Research - (2018) Volume 4, Issue 1
Production and Evaluation of Fruit Wine from Mangifera indica (cv. Peter)
*Corresponding Author: Ogodo AC, Department of Microbiology, Faculty of Pure and Applied Sciences, Federal University Wukari, P.M.B. 1020 Wukari, Taraba State, Nigeria Email:
Abstract
Mangifera indica (Mango) is a fruit with good nutritional attributes but has short shelf-life under the prevailing weather conditions in tropical countries like Nigeria. Therefore, production of wine from this fruit can help increase wine variety and reduce post-harvest losses. Mango fruit of the cultivar commonly known as peter was used to produce two set of fruit wines (A and B) using baker’s yeast (Saccharomyces cerevisiae) and spontaneous fermentations respectively. Exactly 2.5 kg of mango pulp was crushed using laboratory blender for each set-up and mixed with sterile distilled water (1:1 w/v). 0.5 mg/L of sodium metabisulphate was added to must A. The fruit musts were subjected to primary and secondary fermentation for 4 days and 7 days respectively. During primary fermentation, aliquots were removed from the fermentation vessel for analysis of alcohol content, pH, temperature, total solids and total acidity. The result shows increase in alcohol contents (ranging from 0.00% to 7.50%) with gradual decrease in pH (ranging from 4.06 to 3.78). There were fluctuations in temperature between 33°C to 31°C while the total acidity increased in the range of 0.21% to 0.63%. Total soluble solids decreased gradually ranging from 200Brix to 70Brix. The alcoholic content of the final basic wine were 10.5% and 8.5% for A and B respectively. The total acidity was observed to be 0.71% for wine A and 0.8% for Wine B. Sensory evaluation rated the wines acceptability as wine A>wine B and do not show any significant difference (p>0.05) except in clarity. The studies have shown that acceptable fruit wine can be produced from mango fruit (cv. peter) which can help reduce postharvest losses.
Keywords: Fruit wine; Mango; Fermentation; Baker’s yeast
Introduction
Wine, an alcoholic beverage are produced from the fermentation of fruit juices especially grape which have a chemical balance that that allows them to ferment without addition of sugar, acids, enzymes or other nutrients [1,2]. Other than grape, other fruits have been used in wine production by researchers and qualified the wine with the name of the fruit such as mango wine [3], passion fruit wine, pineapple fruit wine, watermelon fruit wine [4], banana wine [5,6] tundu wine [7], sapota fruit wine [8], pawpaw wine [9] and sweet potato wine [10]. Moreover, a combination of one or two of these fruits can be used in mixed fruit wine production. For instance, Ogodo et al. reported the production of mixed fruit wines from pawpaw, banana and watermelon using Saccharomyces cerevisiae isolated from palm wine [2]. Also, reports indicate that home-made wines have been practices with variety of fruits including apple, banana, cashew, watermelon, orange, plum, strawberry, guava, cherries, pawpaw, cucumber etc. using S. cerevisiae which converts the sugar contents of the substrate to other products as alcohol, organic acids and esters [11-13].
Mango (Mangifera indica L. ) is an important tropical fruit distributed worldwide. However, they are highly perishable, with a shelf life of 2-4 weeks at 10-15°C which limits their availability in fresh forms [14,15]. Reddy and Reddy reported that mango contains high concentration of sugar (16-18% w/v), many acids (including, malic, oxalic succinic), antioxidants, carotene, vitamin A [1]. The unripe fruit contains citric acid and are rich in starch, which are hydrolysed to reducing sugars such as sucrose, fructose and glucose during ripening. Mango juice has been recommended because of its aromatic flavour as a restorative tonic as it contains good amount of vitamin A and C which is useful in heat apoplexy. Mangoes with higher initial concentration of b-carotene are helpful as cancerpreventing agents [1,16].
In Nigeria, different mangoes are cultivated in many parts of the country, especially, the Benue State of North central Nigeria. The mangoes are fresh during the harvesting season while the surplus are wasted under the prevailing conditions of temperature and humidity as well as lack of adequate storage facilities. An alternative way of preserving surplus mangoes could be to ferment the juice to fruit wine. This will not only increase wine variety but also add to the economy of the Nation. Therefore, mature and ripe mangoes with their high composition of fermentable reducing sugars such as glucose, sucrose and fructose could serve as substrates for fruit wine production [1] using wine yeast (Saccharomyces cerevisiae ), thus transforming a perishable products to more stable and value added product [4]. Hence, the aim of the present study is to produce and evaluate fruit wines from Mango of the cultivar known as peter using Saccharomyces cerevisiae and spontaneous fermentations.
Materials And Methods
Source of materials
Ripe matured mangoes (Mangifera indica of the cultivar locally known as peter) and baker’s yeast were purchased from the new market, Wukari, Taraba state, Nigeria. The mangoes were transported with clean polythene bags into Biological Sciences Laboratory, Federal University Wukari for identification and analysis.
Preparation of mango musts
The mango musts were prepared following the method described by Ogodo et al. with slight modification . Ripe mangoes fruits were washed thoroughly with distilled water and disinfected with sterile cotton wool soaked in ethanol (70% v/v). They were then peeled and the pulp sliced using stainless steel sterile knife. Exactly 2.5 kg of the mango pulps were transferred to a clean laboratory blender previously disinfected with 70% v/v ethanol for crushing. The crushed samples were transferred to a clean white transparent bucket and mixed with distilled water (1:1 w/v). Exactly 1 g of sodium metabisulphate (Na2S2O2) was dissolve in 100 ml of distilled water and transferred to the must and stirred. The sodium metabisulphate serve as a sterilizer and prevents fermentation before the addition of the starter culture. Two set of musts were prepared (A and B). However, wine B must was prepared without the addition of metabisulphate and starter culture and fermented spontaneously.
Preparation of starter culture
The starter culture was prepared according to the method of Ogodo et al. with slight modification. Exactly 5.00 g of commercial baker’s yeast (Saccharomyces cerevisiae ) was mixed in 200 ml of mango must and stirred vigorously. 2 g of each of yeast nutrients (potassium phosphate, ammonium sulphate and magnesium sulphate) was dissolved in 100 mL distilled water and added to the mixture. The mixture was allowed to stand for three hours after which it was transferred into must A for fermentation.
Fermentation of must to wine, clarification and filtration
Fermentation, clarification and filtration of the wines were carried out following the method described by Ogodo AC et al.. Primary fermentation was initiated in wine A by the addition of the starter culture while wine B was allowed to ferment spontaneously. The primary fermentation was allowed for a period of 4 days within which the musts are stirred at 24 h interval and aliquots collected for analysis of pH, temperature, alcohol content, total dissolved solids and total acidity. After 4 days, the wines were raked in a secondary fermentation tank. Secondary fermentation was done in an air-tight container in which a tube was passed into a clean bottle containing clean water to monitor the course fermentation for a period of 10 days. After secondary fermentation, the wines were racked with minimum exposure to air and then clarified. Clarification was done using bentonite. Exactly 250 g of bentonite was dissolved in 1 litre of boiling water and stirred properly to a gel form. The mixture was allowed to stand for 24 h and then 50 g of the gel-like bentonite was added into each of the wines with vigorous stirring using a sterile glass rod to dissolve properly. A small quantity of the mixture was collected in a clean bottle and both were covered tightly and used to monitor the process of clarification. This was allowed for a period of one month. Filtration of both wines was done using muslin cloth, sieve and syphon tube sterilized by 70% alcohol. The residues were removed and filtrates were allowed to mature for a period of 3 months.
Chemical analysis of the wines
The pH and temperature were determined using pH meter (Hanna H12216-02) and analytical thermometer respectively. Total dissolved solid was determined using a hand-held refractometer. The final alcohol content of both wine were determined using the density method while the total acidity of the wines were determined by titration as described by Giri et al. [16].
Isolation of microorganisms from the fermentation broth
Microbial analysis of the fermentation broth was perform as described by Fleet [11] using nutrient agar (NA), de-Man Rogosa and Sharpe agar (MRS) and potato dextrose agar ( PDA). The nutrient agar was treated with fulcin to suppress fungi growth while the PDA was treated with chloramphenicol to suppress bacteria growth. The cultured plates were incubated at room temperature and pure cultures obtained from successive streaking. Bacteria were identified based on their colonial characteristics, microscopy, biochemical reactions and sugar fermentation tests [17,18] while fungi were identified base on cultural characteristics and microscopy [19].
Sensory evaluation
A panel of 20 judges, comprising students, academic and laboratory staff of Federal University Wukari, were involved in the evaluation process. They assessed the clarity, colour, flavour, taste, and overall acceptability of the mango wines on a 5 point hedonic scale where 1 indicated very poor and 5 indicated excellent. The panellists are familiar with all the attribute and quality of wine.
Statistical analysis
The data obtained for the various analyses were expressed as mean ± standard deviation. Paired T-Test was used to compare the data between wine A and wine B using SPSS version 16.0. Significance was set at P <0.05.
Results
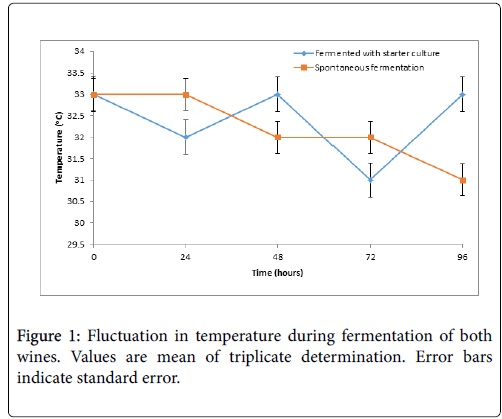
Figure 1 presents the result of the temperature variations during the period of primary fermentation. The temperature fluctuated between 33°C and 31°C for both wines (A and B) as the fermentation time increases.
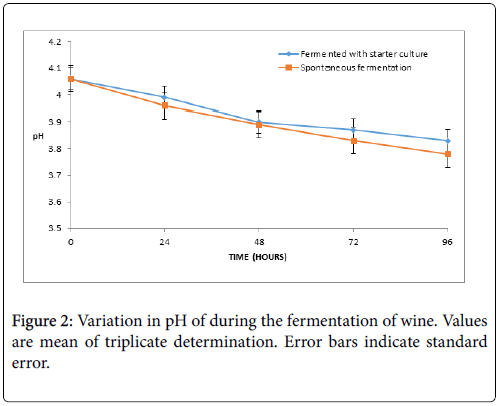
The result of the pH of the fermenting wines is presented in Figure 2. The pH decreased gradually as the fermentation time increases and ranged from 4.06 at 0 h to 3.83 at 96 h (wine A) and from pH from 4.06 (0 h) to 3.78 at 96 h (wine B). The variations did not differ significantly (p>0.05).
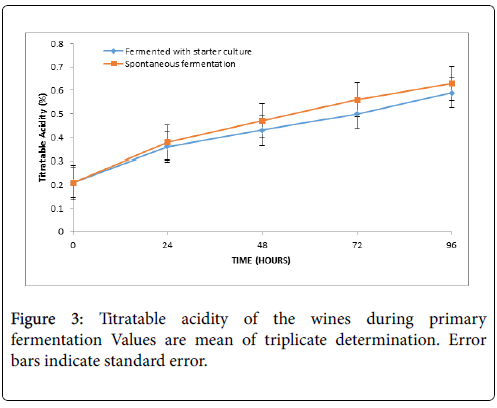
Figure 3 presents the total acidity of the wine during the primary fermentation periods. The titratable acidity increased with increasing fermentation time and ranged from 0.21% to 0.59% (wine A) and from 0.21 % to 0.63% (wine B).
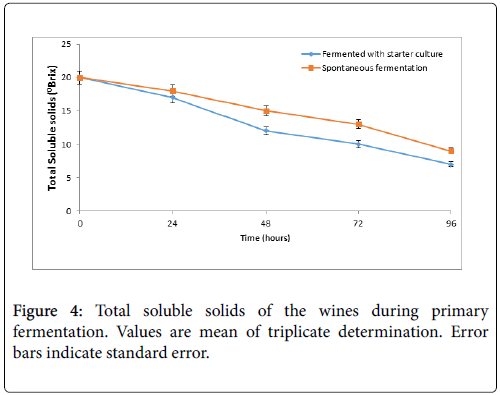
The result of the total soluble solids (°Brix) which is the sugar utilization of the yeast strain over fermentation time is shown in figure 4. The °Brix for both wines decreased with increasing fermentation time for both wines and ranged from 20°Brix to 7°Brix (wine A) and 20°Brix to 9°Brix for wine B.
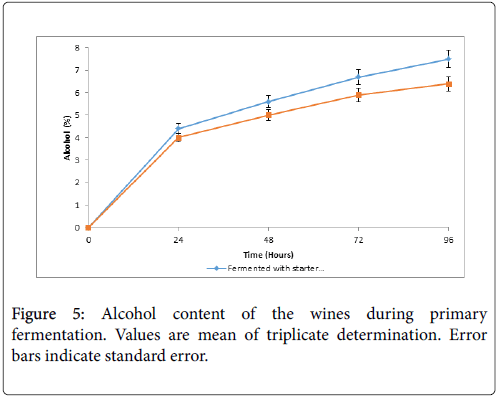
Figure 5 shows the alcoholic content of the wines which increased with increasing fermentation time. The result showed an increase in the alcoholic content with increasing fermentation time. The alcoholic content ranged from 0.00 % to 7.50 % and 0.00 % to 6.40 % for wine A and wine B respectively.
Table 1 shows the final pH, alcohol, total acidity, soluble solids and temperature of the basic wine after secondary fermentation and clarification. The pH was within the acidic range of 3.5 (wine A) and 3.4 (wine B). The titratable acidity was within the acceptable limit of 0.71 and 0.80% for wine A and B respectively. Soluble solids in 0Brix were 5 and 8 for wine A and B respectively while the alcohol contents were 10.5% and 8.5% for wine A and B respectively.
| PARAMETERS | WINE A | WINE B |
|---|---|---|
| pH | 3.5 ± 0.00 | 3.4 ± 0.01 |
| Temperature (°C) | 30 ± 0.00 | 31 ± 0.00 |
| Total acidity (%) | 0.71 ± 0.02 | 0.8 ± 0.00 |
| Total dissolved solids (oBrix) | 5 ± 0.01 | 8 ± 0.00 |
| Alcohol content (%) | 10.5 ± 0.02 | 8.5 ± 0.01 |
Wine A=Fermented with Starter culture; Wine B=Spontaneous Fermentation. Values are mean of triplicate determination
Table 1: pH, temperature, acidity, Brix and alcohol content of the basic final wines.
The result of the sensory evaluation of the wines is presented in Table 2. The result showed that there was significant difference (p<0.05) in the clarity of wine A and wine B while the wines did not differ significantly (p>0.05) in colour, flavour, taste and overall acceptability. However, the overall acceptability shows that wine A is more preferred than wine B.
| PARAMETERS | WINE A | WINE B | P-value |
|---|---|---|---|
| Clarity | 4.05 ±0.686 | 2.95 ± 0.999 | <0.05 |
| Colour | 3.50 ± 0.827 | 3.45 ± 0.887 | >0.05 |
| Flavour | 3.25± 1.020 | 3.35± 0.875 | >0.05 |
| Taste | 3.10± 0.788 | 2.95± 1.099 | >0.05 |
| Overall Acceptability | 3.55 ±0.686 | 3.15 ±1.182 | >0.05 |
Wine A=Fermented with Starter culture; Wine B=Spontaneous Fermentation. Values are mean ± standard deviation of triplicate determination
Table 2: Sensory Evaluation of the basic wines.
Table 3 presents the presence of isolated organisms from the final wines. The result showed that only Saccharomyces cerevisiae was isolated from wine A while Saccharomyces cerevisiae , Lactobacillus sp. and Candida tropicalis were isolated from wine B.
| Organisms | WINE A | WINE B |
|---|---|---|
| Saccharomyces cerevisiae | + | + |
| Lactobacillus species | - | + |
| Candida tropicalis | - | + |
Wine A=Fermented with Starter culture; Wine B=Spontaneous Fermentation.
Table 3: Presence of bacteria and fungi in the final wines.
Discussion
Fermentation for production of beverages like wine depends on the ability and performance of the yeast to convert sugar contents of the substrates to alcohol and esters. Moreover, the species of yeasts that develop during fermentation determine the final characteristics (flavour, taste, aroma etc.) of the product [2,13].
Mango (Mangifera indica ) of the cultivar commonly known as peter was used to produce fruit wine in the present investigation using baker’s yeast (Saccharomyces cerevisiae ) and spontaneous fermentation. The wines showed low pH values (acidic range) throughout the period of fermentation and the final product. The pH values were observed to decrease gradually with increasing fermentation time. This observation is similar to that report of Chilaka et al. on passion, pineapple and watermelon juices and that of Obisanya et al. who reported a steady decrease in pH during the fermentation of mango juice by Saccharomyces cerevisiae and Schizosaccharomyces sp. [4,20]. Ogodo et al. also reported decrease in pH toward acidity during production of mixed fruit wines of pawpaw, banana and watermelon [2]. The present report is also consistent with the report of other researcher for some tropical fruit wines such as tundu [7], sweet potato [10], sapota fruit [8,21], banana [6] and orange juice [22]. The decrease in pH toward acidity could be attributed to production and accumulation of organic acids during fermentation. Moreover, low values in wine have been reported to inhibit spoilage bacteria and creates favourable environment for the growth of desired organisms [1,4]. Therefore the wines will have good keeping quality.
In the present study, the steady decrease in pH observed during the primary fermentation of the musts was followed by steady increase in the total acidity. The acidity ranged from 0.21% to 0.63% during primary fermentation and in the final wines it was 0.71% (wine A) and 0.80% (wine B). The acidity was found to be more in wine B than wine A, although, the variations did not differ significantly (p>0.05). Similar trends have been reported by Okunowo et al. on orange juice and Ogodo et al. on pawpaw banana and watermelon mixed fruit wines [22,2]. However, the acidity in the present study is lower than the report for sapota fruit, sweet potato wine and higher than 0.15 ± 0.07 g/100 ml on bael wine [8,10,21]. Organic acids such as acetic, lactic, formic etc. as well as phenolic compounds, carbon dioxide and esters and are reported to contribute in lowing the pH and concomitantly increase the total acid content of musts during fermentation [23-25]. Generally, pH and acidity influences the tastes of wines by imparting sour tastes to the end product [2]. Chilaka et al. reported that total acidity of wine should fall within 0.5% and 1.0 % and the report of the present study fell within this limit [4].
Fluctuations in temperature of the must were observed during primary fermentation within the range of 30-33°C. The final temperature of the fruit wines ranged are 30°C and 31°C for wine A and B respectively. This observation is in agreement with the report of Ogodo et al. who reported fluctuation in temperature during the fermentation process of mixed fruit wines of pawpaw banana and watermelon [2]. This could be attributed to biochemical changes that occurred as fermenting organism metabolizes the substrates.
There was a gradual decrease in the total soluble solids (oBrix) during the primary must fermentation from initially 20°Brix for both wines. The wine with commercial yeast (wine A) was observed to utilize more sugar (ranging from 20°Brix at 0 h to 7°Brix at 96 h) than the spontaneously fermenting wine ( wine B) (ranging from 20°Brix at 0 h to 9°Brix at 96 h). The final wine shows that the total dissolved solids for A and B were 5oBrix and 8°Brix respectively. The variations differ significantly (p<0.05) when A is compared to B. Similar observation has been reported by other researchers [2,23]. The decrease in the sugar and dissolved solids during fermentation can be attributed to the breakdown of substrates by the fermenting organisms [23,25].
The alcoholic content of the wines in the present study ranged from 0.00% to 7.50% and to 0.00% to 6.40% in wine A and wine B respectively during primary fermentation. The alcohol content of the final wines are 10.5% and 8.5% 10 for wine A and B respectively. The alcoholic content of the present study as produced by the commercial baker’s yeast compared favourably with 10.46% reported by Chilaka et al. produced during mango wine fermentation by commercial yeasts [4]. Alcoholic fermentations are known to lead to the production of ethanol, esters, carbonyl compounds, acids and acetyls which affects the quality of the final product and associated with pleasant aromas. However, the concentration of these by-products can widely vary. Duarte et al. [13], Clement-Jimenez et al. [26] and Reddy and Reddy [27] reported that the concentration of ethanol contribute to the whole characteristics quality and flavour of produced wine. Hence wine A could be preferred to wine B.
The colours of the musts in the present study reduced in both wines as fermentation progressed from the typical yellow pigments of mango fruits to pale-yellow and light yellow for wine A and wine B respectively. This observation is an indication that good fruit wines can be produced from mango of the cultivar ‘peter’. Also, the wines were observed to have good aroma which can be attributed to the alcoholic contents and other aromatic compounds such as esters [26].
Sensory evaluation rated the wines acceptable with Wine A>Wine B. There was no significant difference (p>0.05) in all the sensory parameters analysed between A and B except clarity. The wines produced can be compared to other fruit wine in sensory attributes such as mixed fruit wine from pawpaw, banana and watermelon [2], banana wine [5], sweet potato wine [10] and tendu wine [7].
In this study, Saccharomyces cerevisiae was the only organism isolated from wine A while in wine B, Saccharomyces cerevisiae , Lactobacillus species and Candida tropicalis were isolated. The organisms have been reported to be associated with plant material, hence an indication of good quality [28]. This may be as a result of the low pH and alcoholic content which may inhibit the growth of other pathogenic and spoilage organisms and in turn give fermenting yeast a competitive advantage [1,4].
Conclusion
The present study has shown that acceptable fruit wines can be produced from mango of the cultivar commonly known as peter using Saccharomyces cerevisiae and spontaneous fermentation. However, better wine can be obtained by controlled fermentation using a starter culture. Therefore, large-scale production of mango wines could be an alternative to prolonging the shelf-life of mango fruit and the level of post-harvest loss of the fruit.
References
- Reddy LV, Reddy OVS (2005) Production and characterization of wine from mango fruit (Mangifera indica). World J Microbiol Biotechnol 21: 1345-1350.
- Ogodo AC, Ugbogu OC, Ugbogu AE, Ezeonu CS (2015) Production of mixed fruit (pawpaw, banana and watermelon) wine using Saccharomyces cerevisiae isolated from palm wine. Springerplus 4: 683.
- Reddy LVA, Reddy OVS, Joshi VK (2014) Production of Wine from Mango Fruit: A Review. Inter J Food Ferm Technol 4: 13-25.
- Chilaka CA, Uchechukwu N, Obidiegwu JE, Akpor OB (2010) Evaluation of the efficiency of yeast isolates from palm wine in diverse Fruit wine production. Afri J Food Sci 4: 764-774.
- Akubor PJ, Obio SO, Nwadomere KA, Obimah E (2003) Production and Quality Evaluation of Banana Wine. J plant Foods Human Nutr 58: 1-6.
- Obaedo ME, Ikenebomeh MJ (2009) Microbiology and production of banana (Musa sapientum) wine. Nig J Microbiol 23: 1886-1891.
- Sahu UC, Panda SK, Mohapatra UB, Ray RC (2012) Preparation and evaluation of wine from tendu (Diospyros melanoxylon L) fruits with antioxidants. Int J Food Ferment Technol 2: 171-178.
- Panda SK, Sahu UC, Behera SK, Ray RC (2014) Fermentation of sapota (Achras sapota linn.) fruits to functional wine. Nutrafoods 13: 179-186
- Awe S (2011) Production and Microbiology of pawpaw (Carica papaya L) wine. Curr Res J Biol Sci 3: 443-447.
- Ray RC, Panda SK, Swain MR, Sivakumar SP (2012) Proximate composition and sensory evaluation of anthocyanin-rich purple sweet potato (Ipomoea batatas L.) wine. Int J Food Sci Technol 47: 452-458
- Fleet GH (2003) Yeast interaction and Wine flavour. Inter J Food Microbiol 86: 11-22.
- Isitua CC, Ibeh IN (2010) Novel method of wine production from Banana (Musa acuminata) and pineapple (Ananas cosmosus) waste. Afri J Biotechnol 9: 7521-7524.
- Duarte WF, Dias DR, Oliverira MJ, Teixeira JA, Schwan RJ, et al. (2010) Characterization of different fruit wines made from cocoa, cupuassu, gairoba, jaboticaba and umbu. Food Sci Technol 30: 1-9.
- Yahia EM (1998) Modified and controlled atmosphere for tropical fruits. Horticult Rev 2: 123-183.
- Kumar YS, Varakumar S, Reddy OVS (2012) Evaluation of antioxidant and sensory properties of mango (Mangifera indica L.) wine. CyTA-J Food 10: 12-20.
- Giri KV, Murthy KDV, Rao NPL (1953) Separation of organic acids. J Indian Inst Sci 35A: 77-98
- Fawole MO, Oso BA (1988) Laboratory Manual of Microbiology: Spectrum Books Limited, Ibadan, Pp: 127.
- Onyeagba RA (2004) Identification and characterisation of Microorganisms. In: Laboratory guide for microbiology. Crystal Publishers, Okigwe.
- Barnett JA, Payne RW, Yarrow D (2000) Yeast characterisation and identification. (3rdedn), Cambridge, UK, New York, NY, USA: Cambridge University press.
- Obisanya MO, Aina JO, Oguntimein GB (2002) Production of wine from mango (Mangifera indica) using Saccharomyces and Schizosaccharomyces species isolated from palm wine. J Appl Microbiol 63: 191-196.
- Panda SK, Sahu UC, Behera SK, Ray RC (2014) Bio-processing of bael [Aegle marmelos L.] fruits into wine with antioxidants. Food Biosci 5: 34-41.
- Okunowo WO, Okotore RO, Osuntoki AA (2005) The Alcoholic fermentation efficiency of indigenous yeast strains of different origin on orange juice. Afri J Biotechnol 4: 1290-1296.
- Kamassah AKQ, Saalia FK, Osei-Fosu P, Mensah-Brown H, Sinayobye E, et al. (2013) Fermentation Capacity of Yeasts Using Mango (Mangifera indica Linn.) as Substrate. Food Sci Qual Manag 22: 69-78.
- Onwuka UN, Awam FN (2001) The potential for baker’s yeast (Saccharomyce scerevisiae) in the production of fruit wine from banana, Cooking banana and plantain. Food Sci Technol 1: 127-132.
- Ribéreau-Gayon P, Dubourdieu D, Donèche B, Lonvaud A (2006) Handbook of Enology: The Microbiology of Wine and Vinifications. (2ndedn), John Wiley & Sons, Ontario.
- Clement-Jimenez JM, Mingorance-cazorla L, Martinez-Rodoriguez S, Las Heras VFJ, Rodriguez-vico F (2005) Influence of sequential yeast mixtures on wine fermentation. Int J Food Microbiol 98: 301-308.
- Reddy LV, Reddy OVS (2009) Production, optimization and Characterization of wine from mango fruit (Mangifera indica Linn). Nat Prod Rad 8: 426-435.
- Ugbogu OC, Ogodo AC (2015) Microbial flora, proximate composition and vitamin content of three fruits bought from a local market in Nigeria. Inter J Chem Eng Appl 6: 440-443.
Copyright: © 2018 Ogodo AC, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.