical condition of hypothyroidism, as well as FSH high values recorded for serum #3 suggested polycystic ovary syndrome. Such hypotheses should be studied more thoroughly by cross-referencing integrative analyses with the recording of patients' clinical history to obtain a clearer diagnosis and a more complete understanding of infertility conditions [18].
| Sample | FSH (ng/μL) |
LH (ng/μL) |
TSH (ng/μL) |
|---|---|---|---|
| Serum #12 | 0.96 ± 0.05 | 0.57 ± 0.03 | 0.15 ± 0.01 |
| Serum #18 | 0.67 ± 0.03 | <LOD | 0.12 ± 0.01 |
| Serum #2 | 0.87 ± 0.04 | 0.28 ± 0.02 | 0.12 ± 0.01 |
| Serum #21 | <LOD | <LOD | 0.67 ± 0.03 |
| Serum #23 | 0.05 ± 0.003 | <LOD | 0.53 ± 0.03 |
| Serum #25 | 1.53 ± 0.08 | 0.62 ± 0.04 | 0.21 ± 0.01 |
| Serum #28 | 2.13 ± 0.11 | <LOD | 0.21 ± 0.01 |
| Serum #29 | 0.66 ± 0.03 | 0.01 ± 0.001 | 0.15 ± 0.01 |
| Serum #3 | 4.8 ± 0.24 | <LOD | 0.53 ± 0.03 |
| Serum #31 | 1.34 ± 0.07 | 0.03 ± 0.002 | 0.14 ± 0.01 |
| Serum #32 | 0.02 ± 0.001 | <LOD | 0.68 ± 0.04 |
| Serum #40 | 1.06 ± 0.05 | <LOD | 0.22 ± 0.01 |
| Serum #44 | <LOD | <LOD | 1.94±0.12 |
| Serum #48 | 0.53 ± 0.03 | <LOD | 0.12 ± 0.01 |
Abbrevations: FSH: Follicle-Stimulating Hormone, LH: Luteinizing Hormone, LOD: Limit Of Detection, TSH: Thyroid-Stimulating Hormone
Table 5: FSH quantification in the analyzed serum samples.
Finally, more samples are needed to define a normal range of physiological dosages of both men and women in fertile age to better detect pathological conditions. On the other hand, this method allows the quantification of the three target proteins in a single analysis lasting 60 min, bypassing the problems linked to the complexity of the biological matrix, the high sequence homology in the dimers and the presence of numerous glycoforms.
Matrix effect
In order to define the recovery in matrix, a standard solution of each target protein was prepared by spiking known amount of standard in mice pooled sera. The amount of spiked standard proteins is at least 3-4 orders of magnitude higher than the human endogenous one, therefore, the contribution of the endogenous FSH, LH and TSH of the mice serum can certainly be assumed to be zero. Recovery is calculated by comparing the area of extracted peptide yield before and after the addition of the different FSH, LH and TSH standard solution into the mice pooled sera. In Figure 1 was reported as an example the XIC of FSH 72-79 K.ELVYETVR.V peptide monitored in serum not spiked, 0.5 μg/μL and 1 μg/μL spiked samples. Quantitation was achieved by using the calibration curves. Recovery value ranged between 75-90%.
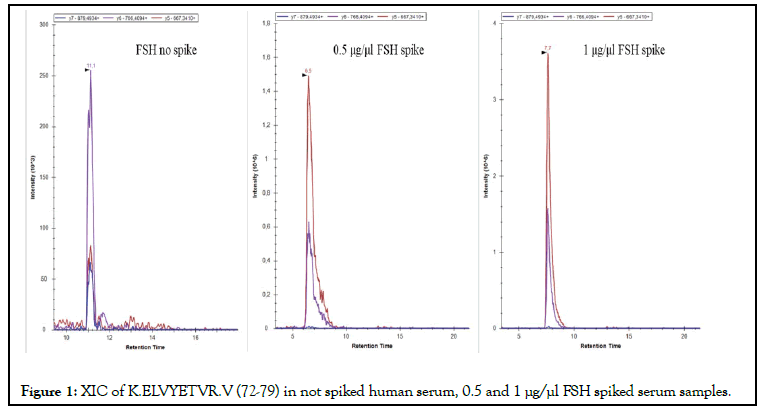
Figure 1: XIC of K.ELVYETVR.V (72-79) in not spiked human serum, 0.5 and 1 μg/μl FSH spiked serum samples.
Instrument assessment
Pooling of samples in proteomics experiments might help to overcome resource constraints when many individuals are analysed (as the measured biological variation should be reduced). The pools were prepared by taking 2 μL from each 14 sera and treated with the same protocol described in material and method section. For each pool, 1 μL of the obtained peptide mixture was analysed with the developed MRM method. As an example, the XIC for the quantifier FSH peptide of the #1 pool was reported and the concentration of target protein was evaluated. Figure 2 showed the XIC profile for K.ELVYETVR.V [72-79], quantifier peptide of FSH β-chain. The transitions 504.77++ → 667.34+; 504.77++ → 766.40+; 504.77++ → 879.49+ were presented in red, violet end blue, respectively, and transition peaks were co-eluted at 12.6 min. By integrating the quantifier’s areas underlying the peaks on the calibration lines, the concentration of the protein target in the sera pool was measured. FSH concentrations in sera pool #1 resulted to be 0.23 ng/mL.
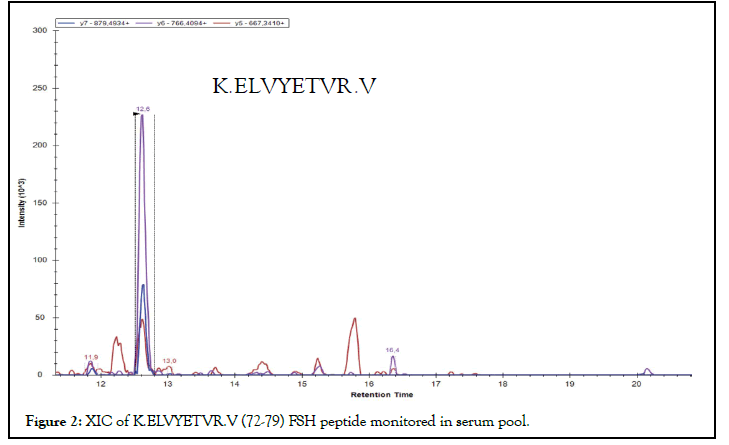
Figure 2: XIC of K.ELVYETVR.V (72-79) FSH peptide monitored in serum pool.
Tandem mass spectrometry in MRM mode is proposed as an alternative and complementary method to immune-enzymatic assays for the quantification of hormonal proteins contained in very low amount in biological fluids, thanks to the comparable sensitivities (<1 ng/mL) and the high selectivity and specificity. In this study, an MRM-MS method to simultaneously quantify FSH, LH and TSH in serum sample was developed. The method involves the identification and quantification of target proteins by monitoring 3 specific proteotypic peptides (precursor-ion) and 3 to 5 best fragments (product-ion) for each hormone, and it was successfully applied to the analysis of 14 sera samples from women of different ages, showing a great variability of the amount of the hormone. Results show comparable sensitivity to ELISA assays, with the advantage of being faster and more selective. Furthermore, this method can be easily implemented for a simultaneous analysis of several other serum proteins. These aspects make the MRM-MS technique advantageous in terms of time and cost.
[CrossRef][Google Scholar] [PubMed]
[CrossRef][Google Scholar] [PubMed]
[CrossRef][Google Scholar] [PubMed]
[CrossRef][Google Scholar] [PubMed]
[CrossRef][Google Scholar] [PubMed]
[CrossRef][Google Scholar] [PubMed]
[CrossRef][Google Scholar] [PubMed]
[CrossRef][Google Scholar] [PubMed]
[CrossRef][Google Scholar] [PubMed]
[CrossRef][Google Scholar] [PubMed]
[CrossRef][Google Scholar] [PubMed]
Citation: Illiano A, Melchiorre C, Cioci C, Pinto G, Rella FD, Conforti A, et al. (2022) Quantitative Analysis of Gonadotropins and Thyrotropin in Sera Samples by LC-MRM Mass Spectrometry. Andrology. S1:002.
Received: 22-Feb-2022, Manuscript No. ANO-22-15962; Editor assigned: 25-Feb-2022, Pre QC No. ANO-22-15962(PQ); Reviewed: 11-Mar-2022, QC No. ANO-22-15962; Revised: 18-Mar-2022, Manuscript No. ANO-22-15962(R); Published: 25-Mar-2022 , DOI: 10.35248/2167-0250.22.S1.002
Copyright: © 2022 Illiano, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Sources of funding : NO