Clinical Pediatrics: Open Access
Open Access
ISSN: 2572-0775
ISSN: 2572-0775
Research Article - (2023)Volume 8, Issue 2
Ethno pharmacological relevance: Qingfei Tongluo formular (QT) is a homemade agent for the Mycoplasma Pneumonia (MPP) treatment developed by pediatrics of Shanghai Longhua hospital, which combining with azithromycin has better clinical curative effect. This study aims at exploring the chemical components and clarifying the possible mechanism involved in.
Materials and methods: LC-MS was used for chemical analysis. The mycoplasma pneumoniae infected mice were treated with AZM and QT (46.25 and 92.5 mg/g) for 3 days. Polymorph nuclear neutrophils (PMNs) and monocytes in Bronchoalveolar Lavage Fluid (BALF) were measured by haemacytometer. IL-6, IL-10, IL-1β and IL-13 in BALF were identified by ELISA assay. HE staining was employed for histological examination. TLR4, COX-2 and NF-κB expressions were assessed by western blot.
Results: 10 compounds were identified in QT. After QT treatment, PMNs and monocytes in BALF were reduced and release of inflammatory cytokines was also mediated. HE staining showed that QT effectively arbitrated the inflammation and embolism in lung tissue. QT also blocked the TLR4-NF-κB-COX-2 signaling.
Conclusion: QT showed favorable effect in MPP treatment by diminishing the inflammation symptoms and regulating the TLR4-NF-κB-COX-2 signaling, which could act as a new agent for MPP therapy.
Pneumonia; Mouse; Embolism; TLR4; NF-κB; Signaling
Mycoplasma Pneumoniae (MP) is one of the main pathogens of community-acquired pneumonia in children, and the incidence of Mycoplasma Pneumoniae Pneumoniae (MPP) increased gradually [1]. Resent years, MP gradually tends to be resistant to antibiotics, which leads to hard to cure and easy to recur and MPP increased year by year. Furthermore, MPP tends to cause asthma by affecting the respiratory epithelium and also can result in pulmonary fibrosis [2]. Therefore, it is urgent to explore effective prevention and treatment for pediatric MPP from perspective of Traditional Chinese Medicine (TCM).
Qingfei Tongluo formular (QT) is a homemade agent for the MPP treatment developed by pediatrics of Shanghai Longhua hospital, which has better clinical curative effect. Qingfei Tongluo formular contains cortex mori from Morus alba L. (9 g), cortex lycii Lycium chinense Mill. (9 g), peach kernel from Prunus persica L. (9 g), aidicha from Ardisia japonica (Thumb.) Blume (9 g), lumbricus from Pheretimaas pergilum (E Perrier) (9 g), almond from Amygdalus communis Vas (9 g), perillaseed from Perilla frutescens L. Britt. (9 g), semen lepidii from Heleocharis dulcis (Burm. f.) Trin. (9 g) and liquorice from Glycyrrhiza uralensis Fisch (3 g) [3]. From our previous studies, we found that the clinical cure rate was 94.67% by QT combining with azithromycin treatment [4]. Current years, TCM research towards MPP mainly focuses on the introduction of clinical experience, while studies based on animal models and the mechanisms involved in is imperative to carry out.
In this study, a BALB/c mouse model of MP infection was established and employed for studying the curative effect of QT on the MPP, the chemical constituents of QT were also identified.
Preparation of MP extracts
MP, ATCC15531 strain (American Type Culture Collection, Rockville, MD) was cultured in modified Hayflick medium (GZBIOTEST Co., Ltd, Guangdong, China) containing PPLO broth, horse serum, 25% yeast extract, which was added with penicillin G (1000 u/ml), thallium acetate (0.025%), glucose (0.5%) and phenol red (0.002%), pH=7.6. MP was cultured at 37°C under 5% CO2 for 7 days.
Qingfei tongluo formular water extracts
Qingfei Tongluo formular and was purchased from Longhua hospital affiliated to Shanghai University of traditional Chinese medicine. All medical materials were decocted by boiling in distilled water to obtain QT water extracts, and water extracts was adjusted to 3.7 g/ml.
Animals
BALB/c mice (3-week-old, 15 ± 1 g) were purchased from Shanghai sippr bk laboratory animals Co. Ltd. (Shanghai, China) and fed with feedstuff and water in a specific pathogen free (SPF) environment. All mice were with ether anesthesia and divided into five groups as control, model, AZM, QT (46.25 mg/g) and QT (92.5 mg/g) randomly. Mice in control group were treated with 100 μl normal saline by nasal drip [5]. Model, AZM, QT (46.25 mg/g) and QT (92.5 mg/g) groups were given with nasal drops containing 100 μl MPFH (1 × 107 ccu/ml) for 2 days. Control and model groups were given by gavage once a day with 0.25 ml/20 g normal saline. AZM, QT (46.25 mg/g) and QT (92.5 mg/g) groups were given by gavage with 46.25 mg/g AZM (Pfizer, New York, USA, 1164096), 46.25 mg/g QT water extracts and 92.5 mg/g QT water extracts 3 days, respectively. Ethical approval for the study was provided by the independent ethics committee, Shanghai University of traditional Chinese medicine.
LC-MS analysis of QT
An Agilent 1100 HPLC system, equipped with a quaternary pump, an autosampler, a degasser, an automatic thermostatic column compartment , a DAD and an LC/MSD Trap XCT ESI mass spectrometer (Agilent Technologies, MA, USA), was used for the separation. The separation was performed on a GS-120-5-C18-BIO chromatographic column (5 mm, 250 × 4.6 mm i.d.) with the column temperature set at 35°C. A linear gradient elution of A (0.1% formic acid water) and B (acetonitrile) was used with the gradient procedure as follows: 0 min, B 5%, to 60 min B 40% (v/v). The flow rate was 1.0 ml/min and the injection volume was 10 mL. DAD was on and the target wavelength was simultaneously set at 210 nm. The split ratio to the mass spectrometer was 1:3. The acquisition parameters for negative ion mode were: collision gas, ultra high-purity helium (He), nebulizer gas (N2), 35 psi, drying gas (N2), 10 l/min, drying temperature, 350°C, HV, 3500 V, mass scan range, m/z 100-2200, target mass, 500 m/z, compound stability, 100%, trap drive level, 100%. All the data were analysis by Chemstation software.
Inflammatory cells
Leukocyte recruitment to alveoli was determined in the Broncho Alveolar Lavage Fluid (BALF). Briefly, animals were sacrificed under ether anesthesia and trachea was exposed and intubated with a catheter, and then repeated 1 ml injections of PBS were made until a total of 3 ml of BALF was recovered. BALF was centrifuged at 3,400 × g for 10 min, and supernatant was frozen at -80°C until analysis of inflammatory mediators. Cells in the pellet were resuspended in PBS for quantification of leukocytes with a haemacytometer, and cell populations were enumerated from Diff-Quik Stain kit (Thermo Fisher Scientific Inc.).
Histological examination
Inferior lobe of right lung isolated on the 3th day was fixed with 4% paraformaldehyde, embedded with paraffin and cut into slices for HE staining. Morphometric analysis was performed by optical microscope (LEICA DMLB, Germany).
Elisa
Cytokine (IL-6, IL-10, TNF-α and IL-13) was measured by ELISA assay. Briefly, lung homogenates were lysed in lysis buffer pH 7.4 which is consisted of 300 mM NaCl/L, 15 mM TRIS/L, 2 mM MgCl2/L, 2 mM Triton X-100/L, 20 ng pepstatin A/mL, 20 ng leupeptin/mL, and 20 ng aprotinin/mL. Then lung homogenates were centrifuged at 1500 ×g for 15 min at 4°C; the supernatant was frozen at -20°C, until cytokine measurement by ELISA as per manufacturer’s protocol (Ray Biotech).
Western blot
Lung tissues were harvest and washed twice with PBS and lysed in ice-cold radio immunoprecipitation assay buffer (RIPA, Beyotime, Shanghai, China) with freshly added 0.01% protease inhibitor cocktail (Sigma, St. Louis, MO, USA) and incubated on ice for 30 min. Tissue lysis was centrifuged at 13,000 rpm for 10 min at 4°C. The supernatant (20-30 μg of protein) was run on 10% SDS-PAGE gel and transferred electrophoretically to a polyvinylidene fluoride membrane (Millipore, Bredford, USA). The blots were blocked with 5% skim milk, followed by incubation with primary antibodies. Antibodies against COX-2, TLR2 and TLR4 were purchased from Abcam. Antibodies against NF-κB, β-actin and H3 were purchased from Santa. Blots were then incubated with goat anti-mouse secondary antibody (Beyotime, Shanghai, China) or goat anti-rabbit secondary antibody (Beyotime, Shanghai, China) and visualized using enhanced chemiluminescence (ECL, Millipore).
Statistical analysis
The Graph Pad Prism 5.0 software system was employed for statistical analysis. Data are expressed as the mean ± standard error. Student‘s t test was used to compared the differences between two groups, while one-way analysis of variance was used when more than two groups were compared. P<0.05 was taken as statistical significance.
The identification of ten compounds
The aqueous extract of mixed nine Chinese medicinal materials was measured by high-performance liquid chromatography coupled with electrospray mass spectrometry (HPLC/ESI-MS) in negative-ion mode. Ten compounds 1-10, with the retention time at 12.0 min, 14.0 min, 14.7 min, 15.1 min, 17.4 min, 26.0 min, 29.7 min, 31.9 min, 48.7 min, and 51.8 min, were identified as kukoamine A (1), bergenin (2), mulberroside A (3), isorhamnetin-3-O-b-D-glucoside (4), amygdalin (5), liquiritin (6), kaempferol-7-O-b-D-glucopyranoside (7), rosmarinic acid (8), licoricesaponin G2 (9), and glycyrrhizinic acid (10), on the basis of the observation of the pseudomolecular ion peak at m/z 529.3 [M-H]-(1), m/z 326.9 [M-H]-(2), m/z 567.1 [M-1]-(3), m/z 477.1 [M-1]-(4), m/z 502.1 [M+Cl]- and 913.1 [2M-1]-(5), m/z 417.0 [M-1]-(6), m/z 446.9 [M-1]-(7), m/z 358.9 [M-1]-(8), m/z 837.4 [M-1]-(9), m/z 821.3 [M-1]-(10) in HPLC/ESI-MS chromatogram, in accordance with the molecular weights of ten compounds (kukoamine A, bergenin, mulberroside A, isorhamnetin-3-O-b-D-glucoside, amygdalin, liquiritin, kaempferol-7-O-b-D-glucopyranoside, rosmarinic acid, licoricesaponin G2, and glycyrrhizinic acid) (Figures 1a-1m and Figure 2).
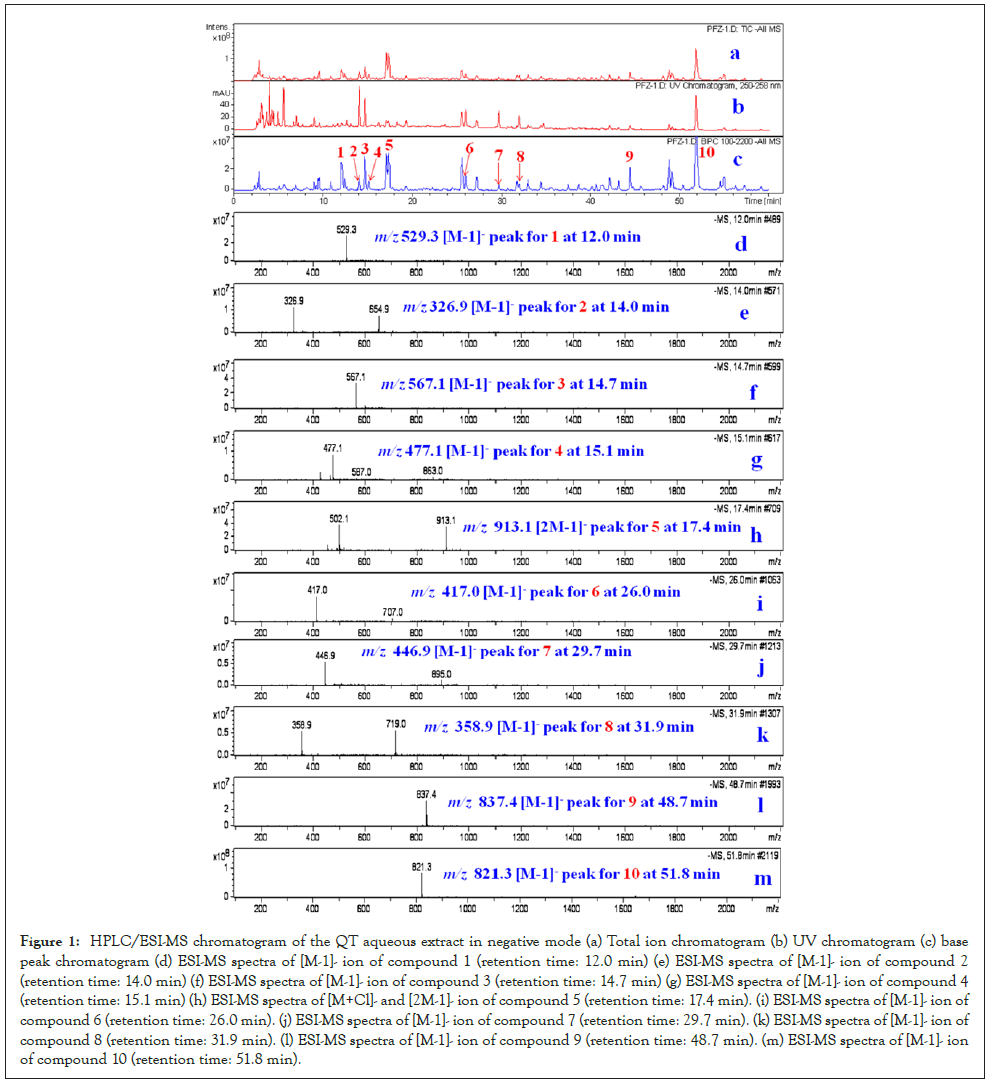
Figure 1: HPLC/ESI-MS chromatogram of the QT aqueous extract in negative mode (a) Total ion chromatogram (b) UV chromatogram (c) base peak chromatogram (d) ESI-MS spectra of [M-1]- ion of compound 1 (retention time: 12.0 min) (e) ESI-MS spectra of [M-1]- ion of compound 2 (retention time: 14.0 min) (f) ESI-MS spectra of [M-1]- ion of compound 3 (retention time: 14.7 min) (g) ESI-MS spectra of [M-1]- ion of compound 4 (retention time: 15.1 min) (h) ESI-MS spectra of [M+Cl]- and [2M-1]- ion of compound 5 (retention time: 17.4 min). (i) ESI-MS spectra of [M-1]- ion of compound 6 (retention time: 26.0 min). (j) ESI-MS spectra of [M-1]- ion of compound 7 (retention time: 29.7 min). (k) ESI-MS spectra of [M-1]- ion of compound 8 (retention time: 31.9 min). (l) ESI-MS spectra of [M-1]- ion of compound 9 (retention time: 48.7 min). (m) ESI-MS spectra of [M-1]- ion of compound 10 (retention time: 51.8 min).
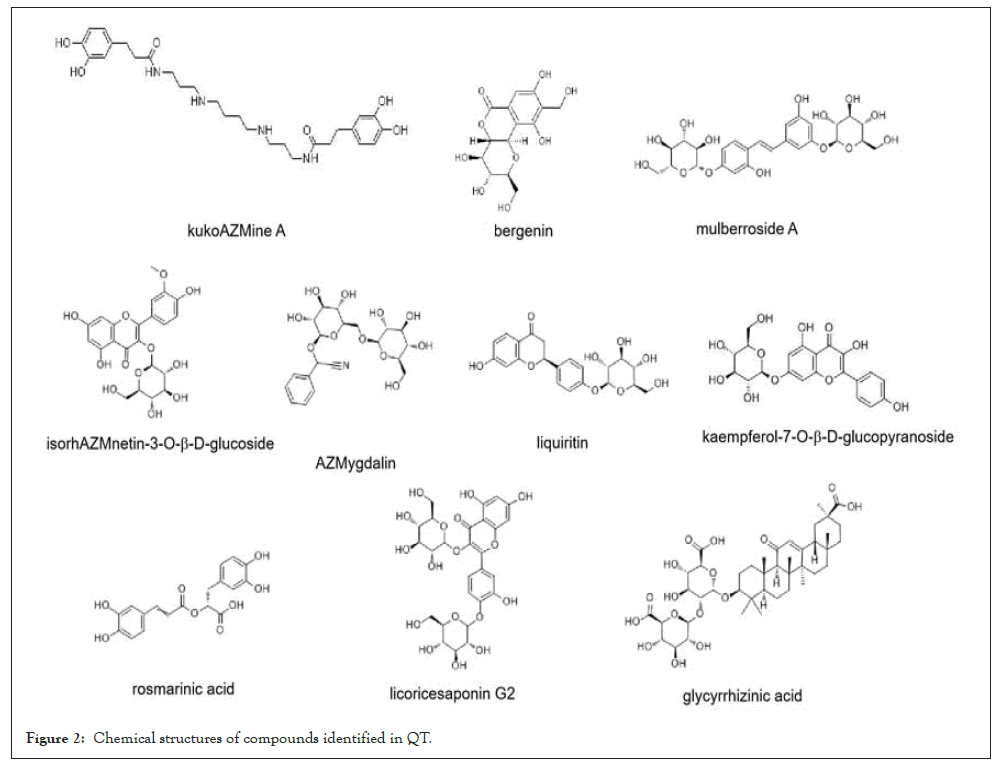
Figure 2: Chemical structures of compounds identified in QT.
Estimation of inflammatory cells in BALF
Leukocyte recruitment to alveoli was determined in the BALF. Compared to the untreated control group, MP-treated group exhibited steady drop in PMN counts in BALF. AZM, QT (46.25 mg/g) and QT (92.5 mg/g) were effective in down regulating PMN counts (Figure 3A). As for the monocyte recruitment in alveoli (BALF), a significant increase was noted in MP treated mice. A significant reduction in those cell counts was observed after AZM, QT (46.25 mg/g) and QT (92.5 mg/g) treatment compared to the model group (Figure 3B).
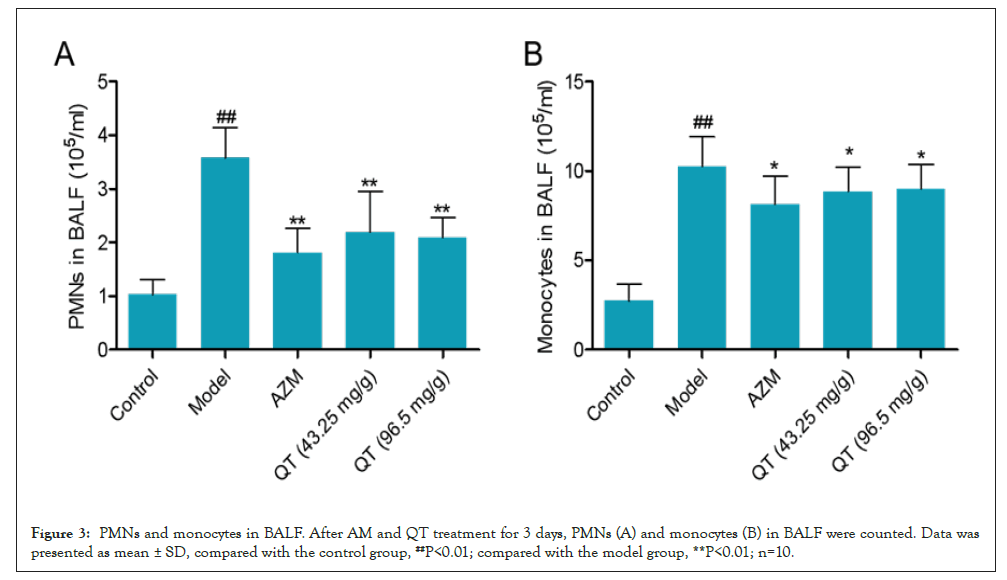
Figure 3: PMNs and monocytes in BALF. After AM and QT treatment for 3 days, PMNs (A) and monocytes (B) in BALF were counted. Data was presented as mean ± SD, compared with the control group, ##P<0.01; compared with the model group, **P<0.01; n=10.
QT mediated the cytokines in BALF
Levels of cytokines in lung homogenates were measured. An increase in the levels of cytokines as IL-6, IL-13 and TNF-α was seen in the lungs of MP treated mice, and was reduced after AZM, QT (46.25 mg/g) and QT (92.5 mg/g) treatment (Figures 4A, 4C and 4D). In addition, the lung IL-10 was after MP treatment, when compared to untreated mice. AZM, QT (46.25 mg/g) and QT (92.5 mg/g) effectively increased the IL-10 level in BALF (Figure 4B).
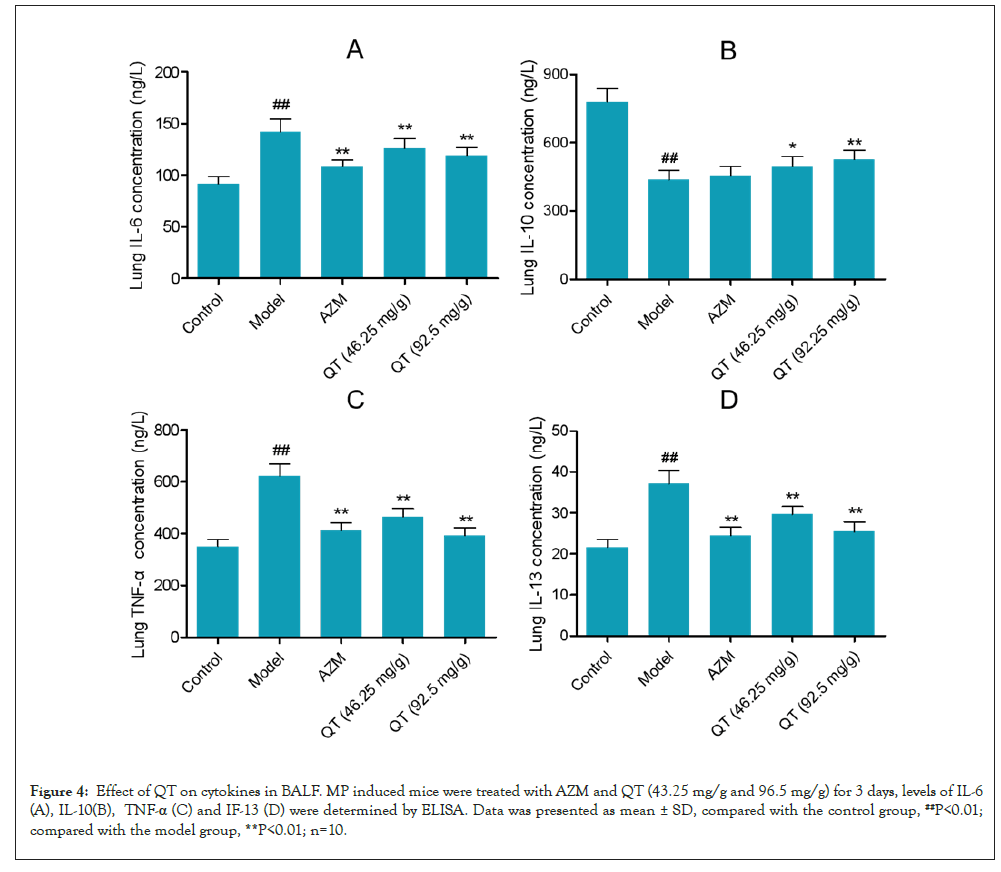
Figure 4: Effect of QT on cytokines in BALF. MP induced mice were treated with AZM and QT (43.25 mg/g and 96.5 mg/g) for 3 days, levels of IL-6 (A), IL-10(B), TNF-α (C) and IF-13 (D) were determined by ELISA. Data was presented as mean ± SD, compared with the control group, ##P<0.01; compared with the model group, **P<0.01; n=10.
Lung histopathology
To investigate the histopathological changes underlying MP induced experimental pneumonia in mice lungs and subsequent recovery from this disease state using AZM and QT. Figure 5A shows normal lung histology of mice. The sections of normal lungs shows alveoli is composed of a single layer of squamous epithelium, bronchioles are lined by ciliated columnar epithelium (larger bronchioles) or cuboidal epithelium (smaller bronchioles leading to alveoli). Between the alveoli a thin layer of connective tissue and numerous capillaries also lined with simple squamous epithelium. Figure 5B shows MP infected lung histology of mice. There are congestion and edema of pulmonary interstitial around vessels, pulmonary embolism was observed. In the segmental bronchi, lymphocytes and plasmacytes are infiltrative peripherally. The alveolar walls are thickened and damaged, and a narrowing of the bronchial tubes is seen. Figure 5C shows lung histology as a result of treatment with AZM. The inflammation was dramatically reduced, and few residual inflammatory cells were observed. The alveolar structure is still maintained, few bronchus is narrowed. Figure 5D shows histological changes in lungs of mice treated with QT (46.25 mg/g). Inflammation response is reduced. Congestion and edema of pulmonary are interstitial around vessels. In the segmental bronchi, a part of lymphocytes and plasmacytes are infiltrative peripherally. The alveolar walls are thickened, and a narrowing of the bronchial tubes is seen. In Figure 5E, QT at dose of 92.5 mg/g effectively arbitrates the pulmonary inflammation. Less congestion and edema of pulmonary are interstitial around vessels, and few lymphocytes and plasmacytes are infiltrative peripherally. Pulmonary embolism is disappeared. The alveolar structure is almost perfectively maintained (Figures 5A-5E).
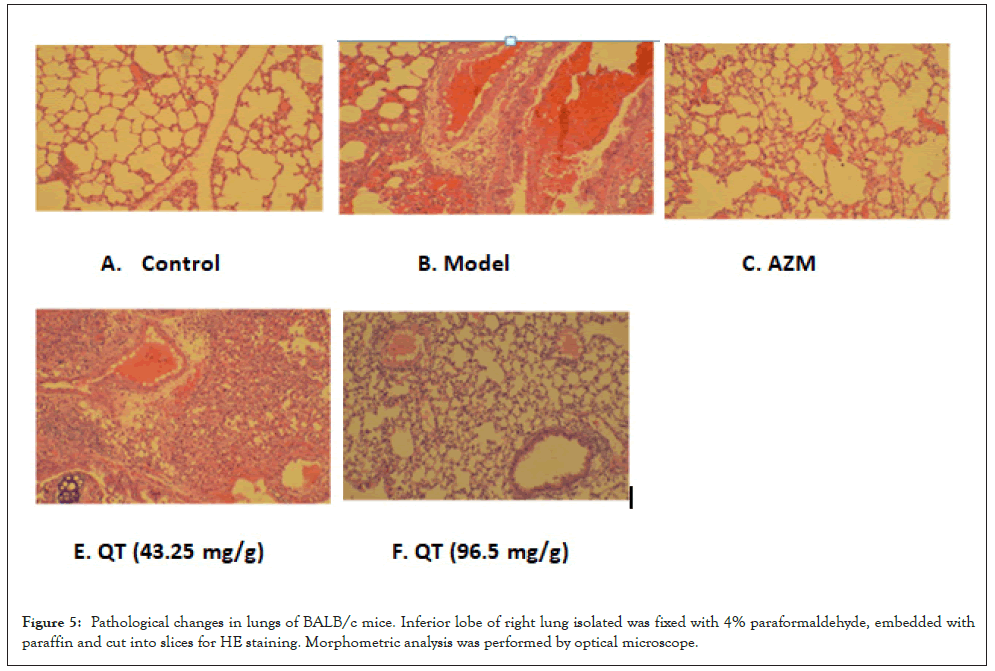
Figure 5: Pathological changes in lungs of BALB/c mice. Inferior lobe of right lung isolated was fixed with 4% paraformaldehyde, embedded with paraffin and cut into slices for HE staining. Morphometric analysis was performed by optical microscope.
Effects of QT on COX-2, TLR4 and NF-κB expression
Expression of COX-2, TLR4 and NF-κB in lung tissue of mice was identified by western blot. As shown in Figures 6A-6C, MP notably induced high expression of COX2 and TLR4 in lung tissue compared with the control group. Dramatic falls of COX-2 and TLR4 were observed in AZM, QT (46.25 mg/g) and QT (92.5 mg/g) groups (Figures 6A and 6B). NF-κB expression in cell nucleus was also measured. MP treatment induced an immediate increase of NF-κB expression in lung tissue, and AZM, QT (46.25 mg/g) and QT (92.5 mg/g) effectively reduced the NF-κB expression (Figures 6C and 6D).
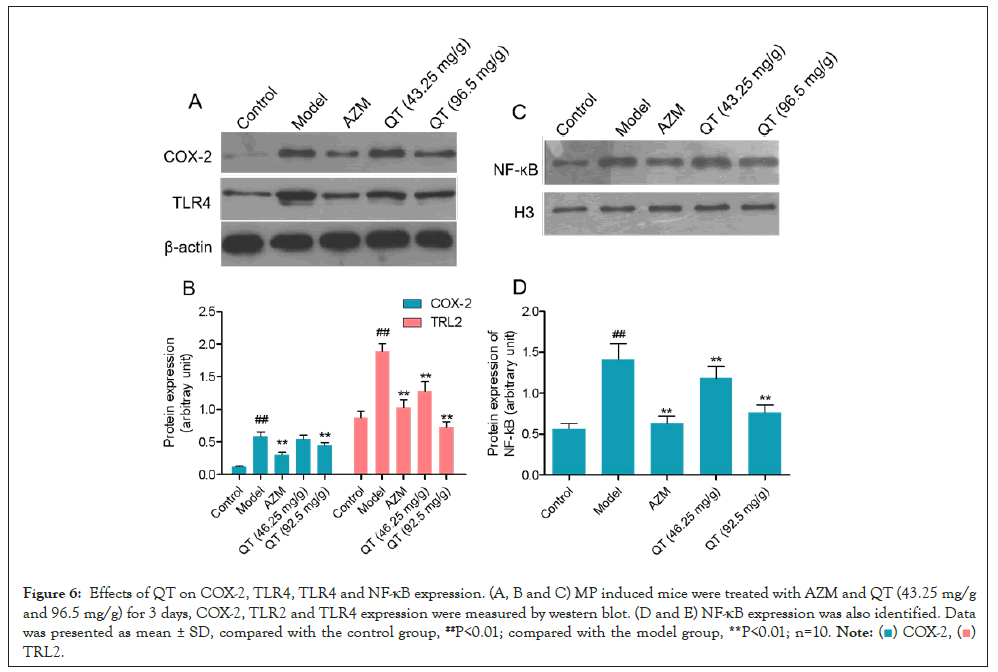
Figure 6: Effects of QT on COX-2, TLR4, TLR4 and NF-κB expression. (A, B and C) MP induced mice were treated with AZM and QT (43.25 mg/g and 96.5 mg/g) for 3 days, COX-2, TLR2 and TLR4 expression were measured by western blot. (D and E) NF-κB expression was also identified. Data was presented as mean ± SD, compared with the control group, ##P<0.01; compared with the model group, **P<0.01; n=10.
Pneumonia is identified as a common disease occurred in children, which has been listed as one of the three most serious global pediatric diseases worldwide by the World Health Organization (WHO) [6,7]. 19% of children under 5 die of pneumonia from the WHO statistics [8]. Pneumonia is an inflammatory condition of the lung affecting primarily the microscopic air sacs known as alveoli. It is usually caused by infection with viruses or bacteria and less commonly other microorganisms, certain drugs and other conditions, such as autoimmune diseases. Pneumonia presumed to be bacterial is treated with antibiotics. Pneumonia infected by mycoplasma can give rise to respiratory tract injury and other extrapulmonary complications. Researches towards MMP almost focus on exploring the physiological and biochemical characteristics of patients and treatment experience of MPP [1,8,9]. Studies on the treatment by Chinese herbs, treatment mechanism and animal model of MP infection are less reported.
QT formula containing 9 kinds of herbal medicines is a TCM compound developed by pediatrics of Shanghai Longhua hospital used for MPP treatment [3]. We previously reported that QT can effectively assist MPP children in the restoration of health, and the cure rate was 94.67% which is much higher than AZM treatment [3]. In the present study, we firstly clarified the main compounds involved in QT by LC-MS. 10 compounds involved in the QT were identified by LC-MS, which is the basis for the further study. We then successfully established the mouse model of MP infection and identified the curative effect of QT.
We established the mouse model of MPP by nasal intubation drip, and AZM and QT were employed to study the curative effect. From the results of histological examination, symptom of pneumonia the model group was the most serious, and AZM and QT effectively reduced the pulmonary interstitial inflammation. QT played better role in inflammation reduction than AM, and combination of QT and AZM provided the best. In the MP-DNA detection, QT and AT contributed equally to MP-DNA reduction, while QT + AZM showed the better effect than either of them.
PMNs and monocytes in serum directly imply the degree of inflammation symptoms and promote vascular inflammation and atherosclerosis [8]. In our study, compared with the control group, MP infection caused a marked increase of PMNs and monocytes in BALF of model group. QT can significantly reduce PMNs and monocytes in BALF.
IL-1β is a predominant cytokine and an important mediator in pulmonary inflammation and bacterial pneumonia [10]. IL-6 and IL-13 act as proinfammatory cytokines in human lung inflammation [11-13]. Our findings showed that QT reduces IL-1β, IL-6 and IL-13 in MP induced mice. IL-10 appears to be valuable for attenuating inflammatory damage to human lung [14,15]. IL-10 play important role in inhibiting the release of proinfammatory cytokines [16]. Thomas et al. demonstrated that IL-10 is an endogenous regulator of chemokine expression in acute lung inflammation [15]. Loebbermann et al. reported that IL-10 inhibited disease and inflammation in mice infected with RSV, especially during recovery from infection [16]. We chose to focus on IL-10 as a representative of cytokine in this class. In the present study, QT effectively increased the IL-10 level in BALF of MP-induced mice, which indicated that QT show attenuated effect in lung inflammation.
Toll-like receptors (TLRs) are known to recognize pathogen-associated molecular patterns and damage-associated molecular patterns that initiate intracellular cell signaling that subsequently activates an inflammatory response and recruits inflammatory cells [17]. Furthermore the TLR4-NF-κB pathway has been exploited as a target to prevent inflammation and carcinogenesis, and TLR4 signaling also induces COX-2 expression [18-20]. COX, officially known as Prostaglandin-Endoperoxide Synthase (PTGS), is an enzyme that is responsible for formation of prostanoids which promote inflammation.
From our study, TLR4, NF-κB and COX-2 expressions in MP-induced mice lung tissue were significantly down regulated by QT treatment. Altogether, QT can effectively reduce the inflammatory response by down regulating the PMNS and monocytes in BALF, mediating inflammatory cytokines and blocking the TLR4-NF-κB-COX-2 signaling, which showed serviceable effect in MPP treatment. All in all, our results indicate that QT showed favorable effect in MPP treatment by diminishing the inflammation symptoms and regulating the TLR4-NF-κB-COX-2 signaling, which could act as a new agent for MPP therapy.
The authors have declared that no competing interests exist
Yonghong Jiang experimental design, Cell experiment, analysis and interpretation of data and writing of the manuscript; Zhen Xiao, study supervision, revision of the manuscript, provide the fund for the study; Zhiyan Jiang, conception and design, revision of the manuscript; Xiaoyang Zhao, Animal experiment, acquisition of data, analysis of data; Jiajia Lv, Specimen detection, check of the manuscript and grammar and spelling; Besides, all authors read and agreed to the final manuscript.
This study was supported by National Natural Science Foundation of China Project (No. 81674024).
The research data used to support the findings of this study are available from the corresponding author upon request.
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
Citation: Jiang Y, Xiao Z, Jiang Z, Zhao X, Lv J (2023) Qingfei Tongluo Formula Attenuated Pulmonary Inflammation and Embolism in Mycoplasma Pneumonia Mice. Clin Pediatr. 8:232.
Received: 01-Feb-2023, Manuscript No. CPOA-22-20544; Editor assigned: 03-Feb-2023, Pre QC No. CPOA-22-20544 (PQ); Reviewed: 17-Feb-2023, QC No. CPOA-22-20544; Revised: 24-Feb-2023, Manuscript No. CPOA-22-20544 (R); Published: 06-Mar-2023 , DOI: 10.35248/2572-0775.23.8.232
Copyright: © 2023 Jiang Y, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.