Pancreatic Disorders & Therapy
Open Access
ISSN: 2165-7092
ISSN: 2165-7092
Research Article - (2022)Volume 12, Issue 3
The impact of delayed graft function on outcomes following various solid organ transplants is well documented and addressed in the literature. Delayed graft function following various solid organ transplants is associated with both short- and long-term graft survival issues. In a retrospective cohort study including 106 patients we evaluated whether pancreas graft survival differs according to moment of insulin therapy following simultaneous pancreaskidney transplant. As a result, we aimed to identify possible risk factors and build a machine-learning-based model that predicts the likelihood of dysfunction following SPK transplant patients based on day zero data after transplant, allowing to enhance pancreatic graft survival. Feature selection by Relief algorithm yielded donor features, age, cause of death, hemoglobin, gender, ventilation days, days in ICU, length of cardiac respiratory arrest and recipient features, gender, long-term insulin, dialysis type, time of diabetes mellitus, vPRA pre-Tx, number of HLA-A mismatches and PRDI, all contributed to the models' strength.
Pancreas transplantation; Rejection; Graft survival
ASA: American Society of Anesthesiologists Classification; AUC-ROC: Area Under the Curve- Receiver Operating Characteristics; BMI: Body Mass Index; CA: Classification Accuracy; CDC-CMx: Crossmatch; CHULC: Centro Hospitalar Universitário de Lisboa Central; dEPGF: Delayed Endocrine Pancreas Graft Function; DGF: Delayed Graft Function; DM: Diabetes Mellitus; DRB: Protein Encoded by the HLA-DRB1 Gene; DSA: Donor-specific Antibody; EIS: Exogenous Insulin Support; Hb: Hemoglobin; HbA1c: Glycated Hemoglobin; HLA: Human Leukocyte Antigen; ICU: Intensive Care Unit; IV: Intravenous; IVC: Inferior Vena Cava; MFI: Mean Fluorescence Intensity; MM: Mismatch; PRA-CDC: Panel-Reactive Antibodies-Complement-Dependent Cytotoxicity; PRDI: Pancreas Risk Donor Index; rATG-Thymoglobulin: Polyclonal Rabbit Antithymocyte Globulin; RReliefF: Relief for Regression; SPK: Simultaneous Pancreas-Kidney; Tx: Transplant; vPRA: Virtual Panel Reactive Antibodies
The effects of delayed graft function on outcomes after various solid organ transplants are well known and discussed in the literature. It is widely understood that delayed graft function following renal transplantation is linked to both short and longterm graft survival adverse effects [1,2]. However, to date, evidence for pancreas transplantation is lacking. Furthermore, there are no established diagnostic criteria for delayed endocrine pancreatic graft function (dEPGF) after pancreas transplantation that can be used to compare and interpret outcomes across institutions [3].
Pancreatic cells have been shown to begin producing insulin as soon as five minutes after reperfusion. As early as twenty-five minutes after reperfusion, the restoration of a physiological axis (correlation between blood glucose levels and C-peptide release) has been observed [2]. Nevertheless, the percentage of islet cell function required to establish euglycemia soon after transplantation is uncertain. Some centers have reported satisfactory endocrine function shortly after pancreas reperfusion, but further details have not been provided [1,2].
Delayed graft function requires temporary graft substitution or medical treatment: Post-transplant dialysis (kidney transplant), plasmatic coagulation factor substitution (liver transplant) or post-transplant support with inotropic drugs (heart transplant). Exogenous insulin is the treatment of choice for selected individuals with type I insulin-dependent diabetes mellitus and delayed endocrine graft function after pancreatic transplantation.
Several groups have tried to address dEPGF, however and by opposition to other solid organ transplantation no consensus has been achieved. For instance, Troppmann et al. defined dEPGF as a total, cumulative insulin demand of >30 U between days 5 and 10, and/or >15 U between days 11 and 15. This group reported a 69% incidence of dEPGF using this definition. This percentage appears to be high, especially when compared to other solid organ grafts [3]. Tan et al. defined dEPGF as the requirement for exogenous insulin at the time of hospital discharge, with a 33 percent overall incidence [3]. Maglione et al. in 2010 characterized dEPDF as the need for temporary medical care for insufficient graft function with a lower incidence (18.6%) [4]. In 2012, Qureshi et al. defined dEPGF as the requirement for exogenous insulin to control hyperglycemia within the first week following transplantation, without specifying the amount of insulin required [5]. The relationship between the prevalence of dEPGF and graft survival is uncertain.
We aimed to evaluate whether pancreas graft survival differs according to the moment of insulin necessity following simultaneous pancreas-kidney transplant. We also aimed to identify possible risk factors and develop a machine-learning-based model that would measures the likelihood of dysfunction after SPK transplant patients on day zero after transplant allowing to take actions to optimize and improve pancreatic graft survival.
We performed a single-center retrospective cohort study. Between March 2011 and January 2020, all recipients of technically successful simultaneous pancreas-kidney transplants obtained from systemic-drained, whole-organ brain-dead donors at Hospital Curry Cabral-Centro Hospitalar e Universitário de Lisboa Central who had complete documentation of post-transplant endocrine parameters (n=106) were identified in this retrospective cohort study. Patients that presented primary non-function (n=5), missing donor data (n=4), splenic artery thrombosis (n=4), splenic venous thrombosis (n=17), renal artery thrombosis (n=1) and stage V Clavien-Dindo classification (n=2) were excluded (Figure 1). A total of 73 participants were included and evaluated until March 2021 in this study. Patients data was retrieved from the SClinic database and all patients had given their informed consent to participate in the study. This study was approved by the CHULC ethics committee n º 985/2020. There were 42 overall features considered, 12 categorized and 30 numerical, grouped into three categories: Recipient, donor, and peri-operative. Recipient features: Age at transplantation, gender, body mass index (BMI), type of dialysis (hemodialysis, peritoneal or pre-emptive), dialysis time until transplantation, time of diabetes until transplantation, longterm insulin dose before transplantation, smoking habits, cardiac ejection fraction, previous pregnancy, previous transfusion of red blood cells, panel-reactive antibodies–complement-dependent cytotoxicity (PRA-CDC), number of previous transfusions of red blood cells, ethnicity, HLA-ABDR, donor-specific antibodies pretransplant, virtual panel reactive antibodies (vPRA), creatinine, and glycated hemoglobin; donor features: Age, gender, BMI, cause of death (hemorrhagic stroke, ischemic stroke, traumatic brain injury or anoxic encephalopathy), days in intensive care unit, ventilation days, cardio-respiratory arrest, catecholamines, hemoglobin, creatinine, urea, c-reactive protein and pancreas donor risk index (PDRI). Total operating time, cold ischemia time, warm ischemia time, intra-operative blood supply, gastroduodenal to hepatic artery reconstruction, American Society of Anesthesiology Classification (ASA) and insulin dose at discharge were all surgical or intra-operative aspects. After transplant, we monitored qualitative aspects of endocrine pancreas graft performance. During the first two weeks, all patients with poor graft function who required exogenous insulin administration were assessed for dEPGF risk factors. As a result, we looked at the longterm results of grafts with and without dEPGF. The Kaplan-Meier estimator was used to examine post-transplant patient, graft, and death-censored graft survival (IBM SPSS Statistics v.28.0). The goal of feature selection was to find the subset of parameters that had the greatest impact on the predictive model design. This is a critical part of model development because unnecessary factors can significantly degrade prediction. Information gain, chi-square, information gain ratio, Gini coefficient, and quick correlation based filter were all employed in this study to select and rank features. Due to the limited size of the training set, a leave-one-out cross-validation procedure was used; this model was evaluated for each held-out observation (n-1 observations) and the final result was obtained by calculating the mean of all individual evaluations [4].
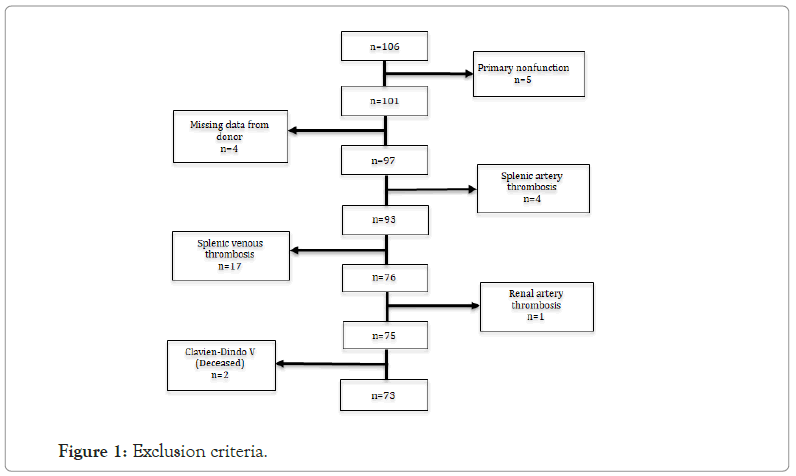
Figure 1: Exclusion criteria.
The area under the curve-receiver operating characteristics (AUCROC) was used to evaluate model fitting; however, classification accuracy (CA), f-1 score, precision and recall were also taken into account. All experiments in this work were carried out with the Orange data mining open-source machine learning software (version v3.29.3) (University of Ljubljana, Ljubljana, Slovenia) [6].
Algorithms
Several machine learning techniques (supplementary data) were examined as well as two different types of supervised classification models: AdaBoost (AB) and Decision Tree (Tm). The performance, AUC and CA of these models were used to select them for inclusion in this study. These classification models are also given a brief description.
Adaboost
The AdaBoost algorithm, also known as Adaptive Boosting, is used to develop a very accurate prediction rule. The algorithm combines numerous weaker and inaccurate rules to achieve a highly accurate prediction rule.
Decision tree
The decision tree algorithm is one of the most popular and intriguing models in machine learning, mainly due to its simplicity and ability to divide data into nodes based on class purity. The idea is to create a tree split with decision nodes related to each other by branches from the root node to the leaf node (end) (Figure 2). At the decision node, the attribute is evaluated and each conclusion results in a branch. Each branch is guided to another node or to the end node to generate a decision [7-9].
Figure 2: Tree model.
Procurement Operation, back-table bench and recipient surgery have been described in detail by our group in a previous publication [10].
Immunosuppression protocol
T cell-depleting antibodies (such as polyclonal rabbit antithymocyte globulin rATG-thymoglobulin) at 1.5 mg/Kg are injected 2-4 hours before surgery and continued intraoperatively if needed. This is followed by 1.5 to 2 mg/kg of rATG-Thymoglobulin each day for the next six days, for a total of seven doses. If the white blood cell count is less than 2000/microL and/or the platelet count is less than 75,000/microL, the rATG-Thymoglobulin dose is lowered or even halted. During the first three days after transplantation, three 500 mg IV methylprednisolone injections are given. The first is given before surgery, while the others are given on day 1 and 2 after surgery. Oral prednisone at a dose of 20 mg once daily is administered after the third day of transplantation for the first 2-3 weeks and then decreased to 5 mg once daily by three months. The antimetabolite mycophenolate mofetil (MMF) is the third immunosuppressive medication in our program. Before surgery, it is administered orally at a dose of 500 mg. A dose of 250 mg is administered IV twice daily after surgery until day 5 post-surgery. We move from IV to enteric-coated mycophenolate sodium (ECMPS) 360 mg orally twice daily once the patient is able to take oral medication (usually on postoperative day 4 or 5).
Regarding rejection treatment, we administer 3 daily pulses of 500 mg methylprednisolone to patients with acute cellular rejection, followed by 1.5 mg/kg rabbit rATG-Thymoglobulin. The major goal of treatment in patients with antibody-mediated rejection is to eliminate the clonal population of B cells or plasma cells that produces the donor-specific antibody (DSA). In case of antibodymediated rejection we perform five plasmapheresis sessions followed by intravenous immune globulin (IVIG), and following the fifth plasmapheresis session, we perform an anti-B cell therapy with a 500 mg Rituximab IV infusion.
Donor and patient HLA typing
Patient typing was done with a polymerase chain reaction sequencespecific oligonucleotide (PCR-SSO) bead-array (One Lambda, Canoga Park, CA, USA) at low/intermediate resolution for loci HLA-A, -B, -C, and DRB1 according to the manufacturer's recommendations. In the past, donors' HLA typing was done using a polymerase chain reaction sequence-specific primer (PCRSSP) for loci HLA-A, -B, -DRB1 (Inno-Train Diagnostik GmbH, Kornberg, Germany), and from 2015 onwards, donors have been tested for loci HLA-A, -B, -C, DRB1, -DQA1, -DQB1, -DPA1 and -DPB (Inno-Train Diagnostik GmbH, Kornberg, Germany).
Crossmatch and detection of DSA
All patients presented a negative complement-dependent cytotoxicity crossmatch (CDC-CMx) transplant utilizing current sera acquired during the previous three months. A prospective flow cytometry crossmatch was performed with peak and current sera; DSA were identified using solid phase assay single antigen beads (LABscreen, One Lambda, Canoga Park, CA, USA), and positivity was considered for HLA-A, -B, -C, -DRB1 and DQB1 whenever the mean fluorescence intensity (MFI) was greater than 1000. These analyses were conducted using single antigen beads according to the manufacturer's specified methodology.
Virtual PRA (vPRA)
The patient's alloreactivity against anti-HLA antibodies identified by single antigen beads is taken into account while calculating vPRA (LS1A04 and LS2A01). All HLA-A, -B, -C, -DRB1, and -DQB1 antibodies with MFI>1000 were examined. These antibodies were evaluated in silico against the HLA genotyping of 2668 Portuguese individuals (HLA-A, B, C, DRB1, and DQB1), with the vPRA calculation equaling the ratio of positive donors/total number of donors *100.
We divided patients into six groups based on when they started taking insulin following SPK transplantation shown in Table 1. The results show which features contribute to the time when each graft starts endocrine function. This preliminary data allowed us to classify patients with grafts that required different levels of exogenous insulin, such as those who required insulin on a daily basis (19%; n=14), never required insulin (15%; n=11), and those who required exogenous insulin for one day (29%; n=21), two days (15%; n=11), three days (14%; n=10) and four days (8%; n=6).
| Mode | Definition of pancreas endocrine DGF | % of patients with DGF (n) | Feature selection (Top scoring method) | Features included (n) description | Top models (AUC-ROC/CA) |
|---|---|---|---|---|---|
| 1 | Need for scheduled exogenous insulin at the time of discharge from hospital after a technically successful SKP transplant | 19% (14) | Information gain |
|
|
| 2 | No need for scheduled exogenous insulin after a technically successful SKP transplant | 84% (62) | Chi-square |
|
|
| 3 | Need for scheduled exogenous insulin at least 1 day after a technically successful SKP transplant | 56% (41) | Chi-square |
|
kNN (0.67/0.63) |
| 4 | Need for scheduled exogenous insulin at least 2 days after a technically successful SKP transplant | 41% (30) | Information gain ratio |
|
Naïve bayes (0.85/0.81) |
| 5 | Need for scheduled exogenous insulin at least 3 days after a technically successful SKP transplant | 27% (20) | Gini coefficient |
|
Naïve bayes (0.85/0.79) |
| 6 | Need for scheduled exogenous insulin at least 4 days after a technically successful SKP transplant | 19% (14) | FCBF (fast correlation based filter) |
|
Naïve bayes (0.88/0.82) |
|
|||||
| 7 | Stop and start | 15% (11) | Gini coefficient |
|
Naïve bayes (0.93/0.89) |
Note: Feature with p<0.05; donor-related features Where (D)=Recipient-related features; (R)=Surgical-related features; (S)=Hb-hemoglobin; DM=Diabetes mellitus; BMI=Body mass index; HbA1c=Glycated hemoglobin; ICU=Intensive Care Unit; ASA=American Society of Anesthesiologists Classification; vPRA=Virtual Panel Reactive Antibodies; N MM=Number HLA mismatch; PRDI=Pancreas Risk Donor Index; AUC-ROC=Area under the curve-receiver operating characteristics; CA=Classification accuracy
Table 1: Stratification of patients in six models based on time of insulin intake after SPK transplantation.
We noticed a "Stop and Start" subgroup in this cohort of patients; these patients stop using exogenous insulin after the transplant but then resume using it for a period of time before stopping while still in the hospital or after discharge. This group (n=11) accounts for 15% of the patients. We then assessed the impact of delayed and non-delayed graft function on each group's graft survival (Figure 3). There is a clear difference between exogenous insulin support and graft survival in these Kaplan-Meier curves. The best pancreatic grafts were those that did not require insulin and had insulin assistance within the first four days after transplantation. It's worth noting that patients who stopped exogenous insulin twentyfour hours after transplantation had a slightly lower survival rate, although it was still above 95% at ten years. At three years, the "Stop and Start" group has a lower survival rate than the other groups, but after that, they have a higher survival rate than the graft groups that continuously required insulin and had a two-day supply of insulin. At 5 years, the 3-day insulin-requiring group has an overall graft survival rate of 80%. In terms of long-term graft survival, the two-days of exogenous insulin support (EIS) were the worst, much beyond the expected group that required constant insulin support. The 14 features identified by RReliefF were: Cause of death, donor's hemoglobin, type of dialysis, DM Previous Treatment Long Term Insulin U/I/day, donor's gender, vPRA Pre-Tx, number of HLA-A mismatches, PRDI, ventilation days, recipient gender, ICU days, donor's age and duration of donor's cardio respiratory arrest, all of which contributed to the models' strength. Based on pre-transplant and peri-operative data, two Machine Learning models (Tree and AdaBoost) were developed to stratify patients according to their EIS days. The R2 for the Tree and AdaBoost models was 0.96 and 0.82, respectively.
Figure 3: Graft survival. Note: Where dark blue=discharge with insulin; green=never need insulin; dark yellow=1 day insulin; pink=2 days insulin; lite yellow=3 days insulin; red=4 days insulin; sky blue=stop and start
Delay in graft function has been shown in several trials to have a negative impact on graft survival in kidney, liver, and heart transplantation [13-20]. Tan et al. found a trend toward lower graft survival in SPK patients with dEPGF at 3 years after transplantation while Maglione et al. found a statistically significant decrease in patient survival in the dEPGF group [12]. In this work, we looked at the temporal demand for exogenous insulin in each pancreas transplant after reperfusion, as well as the impact of this supply on graft survival. After excluding patients with primary nonfunction and patients who had a thrombotic event that impaired graft function (not the goal of this study), the individuals identified in each group showed uneven survival, with an emphasis on the group of individuals who required insulin for two days, where graft survival at three years dropped significantly with survival at the level of grafts that never took off being the worst of all groups. This can be explained by the fact that they are statistically equal distances from the best grafts (those that never required insulin and those that only required twenty-four hours of insulin) and those that required four days of exogenous insulin supply.
We built a model based on the EIS that allows us to know the chance of a given individual falling into one of these survival categories on day 0 post-transplantation. The Tree model, which had an R2 of 96% had the highest accuracy. Based on pre- and peri-operative clinical and immunological parameters, we can estimate the median number of days a certain individual will require exogenous insulin following reperfusion. This is the first translational study to show that using machine learning algorithms, we can identify which grafts require more surveillance and/or exogenous insulin, as well as allow for prompt immunosuppressive management to reduce the likelihood of graft failure. In Figures 4A,4B the most important elements of the Tree model are described and ranked in order of importance. In our study, the median number of days of insulin administration was 3.8 (IQR 38.8). Individuals with the best graft survival (median of days without exogenous insulin after transplantation) had a vPRA of less than 97 percent, were taking less than 46 units of slow insulin per day prior to transplantation, donor did not have cardio-circulatory arrest and had a hemoglobin value of less than 10.7 g/dl, donor's PRDI is greater than 0.75, the number of days in ICU of the donor is fewer than four and the number of mismatches in HLA-A is less than or equal to one.
Figure 4A: Feature importance as per tree model.
Figure 4B: Feature importance as per Adaboost.
An example of a practical use of this model is the capacity to identify a specific group of patients, in this case those who required two days of exogenous insulin (lower survival group), Individuals with a donor PRDI of less than 0.75 have a vPRA of less than 97 percent, were taking less than 46 units of slow insulin per day prior to transplantation, did not have cardio-circulatory arrest, and had a hemoglobin value of less than 10.7 g/dl. The donor PRDI is less than 0.75 if the mismatch number in HLA-A is less than or equal to one.
Interestingly, while never ceasing to have exogenous insulin supply, all of the patients with grafts required less insulin than before the transplant, implying that these grafts can also maintain some residual function, specifically endocrine and exocrine function.
Based on the allograft survival of this cohort of patients, one of the considerations or questions we should ask ourselves is whether we should stop exogenous insulin supply when allograft insulin reaches a certain value or whether we should prolong exogenous insulin supply to give the graft more time to recover from the ischemia vs. reperfusion phenomena, or else, handling immunosuppression in order to reduce the immunological aggression, based on studies performed in the postoperative period (e.g., appearance of DSA), even though this was not the focus of this work.
As strengths of this study we mention the number of patients in the study for a single transplant center and the use of Machine Learning methods that are not often used in data processing. A limitation in the model is that it doesn't identify patients who do not require exogenous insulin anymore but did at one point need it and did not receive it because they were in the hospital or were in an outpatient clinic (Stop and start group). This could be the subject of future research.
In conclusion, the lack of pancreatic grafts for the growing needs of pancreatic transplant waiting lists makes it vital to minimize the loss of post-transplant grafts as much as possible. It is possible to identify recipients at risk of decreased graft survival with high accuracy using features known at the time of transplant from the donor, recipient and transplant, providing versatile and feasible machine learning tools for support in clinical decision-making in order to change protocols, namely the exogenous supply of insulin and/or immunosuppression. So, with the use of versatile and practicable machine learning models it is possible to identify patients at risk of decreased graft survival in order to improve clinical decision-making and adjust the protocols of exogenous insulin administration as well as immunosuppression in the immediate post-transplant period.
[Crossref] [Google scholar] [Pubmed]
[Crossref] [Google scholar] [Pubmed]
[Crossref] [Google scholar] [Pubmed]
[Crossref] [Google scholar] [Pubmed]
[Crossref] [Google scholar] [Pubmed]
[Crossref] [Google scholar] [Pubmed]
[Crossref] [Google scholar] [Pubmed]
[Crossref] [Google scholar] [Pubmed]
[Crossref] [Google scholar] [Pubmed]
[Crossref] [Google scholar] [Pubmed]
[Crossref] [Google scholar] [Pubmed]
[Crossref] [Google scholar] [Pubmed]
[Crossref] [Google scholar] [Pubmed]
[Crossref] [Google scholar] [Pubmed]
[Crossref] [Google scholar] [Pubmed]
[Crossref] [Google scholar] [Pubmed]
Citation: Vigia E, Ramalhete L, Ribeiro R, Barros I, Chumbinho B, Filipe E, et al. (2022) Predicting Function Delay with a Machine Learning Model Improve the Long-term Survival of Pancreatic Grafts. Pancreat Disord Ther. 12:231.
Received: 25-Jul-2022, Manuscript No. PDT-22-18438; Editor assigned: 29-Jul-2022, Pre QC No. PDT-22-18438; Reviewed: 12-Aug-2022, QC No. PDT-22-18438; Revised: 19-Aug-2022, Manuscript No. PDT-22-18438; Published: 26-Aug-2022 , DOI: 10.35248/2165-7092.22.12.231
Copyright: © 2022 Vigia E, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited