
Anesthesia & Clinical Research
Open Access
ISSN: 2155-6148

ISSN: 2155-6148
Review Article - (2022)Volume 13, Issue 11
Background: Chronic Postsurgical Pain (CPSP) remains a continuing challenge in the healthcare systems worldwide due to limited treatment options. Pre-emptive analgesia is reported to be effective for the prevention of postoperative pain. However, there is limited evidence regarding the effectiveness of pre-emptive analgesia on the prevention of CPSP. A comprehensive systematic review and meta-analysis of clinical studies were performed to determine its effectiveness in the prevention of CPSP.
Methods: Relevant articles were searched from EMBASE, PubMed, Medline and Google scholar databases. The main outcome was chronic pain at 3 months and beyond after surgery. The random model was used to estimate the relative Risk Reduction (RR) using the 95% confidence interval. We assessed the routes of administration of analgesics (surgical types categorized under it), time of pre-emptive analgesia administration, pre-emptive vs. prolonged blockade and postoperative follow-up times.
Results: Thirty (27 randomized controlled trials and 3 non-RCT) studies with a total of 2,137 participants were reviewed. Pre-emptive analgesia prevented CPSP in the majority of studies (n=22/30, 73.3%). Pre-emptive analgesia significantly reduced CPSP in comparison to the post-incisional and placebo groups with the Relative Risk (RR) of 0.46 (P=0.0009, 95% CI=0.29-0.73) and 0.54 (P<0.001; 95% CI=0.42-0.68) respectively. Pre-emptive analgesics administered between<1 hr and 48-72 hrs before skin incision in different groups reduced CPSP with the RR of. 62 (P=0.001; 95% CI=0.46-0.83) and 0.42 (P=0.002; 95% CI=0.24-0.73) respectively. Surprisingly, no difference was observed between the pre-emptive and prolonged blockade (pre-emptive plus intraoperative and postoperative analgesia) groups. Subgroup analyses of intravenous route studies showed significant reduction of CPSP at 3 months with RR 0.33 (P=0.03; 95% CI=0.12-0.92), but not at 6 months follow-up. Additionally, oral route studies revealed a reduction of CPSP at 6 months with the RR of 0.44 (P<0.0001, 95% CI=0.29-0.66). Moreover, the overall analysis of epidural studies at 6 months showed a significant reduction of chronic pain with the RR of 0.56 (P=0.007, 95%CI=0.37-0.85). No difference was observed between the pre-emptive analgesia and placebo groups from the trial sequential analyses.
Conclusion: Pre-emptive analgesia reduced CPSP in the majority of studies at 3 months and beyond after surgery. However, pre-emptive analgesia did not show any benefit on the reduction of CPSP in a large number of studies, which could attribute to the heterogeneities in the studies reviewed. A prospective clinical trial studies should be conducted in a large cohort of patients in relation to preoperative risk factors for pain, pre-emptive analgesia, type and extent of surgery, and postoperative follow-up times to strengthen the current findings.
Surgery; Postoperative pain; Chronic postsurgical pain; Pre-emptive analgesia
CPSP: Chronic Postsurgical Pain; DN2: Neuropathic Pain Diagnostic; ETTI: Endotracheal Tube Intubation; FUPQ: Follow-Up Pain Questionnaire; HADS: Hospital Anxiety and DepressionScale ; ICN: IntercostalNerveBlock ; MPQ:McGillPainQuestionnaire ; NPS:Neuropathic Pain Scale; NRS: Numerical Rating Scale; PCA: Patient Controlled Analgesia; PLP: Phantom Limb Pain; PRIR: Pain Rating Index Report; PRISMA: Preferred Reporting Items for Systematic Reviews and Meta-Analyses; RCT: Randomized Controlled Trial; TEA: Thoracic Epidural Analgesia; TSA: Trial Sequential Analysis; VAS: Visual Analog Scale.
It has been advocated for decades that the anti-nociceptive intervention given pre-emptively (before surgical incision) is more effective to prevent both acute and chronic postoperative pains than the same treatment given after surgery as it prevents central sensitization [1]. However, recent reviews showed the controversies with regard to the effectiveness of pre-emptive (pre-incision) versus preventive (intraoperative or postoperative) analgesia for the prevention of postoperative pain, and suggested further study to come up with a firm conclusion [2,3].
Under-treated acute postoperative pain is the main cause for the development of chronic postsurgical pain [4,5]. Acute postoperative pain is caused by noxious stimulation after surgical injury (e.g. skin, tissues and nerves) [3-5]. This nociceptive pain is augmented by the inflammatory mediators released from the surgical wound that reduces the activation threshold of the peripheral sensory afferent nerve fibres (peripheral sensitization) [3-5]. The afferent barrage of impulses coming from the site of injury increases the excitability of neurons in the central nervous system (central sensitization) [3-5].
Chronic Postsurgical Pain (CPSP) is defined as pain that persists beyond 3 months after surgery [3]. CPSP is a common problem after surgery with inadequate treatment due to limited options [6]. It affects 10%-50% of patients after surgery, which imposes huge financial burdens, and significantly affects the quality of life of patients [7]. However, the previous reviews have focused on the effects of pre-emptive analgesia on the prevention of acute postoperative pain, where there are many ins and outs about its efficacy [8-11]. Additionally, there are limited reviews on the effect of pre-emptive analgesia on the prevention of CPSP, which have focused either on drug specific (e.g. ketamine) [12,13] and/or surgery specific effects such as limb amputation and thoracotomy [14,15].
Moreover, there is lack of evidence as to what time the pre-emptive analgesia should be provided before surgical incision in order to obtain a longer analgesic effect than that particular drug actually could have an effect in the context of pre-emptive analgesia to effectively prevent CPSP [2]. Furthermore, there are controversies as to whether pre-emptive analgesia should be followed by post-incisional and postoperative analgesia as compared to pre-emptive analgesia alone to prevent CPSP by considering the possibility of pain resurge when the preventive effect of a particular pre-emptive analgesic drug is worn off. And of course, its effectiveness could also be affected by the type and extent of surgery, routes of administration, duration of actions of analgesic drugs being provided and the sites of pain pathways the drug acts on, etc [2].
In the current comprehensive review, we performed analyses to determine the effect of the timing of pre-emptive analgesia (duration of analgesia administration before surgical incision and type of comparisons: pre-incisional versus post-incisional, pre-incisional with postoperative/placebo/no treatment) on the prevention of CPSP. Additionally, the sub-group analyses were performed based on
The literatures were searched from EMBASE, PubMed, Medline and Google scholar using broad search terms that included the terms ‘’preoperative analgesia’’, ‘’pre-emptive analgesia’’, ‘’postsurgical pain’’, ‘’efficacy of pre-emptive analgesia’’, ‘’pre-emptive analgesia for postsurgical pain’’, ‘’pre-emptive analgesia for chronic postsurgical pain’’, ‘’efficacy of pre-emptive analgesia for postsurgical pain’’ and “preventive analgesia’’ as of 21/08/2021.
Inclusion and exclusion criteriaThe original prospective studies, which assessed the efficacy of pre-emptive analgesia on the prevention of chronic postsurgical pain in adult patients (>18 years old) at 3 months and beyond of surgery with no restriction on the year of publication, study design, surgical speciality and the type of pre-emptive analgesia used were included. The main outcome of interest was the number of patients who received pre-emptive analgesia and experienced pain at 3 months and beyond after surgery compared with the placebo group. In addition, the presence and severity of adverse events, and the number of patents who had withdrawn the intervention due to adverse effects were assessed. Studies which did not report clearly the outcomes after 3 months of surgery were excluded.
Study quality assessmentThe quality of Randomized Controlled Trial (RCT) studies was assessed using the Cochrane Handbook for systematic reviews of interventions [16]. The risk of bias was assessed by employing seven key domains: random sequence generation, allocation concealment, blinding, incomplete outcome data, free of selective reporting, and other bias (Figure 2). Each risk of bias item was presented as a percentage across all included studies (Figure 3). Each study was classified as low risk of bias if all the domains were low risk; high risk if one or more domains were high risk (and/or unclear with one high risk domain) were categorized as high risk, and unclear if one or more domains were unclear [17]. For the non-RCT studies, quality was assessed using the methodological index for the non-randomized studies scale (MINORS) [18].
Data extraction and statistical analysisInformation was extracted on the type of surgery, the numbers of participants, type of pre-emptive analgesia (local, regional, intravenous etc), dose, route of administration, time of administration before surgery, study design (active control or placebo), study duration, outcome reports (pain assessment tool used, the numbers of patients who experienced pain, needed pain treatment, and developed adverse effects after 3 months of surgery). Extracted data were entered in to the RevMan 5.3 software for analysis (Review Manager. Version 5.3 Copenhagen: The Nordic Cochrane Centre, the Cochrane Collaboration, 2014). [19].
Assessment of the outcomes of interventionsThe number of patients who experienced pain at 3 months and beyond, and the numbers of patients who developed adverse effects were included to carryout meta-analyses of risk ratio (RR) with 95% confidence intervals (CIs) [20] and weighted mean difference (the weight of each study in meta-analysis). The analysis was tested using both the random and fixed effect models for meta-analysis of the dichotomous data. The random effect model (the true effect/effectiveness of pre-emptive analgesia in preventing CPSP varied from study to study) was used if there were substantial heterogeneity across studies (I2>50%) [21]. Otherwise, the fixed effect model (the true effect is the same across studies/no substantial heterogeneities) was utilized. The subgroup analysis was performed to determine the heterogeneity with regard to the type of surgery, route of drug administration, type of comparison of pre-emptive analgesia (pre-emptive vs. post-incisional OR pre-emptive vs. placebo OR pre-emptive with pre-emptive/ postoperative), frequency of drug administration (preoperative analgesia only or preoperative analgesia followed by repeated intra and postoperative doses), timing of administration of pre-emptive analgesia before surgery and duration of follow-up after surgery. The heterogeneity was assessed using the Chi-square test (I2 statistic). The I2 value>50% was considered as existence of substantial heterogeneity [21].
Trial Sequential Analysis (TSA)The primary outcome was analyzed using the TSA software (Copenhagen Trial Unit, Center for Clinical Intervention Research, Rigshospitalet, Copenhagen, Denmark to determine the reliability of the results [22]. In the meta-analysis, the sparse data and repetitive testing of accumulating data may increase the random errors and the risk of Type I error [22,23]. The trial sequential monitoring boundaries in the TSA were employed in order to reduce the risk of random errors and to determine the reliability and significance of the meta-analysis [22-24]. If the cumulative Z-curve crosses the trial sequential monitoring boundary, the evidence for reaching a solid conclusion may be sufficient and no further study is needed. The TSA was performed using the α-spending analysis using the O’Brien-Fleming boundaries (α=0.05; 2-sided and β=0.025; power 80%) [25]. The total number of patients in the included pooled trials was used for information size (sample size).
A total of 378 studies were initially evaluated (Figure 1). However, thirty seven duplicates were removed and eighty five studies were excluded by title as they didn’t meet the inclusion criteria; whereas 226 studies that evaluated the effect of pre-emptive analgesia on postoperative acute pain were excluded (Table 1). A total of 27 RCT studies [13-15,26-50] and 3 non-RCT studies [51-53], which consisted of 2,137 participants were reviewed (Table 1). The characteristics of the reviewed studies are summarized in Table 1 (supplementary data 1).
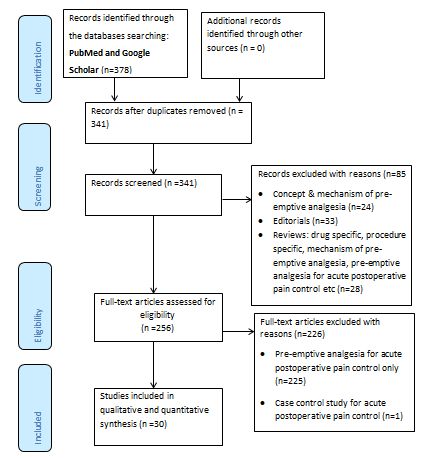
Figure 1: PRISMA flow chart of studies screened for review.
Table 1: Summary characteristics of review studies.
| Study | Sample size at different times | Pre-emptive analgesia | Dose, route and time drug given | Surgery | Pain assessment | Primary outcome: presence of postsurgical pain at>3 months. | Secondary outcome | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Treatment group | Control/active treatment group | |||||||||
| Grigoras et al. | T=17C=19 | Lidocaine | B=1.5 mg/kg IV followed byINF=1.5 mg/kg IV, after ETTI and stopped 60 minutes after skin closure | Received an equivalent saline. | Breast surgery | MPQ,VAS (0-10) | At 3 months: CPSP: lidocaine vs. 2 (11.8%) vs. control 9 (47.4%) (P=0.031). | At 3 months: Worse pain during moment lidocaine vs. controls 0(0%) vs. 8 (41.2%) (P=0.003). Overall intensity of pain (mean + SD): 0.2 (±0.5) vs. 0.9 (±1.4) (P=0.023). Hyperalgesia lidocaine vs. control (mean + SD): 0.2 (±0.8) vs. 3.2 (±4.5) (P= 0.002). | ||
| Lavand’homme et al. | T=20/20/20/20 | Ketamine | G3=EPI-EPI: Ketamine=0.5 mg/kg IV bolus 30 minutes before skin incision followed by epidural 0.25 mg/kg/hr till end of surgery | G1=IV-IVG2=IV-EPIG4=EPI-IV | Digestive surgery | VAS (0-10) | 6 months: Patients treated with preop bolus ketamine G1: 10 (51%), G2:2 (10%), G3:0 (0%) and G4:0 (0%) (P<0.01).12 months: G1:6 (28%), G2: 2 (11%), G3:0 (0%) and G4:0 (0%) (P<0.05) developed pain. | At 6 months: treatment group (G3) 1 (5%) vs. group 2: 2 (10%) and group 4: 3 (15%) patients developed back pain. | ||
| Jahangiri et al. | T=13C=11 | Epidural infusion: diamorphine, bupivacaine, clonidine | INF: diamorphine=5 mg; bupivacaine=75 mg and clonidine=150 µgm (24-5 hrs before surgery) | Received on demand opioids | Lower limb amputation | VAS (0-10) | Phantom pain at 6 months in controls 9 (72.7%) vs. study groups 3 (7.7%) (P=0.002) and 12 months in controls 8 (72.7%) vs. study groups 2 (7.7%) (P=0.002). | - | ||
| Suzuki et a | T=22C=22 | Epidural plusKetamine (IV)-Epidural plus saline | Ketamine=0.05 mg/kg/hr IV infusion after ETTI on top of epidural that continued for 3 days postoperatively | Saline IV infusion on the top of epidural | Thoracotomy | NRS | 3 months: Fourteen (58.3%) patients from the control group and 7 (28%) patients from ketamine group experienced pain (NRS > 1) (P=0.03). Nine (37.5%) patients from the control group and 2 (8%) patients from ketamine group received medication for pain control (P=0.03).6 months: Sharp pain: ketamine 0 (0%) and control 3 (13.6%) patients. | At 3 months (n=14; P=0.56): heavy pain from the wound ketamine 6 (27.3%) and control 9 (40.9%) patients. Sharp pain: ketamine 0 (0%) and control 1 (4.5%) patients.At 6 months (n=12; P=0.4): heavy pain ketamine 7 (31.8%) and control 8 (36.4%) patients. Tingling: ketamine 5 (22.7%) and control 6 (27.3%) patients. | ||
| Salengros et al. | T=18C=20 | Low dose remifentanil with epidural with 0.5% ropivacaine | Low dose remifentanil IV infusion (average effect site concentration 1.99 _ 0.02 ng/mL) with epidural (0.5% ropivacaine)started at the beginning of anaesthesia | High-dose remifentanil IV infusion (average effect-siteconcentration 5.61 _ 0.84 ng/mL) with epidural started at the end of surgery | Thoracotomy | DN4 neuropathic pain diagnostic questionnaire | Numbers of patients experienced pain (remifentanil):-3 months: high dose 15/20 (75%) vs. low dose 5/18 (27.8%) (P=0.013)-6 months: high dose 16/20 (80%) vs. low dose 5/18 (27.8%) (P=0.008)-13 months: high dose 14/20 (70%) vs. low dose 3/18 (16.7%) (P=0.009) | NRS at 3 months (mean + SD): High-Dose Group: 3.00 + 1.51 and low dose: 2.43 + 1.62 (P=0.42).NRS at 6 months (mean + SD): high dose: 2.88 + 1.36 and low dose: 3.50 + 2.28 (P=0.67).At 13 months (mean + SD): high dose: 2.28 + 1.33 and low dose: 4.33 + 2.08 (P=0.10). | ||
| Obata et al. | T=28C=30 | Thoracicepidural | 4 ml of 1.5% mepivacaine bolus started 20 minutes before skin incision followed by 4 ml/hr infusion until 72 hrs after operation | 4 ml of 1.5% mepivacaine bolus started at the end of surgery followed by 4 ml/hr infusion until 72 hrs after operation | Thoracotomy | NRS | 3 months: 14 (50%) and 23 (77%) patients developed long-term pain in pre-group and post group respectively.6 months: In pre-group 9 (33%) and post group 20 (67%) patients developed long-term pain. | At 3 months: In pre-group 11% and in post group 23% patients took medication for pain control.6 months: The NRS was lower in the Pre group at 6 months after operation (P=0.015). Whereas 4% and 6% patients took medication for pain control from pre and post groups respectively | ||
| Karanikolas et al. | T=13/13/13/13C=13 | Epidural/PCA | Epi/Epi/Epi: bupivacaine, 2 mg/ml, and fentanyl, 2 µg/ml, infusion at 4–8 ml/h), started before 48 hrs of surgery.PCA/Epi/Epi: IV fentanyl 25 µg; lockout, 20 min; and no basal infusion) for 48 h and also received epidural normal saline at 2 ml/h.PCA/GA/PCA: IV fentanyl 25 µg; lockout, 20 min; no basal infusion), started at 48 h before surgery. | Received conventional analgesia | Limb amputation | VAS, MPQ-PRIR | At 6 months:PLP prevalence: Epi/Epi/Epi 1 (7.7%), PCA/Epi/Epi 4 (30.7%), PCA/Epi/PCA 7 (58.3%) and PCA/GA/PCA 3 (23%) groups compared to the control group 9 (75%) (P=0.004).PLP VAS score: median (minimum-max): Epi/Epi/Epi 0 (0-20), PCA/Epi/Epi 0 (0-42), PCA/Epi/PCA 20 (0-40), PCA/GA/PCA 0 (0-30) and control 20 (0-58) (P=0.001). | |||
| Comez et al. | T=20/20C=20 | -Epidural (PE)-Epidural + Dexketoprofen (PED) | Epidural (PE): 10-15 ml of 0.125% levobupivacaine before 5 minutes of anaesthesia.Epidural + Dexketoprofen (PED): 10-15 ml of 0.125% levobupivacaine before 5 minutes of anaesthesia. Dexketoprofen 50 mg in 100 m normal saline IV infusion before 15 minutes of skin incision. | No preoperative analgesia was given | Thoracotomy | VAS | 6 months: The incidence of post thoracotomy chronic pain in PED 2 (10%), PE 4 (20%) and Control 6 (30%).The VAS score (mean + SD) control group: 1.85 ± 1.496 vs. PE: 1.15 ± 1.348 vs. PED: 0.60 ± 1.142 (P=0.017). | 6 months: Patient satisfaction score: control 9 (45%) vs. PE 11 (55%) vs. PED 18 (90%) (P=0.008). | ||
| Kairaluoma et al. | T=30C=30 | Paravertebral nerve block (PVB) | 0.5% bupivacaine of 1.5 mg/kg injected at T3 before anaesthesia. | Received saline subcutaneously at the corresponding puncture site | Breast surgery with axillary dissection | NRS | 6 months: the prevalence of pain symptoms (tactile stimulus: median/range) was lower in PVB: 0.5 (0-3) vs. sham group 1 (0-4) (P=0.029).12 months: prevalence of pain symptoms: control 23 (77%) vs. PVB 13 (43%) (P=0.008) | At 12 months NRS median (range): motion related pain: PVB 0 (0–6) vs. 2 (0–8) (P=0.003).Pain at rest: PVB 0 (0–1) vs. control 0 (0–8) (P=0.011). | ||
| Dertwinkel et al. | T=25C=28 | Ketamine | KG=0.5 mg/kg IV bolus immediately after induction of anaesthesia, followed by 0.003 mg/kg/24 hr IV infusion and 0.001 mg/kg/48 hr IV infusion | Not received perioperative ketamine | Amputation of limbs | NRS | 9 months: KG one patient (9.1%) and control group 10 (71.4%) patients developed severe phantom limb pain (P=0.01). | 9 months: Seven (63.6%) and 11 (78.5%) patients developed chronic phantom limb pain in ketamine and control groups respectively. | ||
| Xu et al. | T=62C=79 | Celecoxib, tramadol and acetaminophen | Celecoxib=200 mg BID orally, tramadol 37.5 mg orally and acetaminophen 325 mg orally TID72-1 hr before surgery. | No drug was given to the control group preoperatively | Total knee arthroplasty | VAS | There was low VAS score during moment at 3 months in treatment group compared to the control group (P=0.012) | - | ||
| Remerand et al. | T=72C=70 | Ketamine | B=0.5 mg/kg IV before skin incision, followed by 2 µgm/kg/min for 24 hour infusion | Saline bolus, followed by saline | Total hip arthroplasty | NRS | 6 months: Ketamine group 6 (8%) and placebo group 15 (21%) patients experienced pain at rest (P=0.036). | 6 months: 3 (4.2%) from ketamine group and 10 (13.3%) placebo group reported their pain NRS>3 at rest (P=0.04). | ||
| Senturk et al. | T=22C=24/23 | Thoracic epidural analgesia (TEA) | Pre-TEA: bolus of 10 ml of 0.1% bupivacaine plus 0.1 ml morphine 30 minutes before anaesthesia, followed by 7 ml/hr infusion of the same solution during operation. | In Post TEA group, no epidural medicationwas applied until the postoperative period | Thoracotomy | NRS | At 6 months: Pre-TEA 10 (45%), Post-TEA 15 (63%) and IV-PCA 18 (78%) (P<0.05). | 6 months: The NRS scores (mean+SD) were Pre-TEA ( 0.6 + 0.8), Post-TEA ( 0.9 + 0.9) and IV-PCA ( 1.4 + 1.2) | ||
| Khuranaet al. | T=30/30C=30 | Gabapentin, pregabalin | Gabapentin=300 mg orally and Pregabalin=75 mg orally before 1 hr of surgery and every 8 hr for 7 days postoperatively | Received placebo before 1 hr of surgery | Spinal surgery | VAS | 3 months: VAS score (mean) in pregabalin group was significantly low compared with gabapentin and placebo group (P<0.05). | Prolo functional score: excellent score gabapentin 3 (10%), pregabalin 13 (43.3%) and placebo group 9 (30%)ODIS: moderate disability gabapentin 13 (43.3%), pregabalin 3 (10%) and placebo 14 (46.7%). | ||
| Buvanendran et al. | T=113C=115 | Pregabalin | Pregabalin 300 mg orally 1-2 hr before surgery | Received matched placebo tablet orally 1-2 hr before surgery | Total knee arthroplasty | NRS | 3 months: neuropathic pain in pregabalin group 0 (0%) vs. placebo 10 (8.7%) (P=0.001).6 months: the incidence of neuropathic pain in pregabalin 0 (0%) vs. placebo 6 (5.2%) (P=0.014). | 3 months: allodynia on operated leg was low in pregabalin group 2 (2%) vs. placebo group 14 (12%) (P=0.002).6 months: allodynia on operated leg was low in pregabalin group 0 (0%) vs. placebo group 9(8%) (P=0.002). | ||
| Amr et al. | T=50/50C=50 | venlafaxine | Venlafaxine=37.5 mg/day and gabapentin 300 mg/day orally the night before surgery and for 10 days postoperatively | Received placebo the night before surgery and for 10 days postoperatively | mastectomy | VAS | 6 months: stabbing/pricking pain significantly reduced in venlafaxine group 7 (14%) compared to the control group 20 (40%) (P=0.003) and gabapentin group 16 (32%) (P=0.028).Burning pain was also significantly reduced in venlafaxine group 1(2%) compared with the control group 11 (22%) (P=0.0018). | 6 months: more patients used opioids in the control group 18 (36%) (P=0.02) and gabapentin groups 17 (34%) compared to venlafaxine group 8 (16%) (P=0.03)). | ||
| Bouziaet al. | T=31/31C=31 | pregabalin | Group2: Pregabalin=75 and Group3: 150 mg orally before surgery. | Group1: Placebo capsule orally before surgery | Cardiac surgery | VRS | 3 months: analgesia consumption group1: 26/31, group2: 16/31 and group3: 10/31 (P <0.001) | 3 months: VAS score: median (minimum, max): Control 3 (2, 5), group2: 2 (1, 3) and group3: 2 (1, 3) (P<0.001). sleep disturbance group1: 16/31, group2: 5/31 and group3: 3/31 (P <0.001). | ||
| Sen et al. | T=20/20C=20 | Ketamine and pregabalin | Ketamine group: placebo capsule, 0.3 mg/kg IV bolus and 0.05 mg/kg/hr infusion before skin incision until the end of operation. Pregabalin group=1.2 gram orally and bolus plus infusion of saline 1 hr before skin incision | Placebo capsule and bolus plus infusion of saline | hysterectomy | VRS | 3 and 6-months:the incidenceof incisional pain and pain scores were significantly lower in the gabapentin group compared with the ketamine and control groups (P <0.001) | - | ||
| Broglyet al. | T=23C=24 | Gabapentin | Gabapentin=1200 mg orally 2 hr before surgery | Placebo orally 2 hr before surgery | thyroidectomy | DN2 | 6 months: DN2 score was significantly reduced in gabapentin group 1 (4.3%) compared to the placebo group 7 (29.2%) (P=0.04). | - | ||
| Ju et al. | T=50/48/38C=48/43/39 | Epidural | Epidural: initiated before skin incision and maintained with 5-10 ml/hr 0.5% ropivacaine during operation | Cryo group: each intercostal nerve exposed at the end of surgery and received 90 seconds application ofcold (-70 C0) | Thoracotomy | VRS | 3 months: moderate to severe pain epidural 4 (8%) vs. cryo group 11 (22.9%) (P=0.077).6 months: severe to moderate pain-epidural 3 (6.3%) and cryo group 12 (27.9%) (P=0.013)12 months: moderate to severe pain epidural 2 (5.3%) vs. cryo group 9 (23.1%) (P=0.056). | Allodynia like pain:6 months: epidural 1(2.1%) and cryo 6 (16.3%) (P=0.044).12 months: epidural 0 (0%) and cryo 6 (15.4%) (P=0.025).Interference with daily life: 3 months: epidural 6 (12%) and cryo 18 (37.5%) (P=0.003). 6 months: epidural 5 (10.4%) and cryo 15 (34.9%) (P=0.005). 12 months: epidural 3 (7.9%) and cryo 13 (33.3%) (P=0.014). | ||
| Sen et al. | T=30C=29 | gabapentin | Gabapentin 1.2 gm orally 1 hr before surgery | A placebocapsule was taken orally 1 hr before surgery | Herniorrhaphy | VAS | 3 and 6 months: the mean VAS score was lower in gabapentin (1.25) group compared with the placebo group (2.5) (P< 0.05). | - | ||
| Fassoulaki et al. | T=22/20C=22/21 | Gabapentin | Gabapentin 400 mg orally starting the night before the day of surgery and 8 days postoperatively | Placebo capsules orally starting the night before the day of surgery and 8 days postoperatively | Breast surgery | VAS | 3 months: 18/22 (82%) patients in control group and 10/22 (45%) patients in the treatment group developed chronic pain (P=0.028).6 months: 12/21 (57%) patients from control and 6/20 (30%) patients from treatment developed chronic pain (P=0.151). | 3 months: Axillary pain 10 (22%) patient from control and 3 (14%) patients from treatment group (P=0.045). Arm pain 13 (59%) patients from control and 5 (22%) patients from treatment groups (P=0.038). Five (23%) patients from control and 0 (0%) patients from treatment (P=0.048) needed analgesia.6 months: Four/21 (21%) patients from the control and 0/20 (0%) patients the treatment groups needed analgesia (P=0.107). | ||
| Wilson et al. | T=15/15/14C=19/16/15 | Epidural: Ketamine | Epidural: bupivacaine 0.5% of 1 mg/kg with 0.5 mg/kg ketamine bolus before starting surgery. | Epidural bupivacaine 0.5% of 1 mg/kg with saline was given before starting surgery | Lower limb amputation | VAS, MPQ, NPS, HADS | 3 months: Phantom pain in ketamine group 6/15 (40%) and control group 7/19 (37%)(P=0.867). Stump pain ketamine group 5/15 (33%) and saline group 9/19 (43%) (P=0.635).6 months: Phantom pain ketamine 6/15 (40%) and saline 3/16 (19%) (P=0.252). Stump pain ketamine 7/15 (43%) and saline group 5/16 (32%) (P=0.609).12 months: Phantom pain ketamine group 7/14 (50%) and saline 6/15 (50%) (P=0.867). Stump pain ketamine 3/14 (21%) and saline 5/15 (33%) (P=0.682). Median NPS score decreased at all times postoperatively in both groups. | Anxiety significantly decreased in ketamine group compared with the preoperative value till year one (P< 0.001) whereas there was no difference b/n the preoperative and postoperative anxiety value till year one in saline group (P=0.071).Depression level remarkably reduced in ketamine group at 3, 6 and 12 months compared to placebo group (P=0.003). | ||
| Katz et al. | T=36C=36/38 | Ketamine | Group 1: Fentanyl=1 µgm/kg IV bolus and 25 µgm/kg/hr infusion 5 minutes before induction of anaesthesia Ketamine: 0.2 mg/kg IV bolus, followed by 2.5 µgm/kg/hr infusion 10 minutes before skin incision. | Groups 2 and 3: IV fentanyl and saline (bolus plus infusion) before incision (equivalent amount with group 1) | Radical prostatectomy | MPQ,VAS, FUPQ | 6 months: Most intense pain after surgery (VRS: mean +SD): G1 (5.1 + 1.9), G2 (5.6 + 2.2) and G3 (5.1 + 2.5).VAS-rest pain G1 (2.3 + 1.2), G2 (2.8 + 1.1) and G3 (3.4 + 3.0) respectively. | - | ||
| Nikolajsen et al. | T=17/16/12C=20/20/16 | Epidural (morphine and bupivacaine) | Epidural: 0.2 mg morphine and bupivacaine 0.5% of 5-10 ml bolus were given 18 hr before amputation and continued till end of surgery | Epidural: saline and morphine 5-20 mg orally or intramuscularly six times daily before surgery | Lower-limb amputation | VAS | Phantom pain3 months: block 14/17 (82%) vs. control 10/20 (50%) (P=0.09).6 months: block 13/16 (81%) vs. control 11/20 (55%) (P=0.2).12 months: block 9/12 (75%) vs. control 11/16 (69%) (P=1.0). | Phantom pain:3 months: Block vs. control (82% vs. 50%)6 months: Block vs. control (81% vs. 55%)12 months: Block vs. control (75% vs 69%) | ||
| Dualé et al. | T=34C=35 | Ketamine | B=1 mg/kg IV before surgery,INF=1 mg/kg/hr during surgery and 1 mg/kg/24 IV after surgery | Saline during induction, operation and for 24 hrs | Thoracotomy | VAS | 4 months: Ongoing pain (NPSI)=ketamine 8 (23.5%) vs. placebo 12 (34.3%) (P=0.325). | 4 months: Ongoing pain (PNSI) (mean + SD): ketamine group 8 (23.5) and placebo group 12 (34.3) (P=0.325).Evoked pain (NPSI)=ketamine 13 (38.2%) vs. placebo 16 (45.7%) (P=0.529). Neuropathic pain score (>0): ketamine 16 (47.1%) vs. placebo 24 (68.6%) (P=0.070). | ||
| Joseph et al. | T=16C=19 | Ketamine | B=0.5 mg/kg at inductionINF=3 µgm/kg/min during operation and 1.5 µgm/kg/min for 48 h after operation | IV placebo (a saline solution under the same infusion modalities). | Thoracotomy | NRS | 3 months: NRS at rest (mean + SD): ketamine group: 1.1 ± 2.1 and control group: 0.3 ± 0.7 (P=0.385). | 3 months: NRS during abduction (mean + SD): ketamine group: 1.3 ± 2.3 and control group: 1.1 ± 2.5 (P=0.589). | ||
| Can et al. | T=20C=20/20 | Epidural | Pre-group: 10–15 mL of 0.1% levobupivacaine epidural was given before anaesthesia.Post incision group: remifentanil 0.25–0.50 g/kg/hr infusion started 10 minutes after incision | Control group: no epidural analgesia received before and during operation | Thoracotomy | VAS | 3 months: VAS score ( > 3): control 4 (20%), post-incision group 4 (20%) and pre-group 3 (15%) (P=0.896).6 months: VAS score ( > 3): control 6 (30%), post-incision group 5 (25%) and pre-group 4 (20%) (P=0.769) | 3 months: VAS score (mean + SD): Control group (1.90 + 0.96), Group post-incision (1.80 + 1.00) and Pre-emptive group (1.65 + 0.87) (P=0.664).6 months: VAS score (mean + SD): control group (2.10 + 0.96), Group post-incision (1.95 + 0.99) and Pre-emptive group (1.70 + 0.92) (P=0.348). | ||
| Ryu et al. | T=65C=68 | Ketamine added to epidural | G1: 100 mg ketamine and 2 µg/mL fentanyl were given in combination epidural levobupivacaine before surgery | G2: 2 µg/mL of fentanyl was given in combination with epidural levobupivacaine before surgery | Thoracotomy | VAS | 3 months: Pain at rest ketamine group 33/65 (51%) and control group 29/68 (43%) (P=0.348). | 3 months: pain with movement (coughing) ketamine group 44/65 (68%) and control group 50/68 (74%)(P=0.46). | ||
| Hayes et al. | T=15C=17 | Ketamine | Ketamine group: 0.5 mg/kg IV bolus before anaesthesia, followed by 0.15 mg/kg/hr for 72 hrs after operation | Control group: placebo preoperatively, followed by saline infusion for 72 hrs after operation | Below knee amputation | 6 months: phantom pain ketamine group 7 (47%) vs. control 7 (71%) (P=0.28) and stump pain ketamine group 7 (47%) vs. control 6 (35%) (P=0.72). | ||||
Key: T=Treatment group; C=Control/placebo group (s); B=Bolus; INF=Infusion; MPQ=McGill Pain Questionnaire; ETTI=Endotracheal tube intubation; IV=Intravenous; VAS=Visual Analog Scale; NRS=Numerical Rating Scale; PRIR=Pain Rating Index Report; PCA=patient controlled analgesia; GA=general anaesthesia; PLP=phantom limb pain; ICN=Intercostal nerve block; PE=Pre-emptive epidural; PED=Pre-emptive epidural and dexketoprofen; TEA=Thoracic epidural analgesia; NPS= Neuropathic Pain scale; HADS=Hospital anxiety and depression scale; FUPQ; Follow-up pain questionnaire; DN2=Neuropathic pain diagnostic questionnaire.
Risk of bias assessment of studies
Of the 27 RCT studies, 18 studies were deemed of low risk of bias [26-42,50]; five studies were categorized as at high risk of bias [14,46-48,54] and four studies were of unclear risk of bias [13,43-45] (Figure 2, Table 2). The risks of bias for the non-randomised studies are also shown in Table 2. The funnel plot is also symmetrical, which showed the absence of publication bias in the studies reviewed (Figure 3,4).
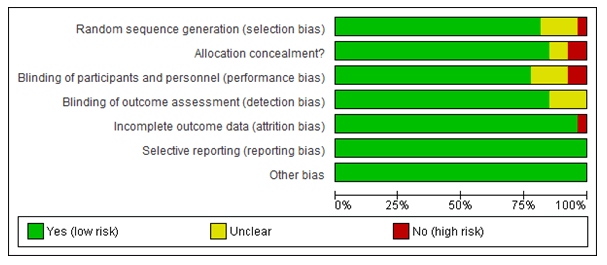
Figure 2: The risk of bias for each item presented as the percentages for reviewed RCT studies (n=27).
Table 2: Methodological items for non-randomized studies (MINORS).
| S.N | Methodological items for non-randomized studies | Jahangiri et al. 1994 | Xu et al. 2017 | Dertwinkel et al. 2002 |
|---|---|---|---|---|
| 1 | A clearly stated aim | 2 | 2 | 2 |
| 2 | Inclusion of consecutive patients | 2 | 2 | 2 |
| 3 | Prospective collection of data | 2 | 2 | 2 |
| 4 | Endpoints appropriate to the aim of the study | 2 | 2 | 2 |
| 5 | Unbiased assessment of the study endpoint | 1 | 2 | 2 |
| 6 | Follow-up period appropriate to the aim of the study | 2 | 2 | 2 |
| 7 | Loss to follow up less than 5% | 0 | 0 | 0 |
| 8 | Prospective calculation of the study size | 2 | 2 | 1 |
| 9 | Adequate control group | 2 | 2 | 2 |
| 10 | Contemporary groups | 2 | 2 | 2 |
| 11 | Baseline equivalence of groups | 2 | 2 | 2 |
| 12 | Adequate statistical analyses | 2 | 2 | 1 |
| Total score | 21/24 | 22/24 | 20/24 |
The items are scored 0 (not reported), 1 (reported but inadequate) or 2 (reported and adequate).
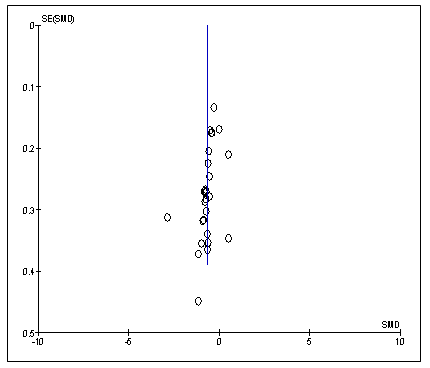
Figure 3: Funnel plot is symmetrical which shows the absence of publication bias in the review studies (95% CI; P>0.05). SE: Standard error; SMD: Standard Error of mean difference.
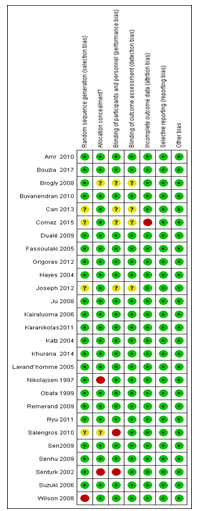
Figure 4: The risk of bias summary of randomized controlled trial studies reviewed (n=27). Green=low risk of bias; Yellow=Unclear and Red=High risk of bias.
Chronic postsurgical pain
The assessment of CPSP varied across studies. Seven studies assessed CPSP at 3 months only [26,30,32,35,41,44,53], seven studies both at 3 and 6 months [27,31,40,42,43,45,50]; and three studies at 3, 6, and 12 months [14,46,47]. In addition, nine studies evaluated the presence of CPSP at 6 months only [13,28,33,34,37,38,48,49,54], whereas four studies evaluated both at 6 and 12 months only. One study assessed CPSP at 12 month only (Table 1). Pre-emptive analgesia reduced CPSP at 3 months and beyond in the majority of the studies [13,26-29,31,32,34-36,38, 39,42,45,46,48-54]. However, no difference was observed between the treatment and non-treatment groups at any time point in the follow-up periods in eight studies [14,30,33,37,41,43,44,47] (Table 1).
Pre-emptive analgesia for the prevention of chronic postsurgical pain
As the comparison of pre-emptive vs. post-incisional analgesia is reported to be appropriate to assess the effectiveness of pre-emptive analgesia on the prevention of postoperative pain [55], the subgroup analyses were performed for the pre-emptive vs. post-incisional; pre-emptive vs. placebo and pre-emptive vs. pre-emptive or postoperative analgesia.
Pre-emptive vs. post-incisional subgroup analysis demonstrated a statistically significant reduction of CPSP with the RR of 0.46 (P=0.0009, 95% CI=0.29-0.73; that is 54% reduction of developing CPSP in patients who received pre-emptive analgesia compared to those who received analgesia after surgical incision. Additionally, pre-emptive vs. placebo subgroup analysis showed a statistically significant reduction of CPSP with the RR of 0.54 (P<0.001; 95% CI=0.42-0.68), implies that 46% reduction of developing CPSP in patients who received pre-emptive analgesia compared to the placebo group (Figure 5). However, there was no difference between pre-emptive vs. pre-emptive or postoperative.
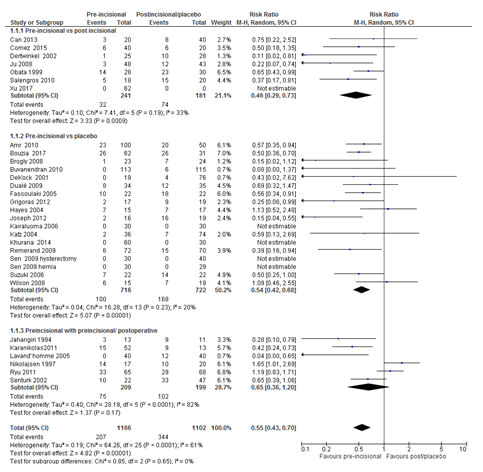
Figure 5: Forest plot of the comparison of pre-emptive analgesia: Pre-emptive vs. post-incisional; Pre-emptive vs. placebo; pre-emptive vs. pre-emptive/post-incisional/postoperative. The overall analysis result favoured pre-emptive analgesia for the prevention of CPSP with a RR of 0.55 (P <0.00001, 95 CI=0.43-0.70). RR=Relative risk, M-H=Mantel-Haenszel; CI=Confidence Interval. All studies were included in the analysis.
There are also controversies regarding the impact of the timing of pre-emptive analgesia on the prevention of postoperative pain. Additionally, there is no clear time frame for the administration of pre-emptive analgesia. Taking this issue in to consideration, the subgroup analyses were performed based on the durations of pre-emptive analgesia administered before the start of surgery. Pre-emptive analgesia, which was given<1 hr before skin incision and/or 48-72 hrs before surgery of different types of operations, significantly reduced the risk of developing CPSP with the RR of 0.62 (P=0.001; 95% CI=0.46-0.83) and the RR of 0.42 (P=0.002; 95% CI=0.24-0.73) respectively (Figure 6).
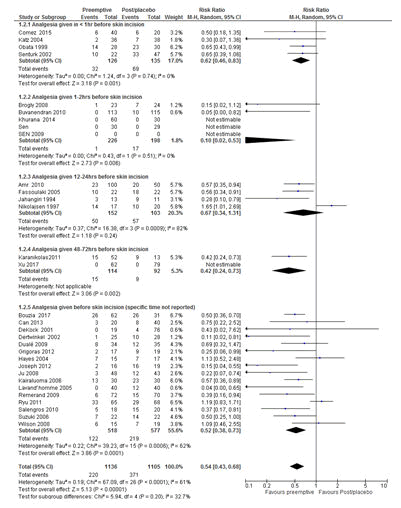
Figure 6: Forest plot of the comparisons of the effect of timing of pre-emptive analgesia administration before surgery on chronic postsurgical pain. The overall analysis result favoured pre-emptive analgesia for the prevention of CPSP irrespective of the time of administration of pre-emptive analgesia with a RR of 0.55 (P<0.00001, 95% CI=0.44-0.69). All studies were included in the analysis. RR=Relative risk, M-H=Mantel-Haenszel; CI=Confidence Interval.
Pre-emptive analgesia vs. prolonged blockade
One of the main challenges in determining the effectiveness of pre-emptive analgesia for the prevention of postoperative pain is the variation in the frequency of pre-emptive analgesia administration. Some clinicians (researchers) compared preoperative single dose with post-incisional/postoperative analgesia/control, whilst others compared repeated analgesia (preoperative analgesia followed by repeated intra and postoperative) with post-incisional analgesia/postoperative/placebo. In the current study, subgroup analyses were performed based on the frequency of analgesia provided. The results showed that the frequency of pre-emptive analgesia didn’t affect the effectiveness of pre-emptive analgesia in preventing the development of CPSP (Figure 7).
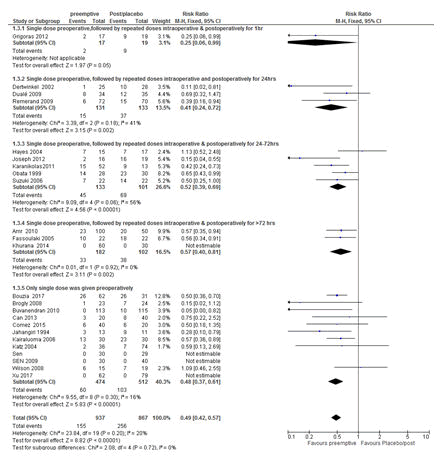
Figure 7: Forest plot of the impact of the frequency of pre-emptive analgesia administration on the prevention of chronic postsurgical pain (CPSP). The overall analysis favoured pre-emptive analgesia in reducing CPSP irrespective of the frequency of pre-emptive analgesia administration (pre-emptive analgesia alone compared with post-incisional/postoperative/placebo and pre-emptive analgesia followed by repeated intraoperative/postoperative doses of the same drug compared with post-incisional/postoperative/placebo) with a RR of 0.49 (P<0.00001, 95% CI= 0.42-0.57). All studies were included in the analysis. RR=Relative risk, M-H=Mantel-Haenszel. CI=Confidence Interval.
The routes of pre-emptive analgesia administration, type of surgery and postoperative follow-up times.
The subgroup analyses were performed on the primary outcome (CPSP) based on the route of administration of pre-emptive analgesia and postoperative follow-up times of studies. The subgroup analysis demonstrated that pre-emptive analgesics, which were provided intravenously at 3 months significantly reduced CPSP with the RRs of 0.33 (P=0.03; 95 CI=0.12-0.92) (Figure 8).
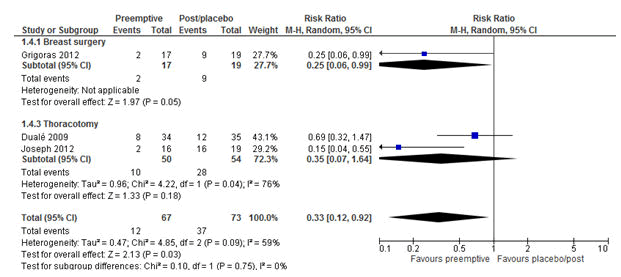
Figure 8: Forest plot of the incidence of chronic postsurgical pain: pre-emptive vs. post-incisional/placebo at 3 months, Intravenous studies only. The overall result favoured pre-emptive analgesia in reducing CPSP at 3 months irrespective of the type of operation with a RR of 0.33 (P=0.03, 95% CI=0.12-0.92). RR= Relative risk, M-H= Mantel-Haenszel. CI=Confidence Interval.
Additionally, the subgroup analysis demonstrated that intravenous administration of pre-emptive analgesia at 6 months significantly reduced CPSP with the RRs of 0.43 (P=0.001; 95% CI=0.25-0.72) (Figure 9).
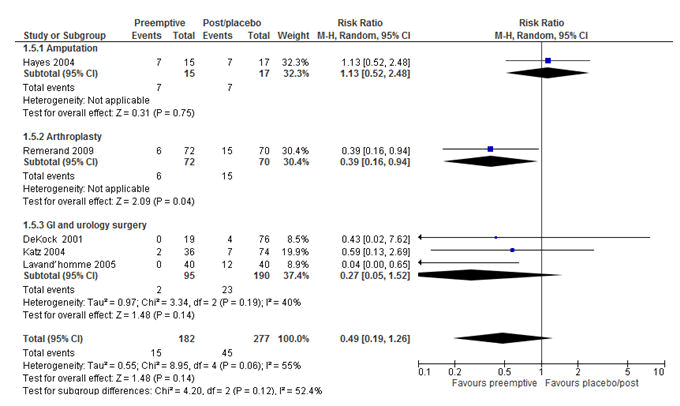
Figure 9: Forest plot of the incidence of chronic postsurgical pain (CPSP): pre-emptive vs. post-incisional/placebo at 6 months, Intravenous (IV) studies only. The overall result did not reach statistically significant. However, from the subgroup analyses IV pre-emptive analgesia was effective in reducing CPSP after arthroplasty only with the RR of 0.39 (P=0.04, 95% CI=0.16-0.94). RR=Relative risk, M-H=Mantel-Haenszel, CI=Confidence Interval.
Moreover, unlike at 3 months, pre-emptive analgesia which was given orally showed a statistically significant reduction of CPSP at 6 months with the RR of 0.44 (P<0.0001; 95% CI= 0.29-0.66) (Figure 10). This indicated that patients who were given oral pre-emptive analgesia were 56% less likely to develop CPSP at 6 months compared to their counter parts.
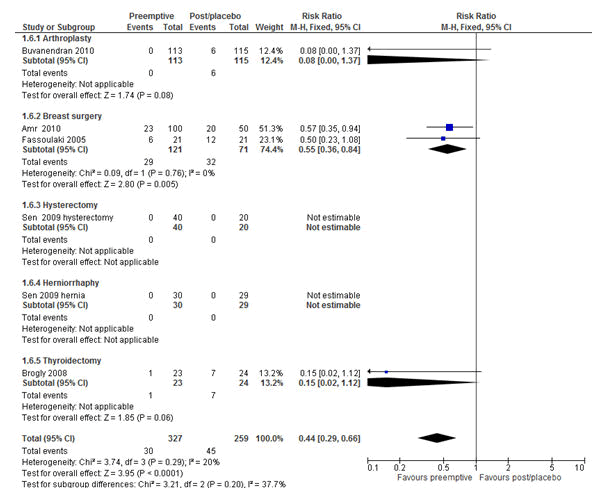
Figure 10: Forest plot of the incidence of chronic postsurgical pain (CPSP): pre-emptive vs. post-incisional/placebo at 6 months, oral studies only. The overall result favoured pre-emptive analgesia in reducing CPSP in patients who received oral pre-emptive analgesia compared to their counter parts with the RR of 0.44 (P<0.0001, 95% CI=0.29-0.66). RR=Relative risk, M-H=Mantel-Haenszel, CI= Confidence Interval.
Furthermore, unlike at 3 and 12 months, epidural pre-emptive analgesia at 6 months significantly reduced CPSP with the RR of 0.56 (P=0.007, 95% CI=0.37-0.85) (Figure 11).
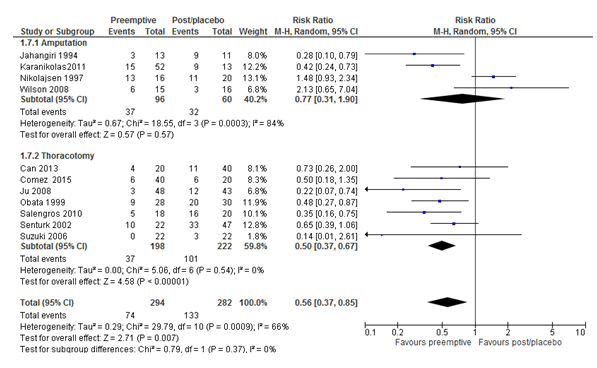
Figure 11: Forest plot of the incidence of chronic postsurgical pain (CPSP): pre-emptive vs. post-incisional/placebo at 6 months, epidural studies only. The overall analysis result favoured epidural pre-emptive analgesia in reducing CPSP with the RR of 0.56 (P=0.007, 95% CI=0.37-0.85). RR=Relative risk, M-H=Mantel-Haenszel, CI=Confidence Interval.
The trial sequential analyses for the routes of administrations of pre-emptive analgesics (where the types of surgery were sub-categorized under it) at different postoperative follow-up times was performed. The results revealed that no difference was observed between the pre-emptive and the placebo groups at any point postoperative follow-up times (P>0.05) (supplementary data 2). However, the TSA analysis was not employed for the other subgroups as the number of studies in each of the other groups was less than ten (the number of studies in each category should be at least ten to run the TSA analysis).
Pre-emptive analgesia is reported to be effective in preventing postoperative pain [5]. However, there is limited up-to-date evidence and of course, the results are contradictory with regard to the effectiveness of pre-emptive analgesia on the prevention of CPSP [2,3,55]. In the present review, the effectiveness of pre-emptive analgesia on the prevention of CPSP was evaluated with a particular focus on the parameters that determine the efficacy of pre-emptive analgesics like the timing of pre-emptive analgesia (duration of analgesia administration before skin incision), frequency of pre-emptive analgesia administration ( pre-emptive single dose vs. post-incisional/intraoperative/postoperative or pre-emptive analgesia followed by repeated intra and postoperative doses), routes of analgesia administration along with surgical types sub-categorized under it and postoperative follow-up times [55,56].
In the current review, whether the type of comparison in different clinical trials in terms of the initiation of pre-emptive analgesia in relation to the start of surgical incision such as pre-emptive analgesia vs. placebo vs. post-incisional analgesia vs. intraoperative/postoperative [55] could affect the effectiveness of pre-emptive analgesia in preventing CPSP was explored using the random effect model. In the present review, pre-emptive vs. post-incisional subgroup analysis demonstrated a statistically significant reduction of CPSP with the RR of 0.46 (P=0.0009, 95% CI=0.29-0.73) [15,34,40,43,46,52,53]. This implies that there is a 54% reduction of developing CPSP in patients who received pre-emptive analgesia compared to those who received analgesia after surgical incision. Additionally, pre-emptive vs. placebo subgroup analysis showed a statistically significant reduction of CPSP with the RR of 0.54 (P<0.001; 95% CI=0.42-0.68) [13,14,26-33,35-37,42,44,45,49,50]. However, no difference was observed between the pre-emptive and postoperative groups [38,39,41,47,48,51]. This lack of difference could due to the heterogeneities of the studies with regard to the type and/or extent of surgery, factors related with pre-emptive analgesia (type, route of administration), and time of pre-emptive analgesia administration in relation to the start of skin incision, postoperative analgesia and study designs [2]. And of course, this could also be due to the relative more efficacy of postoperative analgesia, not due to the inefficacy of pre-emptive analgesia [2].
Additionally, there is no evidence at what time the pre-emptive analgesia should be provided before surgical incision in order to obtain a longer analgesic effect than that particular drug actually supposed have in the context of pre-emptive analgesia to effectively prevent CPSP [2,55]. In the current review, pre-emptive analgesics which were given in<1 hr before skin incision [37,40,48,54] and 48-72 hrs before skin incision [38,53] significantly reduced the risk of developing CPSP with the RRs of 0.62 (P=0.001; 95% CI=0.46-0.83) and 0.42 (P=0.002; 95% CI= 0.24-0.73) in different types of operations respectively as tested using the random effect model. However, analgesics which were provided between 1-2 hrs [13,27,35,42,45] and 12-24 hrs before surgical incision in different types of operations [28,31,47,51] did not show any benefit. This could be due to the difference in the studies with regard to the type and efficacy of analgesics, type of surgery and other perioperative factors [2,55]. This warrants further study to determine optimal time for analgesia administration before surgical incision in the context of the nature of surgery and analgesia (type, duration of action and route of administration). On the other hand, in a large numbers of studies, the specific pre-emptive analgesia administration times before surgical incisions were not reported [14,26,29,30,32-34,36,39,41,43,44,46,49,50, 52]. This could make the assessment of the effectiveness of pre-emptive analgesics difficult [55].
Furthermore, the administration of adequate perioperative analgesia is crucial to effectively control postsurgical pain [5]. However, there is lack of evidence in the literature whether repeated administration of the same analgesic drug during the intraoperative and postoperative periods following a pre-emptive analgesia (prolonged blockade) could have an impact on the effectiveness of pre-emptive analgesia on the prevention of CPSP as compared to pre-emptive analgesia alone. Some studies compared pre-emptive analgesia with post-incisional/postoperative analgesia/placebo [13,14,26,27,29,34,36,37,39,42,43,45-48,51,53,54], whereas others compared pre-emptive analgesia followed by repeated intraoperative and postoperative doses of the same drug for the varieties of postoperative follow-up times with post-incisional/postoperative analgesia or placebo[28,30-33,35,38,40,44,49,50,52]. In the current review, the frequency of pre-emptive analgesia administration had not impact on the effectiveness of pre-emptive analgesia in reducing CPSP as tested using a fixed effect model. This could due to the inhibition of central sensitization by a pre-emptive analgesia [4,5,38].
The factors that could affect the effectiveness of pre-emptive analgesia such as the routes of administrations of pre-emptive analgesics were taken into account, where the types of operations were further sub-categorized under it. The analysis of studies with intravenous analgesia at 3 months follow-up time, which was tested using the random effect model revealed a significant reduction of CPSP with the RR of 0.33 (P=0.03; 95% CI=0.12-0.92). This implies that there was a 67% less chance of developing CPSP in patients who received pre-emptive analgesia intravenously compared with the post-incisional/postoperative/placebo group.
However, the intravenous pre-emptive analgesia was not effective in reducing CPSP after thoracotomy at 3 months follow-up as tested using the random effect model. This could be due to the fact that thoracotomy is one of the most invasive procedures that involve many visceral structures that could increase the risk of more tissue and nerve injuries during operation [34,44]. This could result in severe pain, and necessitates the administration of multimodal analgesia to adequately control pain [34,44].
Additionally, the overall analyses of the intravenous studies at 6 months as tested using the random effect model, showed no benefit on the reduction of CPSP with the RR of 0.49 (P=0.14, 95% CI=0.19-1.26). This negative result is difficult to explain as there were some studies with zero events, which were included in the analysis. However, further subgroup analyses based on the surgical types, the intravenous pre-emptive analgesia significantly reduced CPSP in patients who had arthroplasty with the RR of 0.39 (P=0.04, 95% CI=0.16-0.94).
Moreover, the overall analyses of the studies with oral routes as tested using a fixed effect model, pre-emptive analgesia was effective in preventing CPSP at 6 months with the RR of 0.44 (P<0.0001, 95% CI=0.29-0.66). Nonetheless, orally administered pre-emptive analgesia was not effective in preventing post-thoracotomy chronic pain at 6 months (P>0.05). The control of post-thoracotomy chronic pain is difficult due to the complex nature of the procedure, which could cause stretching of the thorax, lungs, pleura and the shoulder, and displacement of the joints of the vertebrae and ribs [34]. This showed that further clinical trial studies are needed by considering the perioperative factors, type of pre-emptive analgesia, routes of administration of analgesics, efficacy of analgesia, type and extent of operation to establish better preventive and treatment strategies for CPSP [2,4,5,55].
Furthermore, the analyses of all studies with epidural pre-emptive analgesia (surgical types sub-categorized under it) at 6 months follow-up using the random effect model, showed significant reduction of CPSP with RR 0.56 (P=0.007, 95% CI=0.37-0.85). Further subgroup analyses based on the surgical type showed that epidural pre-emptive was effective in reducing CPSP after thoracotomy with RR 0.5 (P<0.00001, 95% CI=0.37-0.67) but not after limb amputation, which could due to an already established central sensitization in patients who experienced phantom pain before pre-emptive analgesia [47].
Postoperative pain is caused by noxious stimulation after surgical injury [2,4,5]. This nociceptive pain is augmented by inflammatory mediators released from the surgical wound that reduces the activation threshold of peripheral sensory afferent nerve fibres that leads peripheral sensitization [2,4,5,57]. The afferent barrage of impulses coming from the site of injury increases the excitability of neurons in the central nervous system [2,4,5,57]. Central sensitization can be prevented if the activation of peripheral afferent nerve fibres by surgical injury is blocked by pre-emptive analgesia [2,4,5,57]. However, the efficacy of pre-emptive analgesia in the prevention of CPSP remains a controversial issue particularly in clinical studies as many factors contribute for postoperative pain and on the preventive effect of pre-emptive analgesia for both acute and CPSP [2]. This could be the case in the current review that pre-emptive analgesia was effective in reducing CPSP in the majority of studies [13,26-29,31,32,34-36,38,39,42,45,46,48-54]. However, no difference was observed between the pre-emptive and post-incisional/postoperative/placebo groups in eight studies with regard to the reduction of CPSP [14,30,33,37,41,43,44,47]. To tackle this problem, future clinical studies should focus on the assessment of the preoperative risk factors for pain, careful surgical handling of tissues and nerves, surgical specific evaluation using large sample sizes, and appropriate methodological designs with varieties of analgesic options to reach a firm conclusion [4].
The current review has a few limitations, which were the inherits of the trials reviewed;
This is the largest comprehensive meta-analysis so far with respect to the effectiveness of pre-emptive analgesia on the prevention of chronic postsurgical pain (CPSP). In this review, pre-emptive analgesia reduced CPSP in the majority of studies (n=22/30, 73.3%) at 3 months and beyond after surgery. Additionally, the factors that have not been addressed before such as the typeofpreemptiveanalgesiacomparisons(preemptivevs.pre/postincisional/postop/placebo) and timing of pre-emptive analgesia were explored, where the outcomes varied due to the heterogeneity of the studies reviewed. Pre-emptive analgesia was effective on the prevention of CPSP compared to both the placebo and postincisional groups (P<0.05). Surprisingly, no difference was observed between the pre-emptive analgesia and prolonged blockade (pre-emptive followed by repeated intraoperative and postoperative analgesia), presumably due to the inhibition of central sensitization by the pre-emptive analgesia [4,5,38]. However, variability was observed across studies in the effectiveness of pre-emptive analgesia on the prevention of CPSP with regard to the factors such as the timing of pre-emptive analgesia administration and as well as the routes of drug administrations at different postoperative follow up times.
However, pre-emptive analgesia did not show any benefit on the reduction of CPSP in a large number of studies at any point follow-up times. The efficacy of pre-emptive analgesia on the prevention of postoperative pain including CPSP remains a controversial issue particularly in the clinical studies as many factors contribute to postoperative pain and on the preventive effect of pre-emptive analgesia for postoperative pain [2]. This could be due to the heterogeneities in the clinical studies with regard to the study designs, sample size, type of pre-emptive analgesics, routes of drug administration, timing of pre-emptive analgesia, type/extent of surgery and postoperative follow-up times [2,57].
A prospective clinical trial studies should be conducted in a large cohort of patients in relation to preoperative risk factors for pain, pre-emptive analgesia (type, route, timing, duration of action), type and extent of surgery, postoperative follow-up times and large sample size with appropriate methodological designs to strengthen the current findings. Additionally, meticulous handling of the tissues (and or nerve) during operation along with surgical specific assessment of CPSP with multimodal analgesia in comparison to a single drug (pre-emptive vs. postincisional/postoperative/placebo) will have an immense value in the clinical settings to determine which technique is appropriate to prevent CPSP.
Declarations
Not applicable.
Authors' Contributions: EGG conceived the idea, conducted the literature search, assessed the quality of studies, analysed the data and drafted the manuscript. WMS performed literature search, assessed the quality of studies and drafted the manuscript. All authors have read the final manuscript and agreed to publish it in the BMC Perioperative Medicine.
Funding: The authors declare that there is no funding.
Availability of data and materials: The supporting materials used in this review are contained within the manuscript.
Ethics approval and consent to participate: Not applicable.
Consent for publication: Not applicable.
Competing interests: The authors declare that they have no competing interests.
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
Citation: Gebremedhn EG, Sefefe WM (2022) Pre-emptive Analgesia for the Prevention of Chronic Postsurgical Pain: A Systematic Review and Meta-analysis with Trial Sequential Analysis. J Anesth Clin Res. 13:054
Received: 28-Nov-2022, Manuscript No. JACR-21-14863; Editor assigned: 01-Dec-2022, Pre QC No. JACR-21-14863; Reviewed: 16-Dec-2022, QC No. JACR-21-14863; Revised: 23-Dec-2022, Manuscript No. JACR-21-14863; Accepted: 30-Dec-2023 Published: 31-Dec-2022 , DOI: DOI: 10.35248/2155-6148.22.13.1054.
Copyright: © 2022 Gebremedhn EG, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.