Journal of Sleep Disorders & Therapy
Open Access
ISSN: 2167-0277
ISSN: 2167-0277
Research Article - (2023)Volume 12, Issue 11
Autism Spectrum Disorder (ASD) is a group of neurodevelopmental disorders, with a prevalence rate of approximately 1% to 2% in the general population. Over 80% of individuals with ASD experience comorbid sleep disturbances, exacerbating daytime behavioral problems and significantly impacting the overall prognosis of ASD. Repetitive Transcranial Magnetic Stimulation (rTMS) is a non-invasive treatment method that holds assurance in improving sleep quality, optimizing sleep structure, and maintaining therapeutic effects. However, the effectiveness of rTMS for treating sleep disturbances in ASD patients remains uncertain. In this review, our aim is to summarize the potential causes of sleep disturbances in ASD and explore the existing literature on treating ASD-related sleep issues with rTMS. Current studies suggest that rTMS may regulate abnormal gene expression, slow wave activity, and alleviate core symptoms and mental complications of ASD, which are closely linked to sleep disturbances. Furthermore, our review identified 11 studies on the treatment of sleep disturbances in ASD patients using rTMS. We provide a summary of the characteristics of these studies and analyze the theoretical basis for the use of rTMS in addressing sleep disturbances in individuals with ASD. These findings are encouraging and suggest that rTMS may hold assurance as a potentially effective intervention for managing sleep disturbances in individuals with ASD.
Autism Spectrum Disorder (ASD); Non-invasive therapy; repetitive Transcranial Magnetic Stimulation (rTMS); Sleep disturbances; Insomnia
Autism Spectrum Disorder (ASD) is a lifelong neurodevelopmental disorder with a high prevalence (1%-2%) in the general population [1]. It is characterized by core symptoms such as deficits in social communication and restricted, repetitive sensory-motor behaviors. ASD can be reliably diagnosed as early as 24 months of age [2]. Patients with ASD has significant clinical heterogeneity often combined with many medical and behavioral conditions such as epilepsy, gastrointestinal disturbances, attention deficit hyperactivity disorder, sensory abnormalities, and sleep disturbances. Each of these comorbidities can pose a serious challenge for treatment and long-term prognosis [3,4].
Among the above conditions, sleep disturbances are one of the most prevalent comorbidities, affecting up to 80% of individuals with ASD [5,6]. Sleep plays a vital role in brain structure and activity modulation during early development. It is essential for promoting healthy cognitive and psychosocial development, and disruptions in sleep patterns during early life can lead to atypical development [7-9]. A recent study conducted on a birth cohort of 1096 children discovered a prospective association between the frequency of night awakenings at 12 months of age and ASD screening scores at 24 months of age [10]. Sleep onset difficulties during the first year of life have been associated with abnormal neurodevelopmental patterns in infants [11]. Additionally, sleep disturbances may be exacerbated and aggravated and have a trend to remain persistent and chronic over time due to biological changes in puberty for most patients with ASD [12,13]. Furthermore, there is no unitary form of sleep disturbances, insomnia and circadian sleep-wake rhythm disorders, including difficulty falling asleep, increased sleep onset latency, reduced Total Sleep Time (TST), increased Wake After Sleep Onset (WASO), and poor Sleep Efficiency (SE), bed resistance, parasomnias, obstructive sleep apnea, and sleep anxiety, are the most consistently reported sleep disturbances in ASD [14,15]. Previous studies have found a bidirectional association between daytime behaviors commonly observed in individuals with ASD, such as stereotypy, restricted and repetitive behavior, self-injurious, and sleep disturbances, where worsening or improving one condition will similarly impact the others [16].
Considering the significant impact of sleep disturbances on individuals with ASD, it has become increasingly recognized and addressed as a comorbid condition in the clinical management of children with ASD [17-19]. Understanding the underlying causes of sleep disturbances in this population and exploring novel, safe, and effective treatments to enhance the quality of life for individuals with ASD are now important areas of focus in current research.
Hypotheses for the cause of sleep disturbances in ASD
While the exact causes of sleep disturbances in children with ASD remain unclear, researchers have proposed four potential mechanisms to explain these disruptions [20]. Firstly, studies employing Whole-Exome Sequencing (WES) and Genome- Wide Sequencing (GWS) in ASD have identified genetic factors associated with the disorder. Specifically, a number of mutations have been identified in genes responsible for the synthesis and processing of key neurotransmitters involved in maintaining the sleep-wake cycle, including Gamma-Aminobutyric Acid (GABA), melatonin, serotonin, dopamine, acetylcholine, and glutamate etc. [21-23]. GABA is the primary inhibitory neurotransmitter in the central nervous system, and it helps to facilitate sleep by reducing neuronal activity and promoting relaxation [24]. Activation of the GABA-A receptor by GABA can enhance Non- Rapid Eye Movement (NREM) spindles and suppress voluntary muscle tone during Rapid Eye Movement (REM) sleep [25]. Reduced GABA levels or impaired GABAergic function can lead to difficulties falling asleep, staying asleep, or experiencing non- restorative sleep. Melatonin, another important neurotransmitter, plays a vital role in regulating sleep by modulating the body’s internal clock and promoting the onset and maintenance of sleep [26,27]. Disruptions in sleep-promoting transmitters such as melatonin abnormalities, have been found in ASD children with sleep disturbances which maybe a direct consequence of it [20]. Serotonin plays an important role in promoting and maintaining sleep, particularly the deep, restorative stages of sleep by inhibiting wakefulness-promoting regions of the brain and promoting the activity of sleep-promoting pathways [28,29]. The role of dopamine, acetylcholine, and glutamate are primarily associated with wakefulness and arousal [28]. Imbalances or dysfunctions in these systems can contribute to sleep disturbances in ASD.
Second, sleep disturbances may be caused by core symptoms of ASD [20]. As mentioned above, there is a bidirectional relationship between ASD core symptoms and sleep, core symptoms can compromise sleep and vice versa [16]. On one hand, sleep disturbances like reduced Total Sleep Time (TST), resistance at bedtime, sleep-wake rhythm disorders, Wake After Sleep Onset (WASO) involving screaming, reduced Sleep Efficiency (SE), and parasomnias can directly affect daytime behaviors [30]. When individuals with ASD experience poor sleep quality or quantity, it can exacerbate their daytime symptoms, leading to increased irritability, hyperactivity, or difficulties with attention and social interaction. On the other hand, the daytime behaviors associated with ASD can also influence sleep patterns. Stereotypic or repetitive behaviors, self-injurious behaviors, and other challenging behaviors can interfere with falling asleep or maintaining sleep, leading to increased sleep onset latency, frequent awakenings, or disrupted sleep-wake rhythms [16]. Communication and social defects in ASD, can interfere with socially relevant circadian rhythm cues, making it difficult for children to understand parents’ intention and preventing children from going to bed [20]. Therefore, all these symptoms may lead to difficulty in falling asleep and insomnia.
Third, sleep problems may represent a co-occurring condition independent of ASD and associated with psychiatric comorbidities [20]. All we all known, patients with ASD are at high risk for gastrointestinal disturbances (23-70%), anxiety (39.6%), depressive (26%), Attention Deficit Hyperactivity Disorder (ADHD) (28.2%), and behavioral problems such as irritability and aggression (25%) [3, 31]. Several studies have demonstrated a strong association between sleep disturbances and psychiatric symptoms in individuals with ASD [16,32].
Fourth, sleep homeostasis is a critical mechanism in regulating sleep that generates sleep pressure based on the duration of wakefulness and can be measured by the power of Slow-Wave Activity (SWA) [33]. A higher sleep pressure can lead to deeper Slow-Wave Sleep (SWS) [33]. However, children with ASD may experience disorders in sleep homeostasis, which are characterized by reduced sleep pressure and increased alertness [34]. Ayelet et al. discovered that individuals with ASD exhibited weaker SWA power, shallower SWA slopes, and a reduced percentage of slow- wave sleep compared to normal controls, especially during the first two hours after sleep onset [34]. They hypothesized that reduced sleep pressure indicated by amplitude of SWA power may contribute to sleep disturbances in ASD patients.
The current status of treatment for insomnia in ASD
Considering the important role of sleep in neurodevelopmental processes, various pharmacological and non-pharmacological approaches have been explored to address sleep difficulties in individuals with ASD [35,36].
While no medication has received approval from the U.S. Food and Drug Administration (FDA) specifically for treating sleep disturbances in ASD, melatonin has been recommended as a primary option (Level B) by the American Academy of Neurology when behavioral strategies are not effective [17,19]. Studies have found that taking melatonin supplementation is a potential therapeutic intervention for improving sleep quality in individuals with ASD, including increased total sleep duration, reduced sleep latency, and improved sleep efficiency [37-39]. Recently, pediatric-appropriate prolonged-release melatonin mini-tablets have gained approval for treating insomnia in ASD by regulatory agencies such as the European Medicines Agency (EMA), Swiss Agency for Therapeutic Products (Swissmedic), Therapeutic Goods Administration (TGA), Ministry of Health (MOH), and Medicines and Healthcare products Regulatory Agency (MHRA). It is worth noting that mild side effects like headaches and daytime somnolence have been reported with melatonin use [37].
In addition to pharmacotherapy, non-pharmacological interventions such as Cognitive-Behavioral Treatment (CBT), weighted blankets, and parent-based sleep education have been employed to address sleep disturbances in children with ASD [35]. A pilot study focused on school-aged children with high- functioning ASD demonstrated that CBT is a viable and positive treatment option to enhance sleep and overall functioning for both children and parents [40]. However, it is important to acknowledge that poor treatment response is frequently reported in ASD individuals undergoing CBT, primarily due to the presence of intellectual disability and non-cooperative behavior commonly observed in children with ASD. Furthermore, regarding the use of weighted blankets, randomized controlled trials investigating their efficacy in managing sleep disturbances in ASD found no significant group differences in objective measures (actigraphy) or subjective assessments of sleep [41,42]. As for parent-based sleep education, objective changes in sleep detected by actigraphy is controversial [43-45]. Evidence is insufficient to determine the effect of parental sleep-specific behavioral training [46]. Apart from these limitations, failing to address and target the specific underlying causes of sleep difficulties in ASD may also contribute to treatment failure [47]. Therefore, there is an urgent need to find safe and effective treatments for sleep disturbance in children with ASD.
The effect of rTMS for insomnia
Repetitive Transcranial Magnetic Stimulation (rTMS) is a safe and non-invasive brain stimulation technique, which can stimulate specific brain areas by magnetic pulses and electrical currents through the skull. High-frequency rTMS (≥ 5Hz) and low-frequency rTMS (≤ 1Hz) can modulate neural activity at the stimulated site, leading to various physiological effects [48]. Previous studies have demonstrated the efficacy of rTMS in treating psychiatric disorders, such as depression, schizophrenia, attention-deficit/hyperactivity disorder, and tic disorders [48- 50]. Recent meta-analyses and systematic reviews have further supported the safety and potential of rTMS in alleviating symptoms of insomnia and sleep disturbances associated with conditions such as depression and anxiety [51-53]. When compared to sham rTMS, actual rTMS treatment has shown improvements in the Pittsburgh sleep quality index total score and subscale scores [53,54]. Additionally, patients receiving rTMS treatment have exhibited increased slow-wave sleep and Rapid Eye Movement (REM) sleep when compared to a control group using parameters of Polysomnography (PSG) [53,55,56]. In comparison to pharmacological treatments and Cognitive-Behavioral Therapy (CBT), rTMS has shown assurance in enhancing sleep quality, optimizing sleep structure, and maintaining therapeutic efficacy [57,58]. These findings position rTMS as a positive tool for addressing sleep disturbances.
The effect of rTMS on the potential causes of sleep disturbances in ASD
In addition to its application in treating various psychiatric and neurological disorders, rTMS has also been explored for its potential to improve sleep quality in individuals with ASD [59- 61]. In this regard, we will present current evidence regarding four different aspects related to the potential causes of sleep disturbances in ASD, including gene expression, core symptoms of ASD, comorbidities, and sleep pressure.
The potential role of rTMS in regulation of gene expression
Several studies have investigated the effects of rTMS on gene expression in animal models. For instance, Ikeda et al. discovered that the therapeutic effects of chronic rTMS are associated with the regulation of endoplasmic reticulum stress-related genes and genes encoding glutamate transporters (GLAST, GLT-1), glycine, and GABA transporter proteins in the brains of mice [62]. Other studies have reported changes in the expression levels of monoamine transporter proteins, dopamine receptor 2, Heat Shock Protein 70 (HSP70), and circadian rhythm-related genes following acute and chronic rTMS treatment, which are related to sleep regulation [63,64]. Furthermore, a study conducted on patients with primary insomnia revealed that bilateral low- frequency rTMS applied over the Dorsolateral Prefrontal Cortex (DLPFC) led to improvements in Brain-Derived Neurotrophic Factor (BDNF) levels and Gamma-Aminobutyric Acid (GABA) levels [65]. Moreover, rTMS has been found to increase glutamate/glutamine levels, helping to maintain the balance between the glutamatergic and Gamma-Aminobutyric Acidergic (GABAergic) systems [66,67]. The CB1R (Cannabinoid Receptor 1), which is involved in modulating glutamate concentration in the cerebrospinal fluid, plays an important role in social interaction and sleep regulation. It is also considered a candidate gene for ASD [68-70]. Pharmacological blockade resulting in the reduction of CB1R gene expression can lead to decreased sleep and increased wakefulness [71]. Studies have demonstrated that rTMS exerts its anti-depressant effects by increasing the expression of CB1R in hippocampal astrocytes and neurons [72].
The potential role of rTMS in alleviating core symptoms of ASD
The existing literature on insomnia suggests that effectively treating insomnia can have positive effects on factors such as pain, which also exhibit a bidirectional relationship, and may also benefit comorbid conditions like depression [73,74]. This conceptual framework could be applicable to ASD as well. Therefore, it is plausible that improving the core symptoms of ASD could also lead to improvements in sleep disturbances. Two double-blind, randomized trials have demonstrated that rTMS may have a potential role in reducing social impairment and improving executive function performance in ASD patients [75]. Furthermore, other studies have indicated that rTMS can enhance linguistic abilities, cognition, emotional recognition, and reduce obsessive-compulsive symptoms, with effects that may last for several months after treatment [76-78]. A meta-analysis has also shown that rTMS can significantly alleviate repetitive and stereotyped behaviors, improve social behavior, and reduce errors in executive function tasks [79].The potential role of rTMS in alleviating comorbidities of ASD
ASD often co-occurs with various other conditions, including epilepsy, gastrointestinal disturbances, Attention Deficit Hyperactivity Disorder (ADHD), sensory abnormalities, and hyperarousal [3]. These comorbidities are closely associated with sleep disturbances, and sleep problems may serve as an intermediary phenotype for these conditions [80]. In a study conducted by Gwynette, et al. they carried out an open-label trial to investigate the effects of long-term rTMS on depressive and core autism symptoms in 13 adults with ASD and comorbid depression [81]. The researchers found that rTMS was well tolerated, and 40% of the patient’s achieved remission of depression along with an improvement in core autism symptoms. Another study by Enticott et al. conducted a 2-week prospective, double-blind, randomized, placebo-controlled trial with 28 high- functioning individuals with ASD [82]. The results indicated that rTMS reduced anxiety levels, even in the presence of social and emotional difficulties. Several other studies have also demonstrated the potential of rTMS in alleviating gastrointestinal disturbances, ADHD, Tourette syndrome, irritability, and aggression in individuals with ASD [83-85].
The potential role of rTMS in regulating sleep pressure
Sleep homeostasis plays an important role in regulating sleep, and sleep stress increases with prolonged wakefulness, which can be measured by the power of Slow-Wave Activity (SWA) [86]. Previous research has indicated that rTMS affects SWA and slow- wave sleep. In an open-label pilot study conducted among patients with major depression, it was found that rTMS applied over the left dorsolateral prefrontal cortex increased slow-wave activity at F3 during the initial five sessions of rTMS, but not during the latter half [87]. Similar results have been reported in other studies and meta-analyses, suggesting that rTMS can stimulate slow wave activities and promote deeper sleep [87-89]. However, no study has specifically investigated the role of rTMS in modulating SWA in individuals with ASD. Considering the existing studies, it can be speculated that rTMS treatments may also enhance slow-wave activities and/or increase sleep pressure in individuals with ASD, thereby improving sleep disturbances.The existed evidence for rTMS treating sleep disturbances in ASD
In order to examine the potential efficacy of rTMS in addressing sleep disturbances in individuals with ASD, A comprehensive search of articles published from their inception dates to July 6th, 2023, in Pubmed, Embase, Science Direct, Web of Science, China National Knowledge Infrastructure (CNKI), and WANFANG database. Terms related to “rTMS” (“Transcranial Magnetic Stimulation”, “repetitive Transcranial Magnetic Stimulation”, “rTMS”), “autism” (“Autism Spectrum Disorder”, “ASD” and “autism”) and “sleep” were used in this study. Two authors (Junjie Zhang and Weiping Zhou) independently reviewed the title, abstract, and full text of the publications and determined the suitability for inclusion. Studies were included based on the following criteria: a) Studies conducted to the human population with comorbidity of ASD and sleep disturbance. b) rTMS was administered alone or in combination with other physical therapies to treat sleep disturbance in patients with ASD. c) Studies reported subjective or objective results about sleep disturbance related parameters. d) Written in English or Chinese and full text availability. Studies such as case reports or case series with a sample size of five or less, published repeatedly, data cannot be extracted, animal studies, review articles, conference abstracts, publications without available full text were excluded. Duplicate publications were removed. Two authors (Junjie Zhang and Weiping Zhou) read the title, abstract, and keywords of every article independently to eliminate irrelevant studies. The remaining articles were reviewed relative to the selection criteria. Discrepancies were resolved by discussion or consultation with a third author (Yongqiang Zheng). The flowchart of the study selection process is shown in Figure 1.
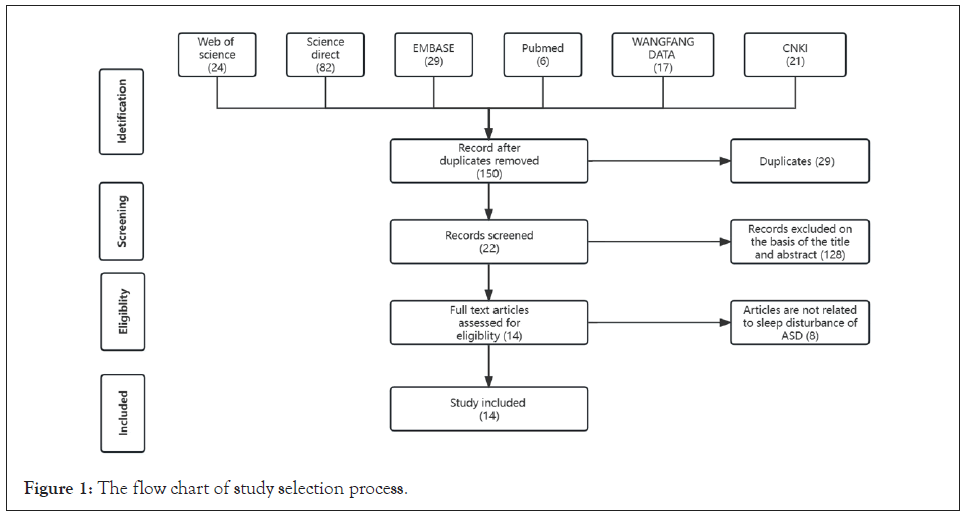
Figure 1: The flow chart of study selection process.
Three authors (Junjie Zhang/Weiping Zhou/ Yongqiang Zheng) independently extracted data from the included studies based on the inclusion criteria. If a disagreement occurred between any two of the authors, Zheng would reconcile the data. The first author, year of publication, study design, diagnostic/assessment tools, baseline demographic information, intervention method, rTMS device, rTMS site/parameters/duration (frequency, intensity of stimulation, number of sessions, number of pulses per session, duration of stimulation), principal findings and adverse effects were extracted from each included study.
A total of 11 articles that investigated the use of rTMS for improving sleep disturbances in ASD met our selection criteria and were included in our review. Comprehensive information regarding these studies is presented in Table 1.
| Study | Diagnostic /assessment tools | Baseline demographic information | Intervention method | rTMS device | rTMS site, parameters, duration | Principal findings (Adverse effects) |
|---|---|---|---|---|---|---|
| Duan, et al. [90] | DSM-IV. | 60 ASD (M: F=34:26; Age: 2-6 years) with sleep disturbances: Patients were randomly divided into the control group (n=30) and the study group (n=30) | Both groups received sensory integration, intellectual training, and acupuncture treatment. On this basis, the children in the study group were given rTMS. | HX-C3 | Frontal, bilateral temporal, occipital, and parietal. 0.5-9Hz. 1 time /day, 20 min/ time, 6 times/ week, 1 month/ course, 3 courses, with an interval of 5-7 days between each course. |
Sleep disturbance improved in both groups of children after treatment. rTMS treatment group improved better than the control group (P<0.05). rTMS treatment group had an effective rate of 70%, while the control group had an effective rate of 33%. (Not report). |
| Dong, et al. [91] | DSM-5, ICD-10, CARS, SDQ, SCQ, RBQ-2, CSHQ | 5 ASD (M: F =34:26; age: 4-8 years) with sleep disturbances. Case report. |
rTMS | CCY-I | The bilateral DLPFC: Left high-frequency (10 Hz, 80%MT, 30 pulses per session of 24 sessions)+right low-frequency (1 Hz, 80%MT, 8 pulses per session of 82 sessions). 1 time/day, 15 minutes in the left/right dorsolateral prefrontal region, respectively. 2 courses, 10 times /course. |
Five cases showed varying degrees of improvement in sleep disturbance (CSHQ) and behavioral problems (SDQ, SCQ, RBQ-2, and SDQ-emotional symptom scores). The CSHQ total scores and behavior problem scores of 5 cases at the endpoint of intervention have decreased to varying degrees, and 3 cases have CSHQ total scores less than 41 points. (Not report). |
| Qiu, et al. [92] | DSM-5 | 54 ASD (M: F =37:17; age: 2-15 years) with sleep disturbances were randomly divided into the control group (n=27) and the study group (n=27) | Both groups received sensory integration, intellectual training, and acupuncture treatment. On this basis, the children in the study group were given rTMS. | NA | DLPFC (low frequency) Parameters: NA 20 min/ time. 1 time /day, 5-6 times/ week, continuous treatment for 3 months |
The total effective rate of the two groups was 70.37% in the study group, which was higher than 55.56% in the control group, the difference was statistically significant (P<0.05). The treatment satisfaction of the study group was 66.67%, which was higher than 48.15% the control group, there was significant difference between the two groups (P<0.05). (Not report) |
| Ni, et al. [93] | DSM-5, CARS, CSHQ, ABC | 80 ASD (M: F =41:39; age: 3-6 years) with sleep disturbances were randomly divided into the control group (n=40) and the study group (n=40) | Both groups received sensory integration, social skills training, and speech therapy. On this basis, the children in the study group were given rTMS. | CCY-I | rTMS site: Right DLPFC. Parameters: 1 Hz, 80%MT, 14 pulses/session, 80 sessions. Duration: 20 min/ time. 1 time/day, 10 days /course followed by a 10-day break before starting the next treatment session, continuous treatment for 3 months. |
After treatment, the scores of CSHQ, CARS, ABC, behavior, language, social interaction and perception were all decreased in both groups, and the study group was lower than the control group (P<0.05). The scores of vestibular imbalance, tactile defense, proprioception and learning ability were increased in the two groups, and the study group was higher than the control group (P<0.05). (Not report) |
| Chen et al. [94] | DSM-5, CARS, ABC, CHSQ | 40 ASD (M: F =29:11; age: 3-6 years) with sleep disturbances were divided into the control group (n=20) and the study group (n=20) | Both groups received sleep behavior education and routine rehabilitation training. On this basis, the children in the study group were given rTMS. | CCY-I | rTMS site: Left DLPFC. Parameters: 1 Hz, 80%MT, 10 pulses/session, 5s between sessions,1200 pulses. Duration: 30 min/ time, 1 time/day, 5 times/week, 8 weeks. |
After 8 weeks of treatment, the average scores of CARS, CSHQ, and ABC for both groups of patients showed significant improvement compared to before the treatment. Additionally, the study group had significantly better total scores on these scales compared to the control group. |
| Wu, et al. [95] | DSM-5, CARS, CSHQ, FISH | 199 ASD (M: F =170:29; age: 2-6 years) with sleep disturbances were divided into the control group (n=98) and the study group (n=101) | Both groups received sleep behavior education. On this basis, the children in the study group were given rTMS. | NA | rTMS site: Left DLPFC. Parameters: 1 Hz, 90%MT, 720 pulses. If the threshold cannot be measured in patients, a fixed stimulus intensity of 40% × 90% is used. Duration: 15 min/ time. 1 time/day, 5 times/week, 4 weeks. |
After treatment, the study group showed better improvement than the control group in total CSHQ score, sleep onset delay, sleep resistance, sleep anxiety, night awakenings, and parasomnias (P < 0.05). (9 children exhibited mild excitement and agitation symptoms during the first week of rTMS treatment). |
| Li T, et al. [96] | DSM-5, CARS, CSHQ | 58 ASD (M: F =47:11; age: 5.51 ± 1.28 years) with sleep disturbances were randomly divided into the control group (n=29) and the study group (n=29) | The study group were given rTMS; the control group were given sham rTMS. | MAGPro R30 with an “8” shape coil. | rTMS site: the bilateral DLPFC: Parameters: Left high-frequency (10 Hz, 80%MT, 30 pulses/session, 35s between sessions,24 sessions)+right low-frequency (1 Hz, 80%MT, 8 pulses/session, 3s between sessions,82 sessions). Duration:5 times/week, 4 weeks. |
The total scores of CARS and CSHQ in the study group were compared at different time points, and the differences were statistically significant, while those in the control group were not statistically significant. The scores of the subscales of CSHQ in the trail group improved to varying degrees with the extension of the intervention. (Not report). |
| Song and Gao, et al. [97,98] | DSM-5, CARS, CSHQ, SDQ, RBQ, SSP, PPVT | 39 ASD (M: F =31:8; age: 9.0 ± 4.4) years with sleep disturbances. Case report. |
rTMS | CCY-I | rTMS site: The bilateral DLPFC. Parameters: Left high-frequency (10 Hz, 25%MT, 32 pulses/session, the intermittent time is 1s, 45 sessions)+right low-frequency (1 Hz, 25%MT, 32 pulses/session, the intermittent time is 10s, 28 sessions). Duration: 1 time/day, 5 times/week, 4 week/course, 2 courses. |
After 4-weeks rTMS intervention, the total CSHQ score was decreased (P<0.05), especially on the aspect of poor sleep habits, sleep anxiety, and irregular sleep duration. After 8 weeks, the total CSHQ score was further decreased, mainly in poor bedtime habits, sleep anxiety, and irregular sleep duration, parasomnias, and daytime sleepiness. The total score of SSP mediated the treating efficacy of rTMS on CSHQ. (Not report). |
| Yang, et al. [99] | DSM-5, CARS, CSHQ, ABC, | 82 ASD (M: F =66:16; age: 1-12 years) with sleep disturbances were randomly divided into the control group (n=41) and the study group (n=41) | Both groups received routine rehabilitation treatment. On this basis, the study group were given rTMS. | Rapid2 with a “8” shape coil. | rTMS site: During the first and second weeks: NA. During the third and fourth weeks, the right DLPFC is primarily stimulated. During the fifth and sixth weeks, both the left and right DLPFC are primarily stimulated. Parameters: 1 Hz, 90%MT, 10 pulses/session, 20s between sessions, 40 sessions. Duration:5 times/week, 6 weeks. |
The scores of CARS, ABC, and ATEC scores in the two groups were lower than those before treatment, and the scores in the observation group were significantly lower than those in the control group (P<0.05). The CSHQ scores in the groups were significantly lower after treatment than before treatment, and the score in the observation group was significantly lower than that in the control group (P<0.05). (Not report). |
| ATEC | ||||||
| Zhao, et al. [100] | CARS, | 60 ASD (M: F =33:27; age: 3-6 years) with sleep disturbances were randomly divided into the control group (n=30) and the study group (n=30) | Both groups received routine rehabilitation treatment. On this basis, the study group were given rTMS. | NA | rTMS site: Right DLPFC. Parameters:1 Hz, 80%MT, 14 pulses/session, 1s between sessions, 80 sessions. Duration: 20 min/ time. 1 time/day. Continued treatment for 10 days, rest for 10 days to continue the next course of treatment. A total of 6 courses will be implemented. |
The study group showed a significantly greater improvement in CSHQ, CARS, and ABC scores compared to the control group after the treatment. Additionally, the treatment effectiveness rate was higher in the study group compared to the control group (P<0.05). |
| ABC, | ||||||
| CHSQ | ||||||
| Xu, et al. [101] | DSM-5, | 90 ASD (M: F =47:43; age: 2-6 years) with sleep disturbances were randomly divided into the control group (n=45) and the study group (n=45) | Both groups received routine rehabilitation treatment. On this basis, the control group were given routine rTMS; the observation group were given individualized rTMS based on ERP. | NA a “8” shape coil | The control group: the bilateral DLPFC: Left high-frequency (10 Hz, 80%MT, 30 pulses/session, 35s between sessions, 24 sessions)+right low-frequency (1 Hz, 80%MT, 8 pulses/session, 3s between sessions,82 sessions). The observation group with high excitability of brain function are given low-frequency rTMS, while those with high inhibition are given high-frequency rTMS; 80% MT, and the stimulation parameters are the same as those of the control group. 15 min/ time. 1 time/day, 1 month/course, 6 courses. |
The CARS and ATEC scores in two groups after treatment showed a significant decrease (P<0.05), and the CARS and ATEC scores in the observation group were significantly lower than those in the control group (P<0.05). The CSHQ scores in the two groups after treatment were significantly decreased (P<0.05), and the FISH scores were significantly increased (P<0.05). Additionally, the CSHQ scores in the observation group were significantly lower than those in the control group (P<0.05), and the FISH scores in the observation group were significantly higher than those in the control group (P<0.05). (Not report). |
| CARS, | ||||||
| CSHQ, | ||||||
| ATEC, | ||||||
| FISH, | ||||||
| ERP |
Note: Duration#: Specifical for the time of rTMS intervention; CSHQ: The Children’s Sleep Habits Questionnaire. SDQ; Strengths and Difficulties Questionnaire; SCQ: Social Communication Questionnaire; RBQ-2: Repetitive Behaviour Questionnaire-2; CARS: The Childhood Autism Rating Scale; ABC: The Autism Behavior Checklist; ATEC: Autism Treatment Evaluation Scale; PPVT: Peabody Picture Vocabulary Test; FISH: The Family Inventory of Sleep Habit; ERP: Event-Related potential; SSP: Short Sensory Profile; DLPFC: Dorsolateral Prefrontal Cortex.
Table 1: The study characteristics for alleviating sleep disturbances in individuals with ASD by rTMS.
Study characteristics
Duan, et al. conducted a study involving 60 ASD patients (Male: Female=34: 26; age: 2-6 years) with co-morbid sleep disturbances [90]. The patients were randomly divided into study group and control groups. Both groups received sensory integration training, intellectual training, and acupuncture treatment, while the study group additionally received rTMS. The rTMS treatment lasted for 3 months, with a frequency of 6 sessions per week, once daily, and each session lasted for 20 minutes. They used the Beijing Huaxing (HX-C3) transcranial magnetic treatment instrument with a children’s treatment cap. The cap had six heads distributed on the Frontal (1), bilateral temporal (2), occipital (2), and parietal (1). The magnetic field intensity used was 3.5 ± 1 mT, with an automatic frequency conversion of 0.5, 1, 3, 5, 7, and 9 Hz. The sleep status of the ASD children was assessed based on parents’ reports on overall impressions of their children’s sleep at baseline and post-treatment. The assessment categories included: Cure (normal sleep with disappearance of symptoms), significantly effective (significantly improved sleep with sleep onset within 30 minutes, sleep duration over 5 hours, improved sleep depth, and good sleep mental state), effective (symptom improvement with longer sleep duration compared to before, less than 3 hours), and unhealed (sleep worsening with no improvement in symptoms). The researchers found that the quality of sleep improved in both groups after treatment. The treatment group reported effective improvement or greater in 70% of cases, compared to 33% in the control group (P<0.05). No adverse effects were reported.
Dong, et al. conducted a case series study involving 5 ASD patients (male: female=3:2; age: 4-8 years) with co-morbid sleep disturbances (CSHQ>41) [91]. All patients received left high- frequency (10 Hz, 80% Metric Ton (MT), 30 pulses per session of 24 sessions)+right low-frequency rTMS (1 Hz, 80% Metric Ton (MT), 8 pulses per session of 82 sessions). The rTMS conducted 1 time/day, 15 minutes in the left/right dorsolateral prefrontal region, respectively, 2 courses, 10 times/course. The study did not provide information about the order in which these treatments were administered. To evaluate the clinical symptoms of ASD and sleep quality, the researchers assessed several measures, including the Child Sleep Habits Questionnaire (CSHQ), Sleep diary, Childhood Autism Rating Scale (CARS), Strengths and Difficulties Questionnaire (SDQ), Social Communication Questionnaire (SCQ), and Repetitive Behavior Questionnaire-2 (RBQ-2), both at baseline and post-treatment. All patients exhibited varying degrees of improvement in sleep disturbances and behavioral problems. There were significant improvements in total sleep time and sleep latency following rTMS treatment (P<0.05). Furthermore, in three out of the five cases, the total scores on the CSHQ were below 41 after the intervention, which no longer met the diagnostic criteria for sleep disturbances according to this study. The scores on the SDQ, SCQ, RBQ-2, and SDQ-mood symptoms decreased to varying degrees in all five cases. No adverse effects were reported for any of the patients.
Qiu, et al. employed an experimental protocol similar to that used by Duan et al [92]. A total of 54 ASD children (Male: Female=37:17, Age: 2-15 years) were randomly divided into the active treatment group and the control group with mean ages of 8.2 ± 1.2 and 8.2 ± 1.6 years, respectively. Both groups received sensory integration training, intellectual training, andacupuncture treatment. The active treatment group also received low frequency rTMS to DLPFC 5 to 6 times per week, 1 time per day, and 20 min per time for 3 months. rTMS parameters was not report. Using the same evaluation criteria as Duan et al, professional doctors evaluate the children’s sleep improvement. With the rTMS treatment the total effective rate was 70.4%, significantly greater than that of 55.6% in the control group (P<0.05). No adverse effects were reported.
Ni, et al. conducted a randomized controlled study aims to evaluate clinical effect of the low-frequency transcranial magnetic stimulation combined with a rehabilitation training in the treatment of children with autism and sleep disturbances [93]. Eighty cases (Male: Female=41: 39; Age: 3-6 years) with ASD combined with sleep disturbances (CSHQ>41) were selected as the study subjects. Using a random number table method, they were divided into a study group and a control group, with 40 cases in each group. The control group received rehabilitation training (Each treatment session lasts for 60 minutes, and the treatment is conducted once a day. The treatment is administered continuously for 5 days, and this 5-day period is considered one cycle. The total duration of the treatment is 4 months); while the study group received a low frequency rTMS (1 Hz, 80% Metric Ton (MT), 14 pulses per session of 80 sessions to right DLPFC) on the basis of the control group. One rTMS treatment course consists of 10 days of treatment followed by a 10-day break. After the break, the second treatment course begins. A total of 6 treatment courses are conducted. Both groups were treated for four months. Compared to before the treatment, both groups of children showed a decrease in scores on the CSHQ, CARS, The Autism Behavior Checklist (ABC), as well as behavior, language, social interaction and perception were all decreased in both groups, and the scores of the study group were lower than those of the control group(P<0.05).
Chen et al. included 40 children with ASD (Male: Female 170: 29; Age: 2-6 years) who had comorbid sleep disturbances (CSHQ>41) were divided into a control group and a study group [94]. Both groups received sleep behavior education and routine rehabilitation training. On this basis, the children in the study group were given rTMS (30 minutes at 80% of the Running Metre (RMT) with 1Hz, 1200 pulses per session to left DLPFC) for first 8 weeks. After 8 weeks of treatment, the average scores of CARS, CSHQ, and ABC for both groups of patients showed significant improvement compared to before the treatment. Additionally, the study group had significantly better total scores on these scales compared to the control group.
Wu, et al. included 199 children with ASD (Male: Female=170: 29; Age: 2-6 years) who had comorbid sleep disturbances (CSHQ>41) were divided into a control group and a study group [95]. Both groups received sleep behavior education for 16 weeks. On this basis, the children in the study group were given rTMS (15 minutes at 90% of the Running Metre (RMT) with 1Hz, 720 pulses per session to left DLPFC) for first 4 weeks. At baseline, after 4 weeks of treatment, and at the end of the 16-week treatment period, CARS, CSHQ and Family Interview for Sleep Habits (FISH) were used to evaluate the children’s symptoms and sleep habits. As a result, rTMS can significantly improve sleep resistance, sleep onset delay, sleep anxiety, night awakenings, parasomnia, and sleep habits in children with ASD.
Furthermore, the therapeutic effects of this treatment persist even after the completion of rTMS therapy. The application of rTMS in preschool-aged children with ASD is safe and well-tolerated, with no reported severe adverse events.
Li T, et al. included 58 children with ASD (Male: Female=47:11; Age: 3-12 years) who had comorbid sleep disturbances (CSHQ>41) were divided into the control group (N=29) and the trail group (N=29) [96]. The trail group received left high-frequency (10 Hz, 80% Metric Ton (MT), 30 pulses per session of 24 sessions)+right low-frequency (1 Hz, 80% Metric Ton (MT), 8 pulses per session of 82 sessions) rTMS stimulation to the bilateral DLPFC in children with ASD by MAGPro R30 instrument, which is manufactured by Medtronic. In contrast, the control group received sham stimulation, with the same duration and site as the experimental group. Both groups underwent a 4-week intervention for 20 days, and assessments were conducted using the CARS and CSHQ before treatment and at 2 and 4 weeks of intervention. There was no statistical difference between the two groups on age, gender composition, CARS score and CSHQ score (P>0.05). Through simple effect analysis and further analysis of the interaction between the group and the course of treatment, it was found that the total scores of CARS and CSHQ in the trail group were compared at different time points, and the differences were statistically significant, while those in the control group were compared at different time points, and the differences were not statistically significant. The scores of the subscales of CSHQ in the trail group improved to varying degrees with the extension of the intervention.
Song and Gao, et al. conducted an uncontrolled study involving thirty-nine ASD patients (Male: Female=30:10; Age: 9.0 ± 4.4 years) with co-morbid sleep disturbances (CSHQ>41) [97,98]. The rTMS intervention was administered five times per week, with each treatment course lasting four weeks. Each child received two courses of intervention, resulting in a total treatment period of eight weeks. All patients received left high-frequency (10 Hz, 25%MT, 32 pulses per session of 45 sessions)+right low-frequency rTMS (1 Hz, 25% Metric Ton (MT), 32 pulses per session of 28 sessions). The order in which the treatments were administered was not specified. To evaluate the effects of the intervention, the researchers utilized the Child Sleep Habits Questionnaire (CSHQ), Strengths and Difficulties Questionnaire (SDQ), Childhood Autism Rating Scale (CARS), and Repetitive Behavior Questionnaire-2 (RBQ-2). These measures were administered before the start of treatment, at the 4th week, and at the 8th week. The results showed that after four weeks of rTMS intervention, the CSHQ scores decreased by 4.59 ± 0.85 (P<0.05), indicating improvements in poor bedtime habits, sleep anxiety, and irregular sleep duration. After eight weeks, the CSHQ score decreased by 6.26 ± 0.98 compared to the baseline, primarily reflecting improvements in poor bedtime habits, sleep anxiety, irregular sleep duration, parasomnias, and daytime sleepiness. No adverse effects were reported during the course of the intervention. Based on these findings, the study concluded that rTMS intervention effectively improved sleep disturbances and emotional symptoms in children with ASD.
Yang, et al. included 82 children with ASD (Male: Female= 66:16; Age: 1-12 years) were randomly divided into the control group (N=41) and observation group (N=41) by a random number table method [99]. Both groups received sensory integration training (once in the morning and once in the afternoon, each session lasting for 30 minutes, last for 6 months). The observation group received low frequency (1 Hz, 90%MT, 400 pulses per session of 10 sessions for 5 days a week) rTMS stimulation to the DLPFC in children with ASD for 6 months. There were no statistically significant differences in baseline characteristics between the two groups of patients (P>0.05). After treatment, the scores of CARS, ABC, and ATEC scores in the two groups were significantly lower than those before treatment, and the scores in the observation group were significantly lower than those in the control group (P<0.05). The scores of four items (vestibular imbalance, resolution defense, proprioception, and learning ability) in sensory integration scale in the two groups after treatment were significantly higher than those before treatment, and the scores were significantly higher in the observation group than those in the control group (P<0.05). The CSHQ scores in the groups were significantly lower after treatment than before treatment, and the score in the observation group was significantly lower than that in the control group (P<0.05).
Zhao, et al. included 60 children with ASD (Male: Female=33:27; age: 3-6 years) were randomly divided into the control group (N=30) and observation group (N=30) by a random number table method [100]. Both groups received routine rehabilitation treatment. On this basis, the study group were given rTMS. On this basis, the children in the study group were given rTMS (20 minutes/day at 80% of the RMT with 1Hz, 14 pulses/session, 1s between sessions, 80 sessions to left DLPFC). Continued treatment for 10 days, rest for 10 days to continue the next course of treatment. A total of 6 courses will be implemented. After treatment, the study group showed a significantly greater improvement in CSHQ, CARS, and ABC scores compared to the control group after the treatment. Additionally, the treatment effectiveness rate was higher in the study group compared to the control group (P<0.05).
Xu, et al. included 90 children with ASD (Male: Female= 47:43; Age: 2-6 years) who had comorbid sleep disturbances (CSHQ>41) were randomly divided into the control group (N=45) and observation group (N=45) by a random number table method [101]. Both groups received routine rehabilitation treatment (Structured teaching model training, behavioral analysis training, speech training, and sensory integration and auditory integration training were implemented. Each training session for each component lasted for 30 minutes, once a day, six times a week, for a total duration of six months.). The control group received left high-frequency (10 Hz, 80% Metric Ton (MT), 30 pulses per session of 24 sessions)+right low-frequency (1 Hz, 80% Metric Ton (MT), 8 pulses per session of 82 sessions) rTMS stimulation to the bilateral DLPFC in children with ASD. The observation group was treated with individualized rTMS according to the results of Event-Related Potential (ERP). CARS, Autism Treatment Evaluation Checklist (ATEC), CSHQ, and Family Sleep Habits questionnaire (FISH) were used to compare and analyze the intervention effects between the two groups. The observational group performed individualized repetitive Transcranial Magnetic Stimulation (rTMS) based on the ERP examination results of the patients. The stimulation sites were selected based on ERP abnormalities corresponding to specific brain functional areas: For patients with abnormal P300, bilateral dorsolateralprefrontal cortex and temporal lobe were stimulated; for patients with abnormal N400, the temporal lobe was stimulated; for patients with Mismatch Negativity (MMN) abnormalities, the temporal and frontal lobes were stimulated; for patients with Contingent Negative Variation (CNV) abnormalities, the frontal and parietal lobes were stimulated. For individuals with high excitability in brain function activity, low-frequency rTMS was administered, while high-frequency rTMS was given to those with high inhibitory activity. The stimulation intensity was set at 80% of the motor threshold, and the stimulation parameters were the same as those in the control group. Treatment duration: Once a day, 15 minutes per session, one month per course, for a total of six courses of treatment. As a result, the autism-related CARS and ATEC scores in two groups after treatment showed a significant decrease (p<0.05), and the CARS and ATEC scores in the observation group were significantly lower than those in the control group (P<0.05). Comparing with those before treatment, the CSHQ scores in the two groups after treatment were significantly decreased (P<0.05), and the FISH scores were significantly increased (P<0.05). Additionally, the CSHQ scores in the observation group were significantly lower than those in the control group (P<0.05), and the FISH scores in the observation group were significantly higher than those in the control group (P<0.05).
Future prospect
The current available results have preliminarily indicated that rTMS can alleviate sleep disturbances in ASD patients, but there are no randomized controlled double-blind studies investigating the clinical effects of it. Additionally, the underlying mechanisms of rTMS in alleviating sleep disturbances in ASD patients are not yet clear. Therefore, it is essential to conduct more high-quality, randomized, double-blind controlled studies to validate the efficacy of rTMS for sleep disturbances in ASD and determine its suitability for general clinical use.
rTMS may holds assurance as a potential intervention for treating sleep disturbances by regulating abnormal gene expression, slow wave activity, and alleviate core symptoms and mental complications in individuals with ASD. The potential causes of sleep disturbances in individuals with Autism Spectrum Disorder (ASD) are multifaceted and may involve a complex interplay of genetic, neurobiological, and environmental factors. The systematic review conducted on the role of repetitive Transcranial Magnetic Stimulation (rTMS) in addressing sleep disturbances in ASD analyze on a positive avenue for intervention. The systematic review also highlights the potential efficacy of rTMS as a non-invasive neuromodulatory intervention to ameliorate sleep disturbances in ASD. Through targeted stimulation of specific brain regions implicated in sleep regulation and sensory processing, rTMS holds assurance in modulating neural activity and potentially improving sleep outcomes in individuals with ASD.
Gabriel Cortazar and Jassel Phelia contributed equally to this article. All authors approved the final manuscript.
This work was supported by Health Commission of Hubei Provincial (WJ2019M065), Hubei Provincial Department of Education research project (B2019025), Hubei Provincial Public Health Youth Elite Talent Training Program ((2021)74) and Yichang Municipal Science and Technology Bureau Medical Health Research Project (NO: A20-2-034).
The authors would like to acknowledge all workers for this job.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Lorenzo G, Kharazhanov D, Cortazar G, Andrea G, Phelia J (2023) Potential Causes of Sleep Disturbances in Autism Spectrum Disorder (ASD) and the Role of Repetitive Transcranial Magnetic Stimulation on it: A Systematic Review. J Sleep Disord Ther.12:492.
Received: 27-Nov-2023, Manuscript No. JSDT-23-28176; Editor assigned: 30-Nov-2023, Pre QC No. JSDT-23-28176 (PQ); Reviewed: 14-Dec-2023, QC No. JSDT-23-28176; Revised: 21-Dec-2023, Manuscript No. JSDT-23-28176 (R); Published: 28-Dec-2023 , DOI: 10.35248/2167-0277.23.12.492
Copyright: © 2023 Lorenzo G, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.