Clinical Pediatrics: Open Access
Open Access
ISSN: 2572-0775
ISSN: 2572-0775
Research Article - (2023)Volume 8, Issue 2
Objective: Retinopathy of Prematurity (ROP) is a sight-threatening disease representing one of the main disabling diseases affecting premature newborns. Accurate and timely diagnosis of the early stages of ROP is needed in order for ophthalmologists to recommend appropriate treatment. The purpose of this study was to evaluate the relationship between platelet parameters and ROP requiring treatment in extremely premature infants.
Methods: A total of 162 extremely premature infants (born with a gestational age less than 28 weeks) were included in this retrospective study. Demographic data, platelet parameters in blood tests, and ROP results were evaluated.
Results: Patients with severe ROP had significantly lower platelet (PLT) counts and significantly higher Platelet Distribution Width (PDW) and Mean Platelet Volume (MPV) levels than those with mild ROP. The regression analyses showed significant association between MPV at postmenstrual 36 weeks and severe ROP (OR=18.551, P=0.002), PLT count at postmenstrual 36 weeks and severe ROP (OR=1.007, P=0.026). MPV higher than 10.05 fL at postmenstrual 36 weeks reflects severe ROP with a sensitivity of 76.9%, specificity of 76.2%; PLT lower than 340 × 109/L at postnatal 36 weeks after birth could detect severe ROP with a sensitivity of 92.0%, a specificity of 84.0%.
Conclusion: This study found significantly positive association between MPV levels at postnatal 36 weeks and severe ROP, as well as negative association between PLT counts at postnatal 36 weeks and patients with severe ROP, suggesting that platelet activation may contribute to severe retinopathy of prematurity. Our findings indicate that a higher postnatal MPV levels (>10.05 fL) and a lower PLT counts (<340 × 109/L) at postnatal 36 weeks might be a predictor for the development of severe ROP in extremely premature infants.
Platelet; Severe; Retinopathy of prematurity; Extremely premature; Infants
Extremely premature infants (gestational age ≤ 28 weeks) are at high risk of Retinopathy of Prematurity (ROP). In the human fetus, retinal vascularization begins during the fourth month of gestation and occurs in the hypoxic uterine environment. In preterm infants, the retina is incompletely vascularized and the exposure to the hyperoxic extra-uterine environment leads to down regulation of proangiogenic factors, and thus resulting in vessel regression characterizing the first phase of ROP. As the metabolic demand of the developing retina increases, the retinal environment becomes hypoxic and this moves ROP into its second phase, in which hypoxia up regulates proangiogenic factors that, in turns, stimulate the outgrowth of pathological blood vessels [1]. Previous study has recognized that platelets play a key role in angiogenesis [2]. Platelets are anucleate cells that circulate in blood andare critical for body’s response to vascular injury, as well as preventing bleeding [3]. Platelet has been demonstrated to be involved in the pathomechanisms of glaucoma in animal and clinical study observed that platelets actively interacted with retinal endothelial cells in the postischemic retina through P-selectin [4]. Some observed an increased platelet aggregation in glaucoma, which negatively influences blood flow in the small branches of the short ciliary arteries supplying the opticdisk [5]. Together, it makes it interesting to consider the contribution of platelets to the pathomechanism of neurodegeneration.
This retrospective study was conducted at the neonatal intensive care unit (NICU) of Shenzhen Maternal and Child Health care Hospital between January 2016 and July 2018. All neonates born before 28 weeks’ gestation at our center were eligible for this study.
Data were retrieved from the electronic medical record, including maternal complications (e.g. maternal age, plurality, and mode of conception), and use of antenatal corticosteroids and so on. The characteristics of neonates, i.e. sex, Birth Weight (BW), and Gestational Age (GA), surfactant treatment, ventilation mode, patent ductus arteriosus (PDA) and early onset neonatal sepsis, Intraventricular Hemorrhage (IVH), Necrotizing Enterocolitis (NEC) , Bronchopulmonary Dysplasia (BPD) and Retinopathy of Prematurity (ROP) were recorded.
Definitions
The subjects underwent complete ophthalmological examination conducted by an ophthalmic specialist. ROP is classified in 5 stages, with stage 1 characterized by a mild disease and stage 5 representing the end stage of the disease with severe visual impairment. Treatment of premature infants with ROP, which is mainly based on surgical interventions, is considered when the disease develops to stage 3 [6]. Stage ≥ 3 was defined as severe ROP, stage <3 were defined as mild ROP.
Statistical analyses
Platelet parameters were displayed as median Interquartile Range (IQR) and analyzed by the nonparametric test. Chi-square or Fisher’s exact test were used to compare categorical data accordingly. Multivariate logistic regression was applied to identify the independent risk factors of severe ROP. The Odds Ratios (ORs) and 95% Confidence Interval (CI) were determined by logistic regression analysis. Subsequently, the Receiver-Operator Curve (ROC) was adopted to calculate the cut off values to dichotomize the continuous variables independently associated with the presence of severe ROP. Statistical analyses were performed using SPSS version 26 (IBM Corporation, NY).
Subject characteristics
A total of 162 inborn extremely premature infants were admitted to our NICU during the study period. Thirty infants were excluded due to missing data. Fifty-six infants were excluded due to congenital abnormalities or without ROP. As a result, 76 infants were included in our analysis. The diagnosis of severe ROP was made in 13 infants (Figure 1). The median gestational age was 25.4 (interquartile range: 24.3,26.0) weeks, the median body weight was 766.0 (interquartile range: 637.5, 825) grams. The clinical characteristics of 162 infants by ROP status were summarized in Table 1.
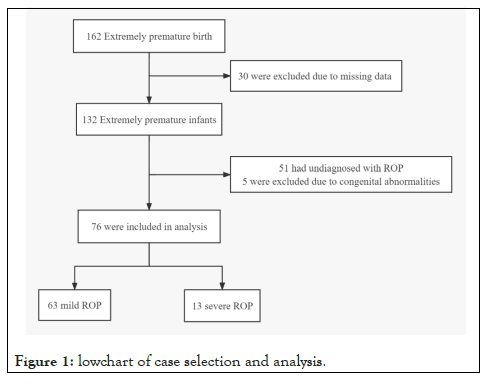
Figure 1: Lowchart of case selection and analysis.
| Variables | Severe ROP (n=13 ) | Mild ROP (n=63) | x2/z | P |
|---|---|---|---|---|
| Gestational age [wk, M (Q1, Q3)] | 24.6 (23.6,25.3) | 26.0 (25.0,26.6) | -3.121 | 0.002 |
| Birth weight [gr, M (Q1, Q3)] | 700 (555,715) | 820 (720,935) | -2.850 | 0.004 |
| Sex (male) | 6 (46.2%) | 40 (63.5%) | 1.356 | 0.244 |
| Antenatal steroid treatment | 13 (100.0)% | 59 (95.2%) | 0.655 | 0.418 |
| cesarean section delivery | 3 (23.1%) | 22 (34.9%) | 0.685 | 0.408 |
| Gestational diabetes mellitus (GDM) | 0 (0.0%) | 15 (23.8%) | 3.856 | 0.050 |
| Maternal hypertension | 4 (30.8%) | 5 (7.9%) | 5.381 | 0.020 |
| PROM | 4 (30.8%) | 31 (49.2%) | 1.474 | 0.225 |
| conception by ART | 4 (30.8%) | 17 (27.0%) | 0.077 | 0.781 |
| 1-minute Apgar score [score, M (Q1, Q3)] | 5 (3,6) | 8 (5,9) | -1.612 | 0.107 |
| 5-minute Apgar score [score, M (Q1, Q3)] | 9 (9,10) | 9 (6,10) | -0.698 | 0.485 |
| Endotracheal intubation in the delivery room, n (%) | 5 (38.5%) | 23 (36.5%) | 0.018 | 0.894 |
| Duration of invasive mechanical ventilation (days) | 11.0 (0.0,52.0) | 0.0 (0.0,25.0) | -0.215 | 0.829 |
| Duration of noninvasive mechanical ventilation (days) | 29.0 (17.3,39.3) | 27.0 (14.0,38.0) | -1.976 | 0.048 |
| PDA requiring therapy | 9 (69.2%) | 28 (44.4%) | 2.650 | 0.104 |
| NEC(Grade ≥2) | 1 (7.7%) | 1 (1.6%) | 1.568 | 0.211 |
| Early-onset sepsis | 3 (23.1%) | 10 (15.9%) | 0.394 | 0.530 |
| Late-onset sepsis | 4 (30.8%) | 3 (4.8%) | 8.716 | 0.003 |
| IVH grade 3 or 4 | 2 (15.4%) | 1 (1.6%) | 5.411 | 0.020 |
| BPD | 8 (61.5%) | 22 (34.9%) | 3.196 | 0.074 |
Table 1: Clinical characteristics by Retinopathy of prematurity status.
Dynamic change of platelet parameters during ROP development
We observed increased PLT and decreased MPV and PDW with advancing postmenstrual age (PMA). Platelet counts at consecutive time points of analysis were significantly lower in infants developing severe ROP compared to infants with mild ROP after PMA of 34 weeks. Severe ROP infants had significantly higher MPV and PDW at PMA of 36 weeks than mild ROPs (Figure 2).
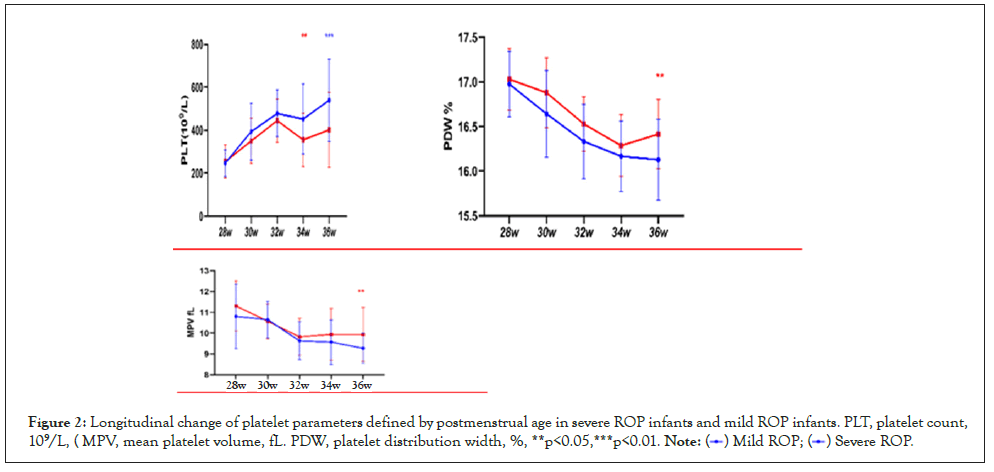
Figure 2: Longitudinal change of platelet parameters defined by postmenstrual age in severe ROP infants and mild ROP infants. PLT, platelet count,
109/L, ( MPV, mean platelet volume, fL. PDW, platelet distribution width, %, **p<0.05,***p<0.01. 
Identifying the independent risk factors for severe ROP
These potential confounders were subsequently entered to the multivariable regression model. We found that the risk of severe ROP was independently associated with birth weight (OR: 0.992, 95% CI: 0.987-0.997, P=0.048), gestational age(OR: 0.414, 95% CI: 0.236-0.727, P=0.002), Late onset neonatal sepsis (OR: 4.737, 95% CI: 1.750-12.826, P=0.002),BPD (OR: 3.446, 95% CI: 0.928- 12.797, P=0.065), MPV at 36 w (OR: 18.551, 95% CI: 2.866- 120.092, P=0.002) and PLT at 36 w (OR: 1.007,95% CI: 1.001- 1.012, P=0.026) (Table 2).
| Variables | b | S.E. | Wald | P | OR (95% CI) |
|---|---|---|---|---|---|
| Birth weight (gr) | -0.008 | 0.003 | 8.847 | 0.003 | 0.992 (0.987,0.997) |
| Gestational age (wk) | -0.881 | 0.287 | 9.428 | 0.002 | 0.414 (0.236,0.727) |
| Late-onset sepsis | 2.185 | 0.843 | 6.713 | 0.010 | 8.889 (1.702,46.413) |
| BPD | 1.237 | 0.669 | 3.415 | 0.065 | 3.446 (0.928,12.797) |
| IVH grade 3 or 4 | 2.695 | 1.331 | 4.097 | 0.043 | 14.804 (1.809,201.227) |
| MPV at 36 week | 2.921 | 0.953 | 9.393 | 0.002 | 18.551 (2.866,120.092) |
| PLT at 36 week | 0.007 | 0.003 | 4.957 | 0.026 | 1.007 (1.001,1.012) |
| PDW at 36 week | 0.990 | 0.921 | 1.156 | 0.282 | 2.692 (0.443,16.369) |
Table 2: Multivariate logistic regression of independent risk factors of severe ROP.
Calculation of the cut off value for MPV and PLT at PMA of 36 weeks
The receiver-operator curve was used to calculate the cut off value of MPV and PLT at PMA of 36 weeks for assessing the risk of severe ROP (Table 3). In our study population, the ROC of MPV and PLT at PMA of 36 weeks had an Area Under Curve (AUC) of 0.840 and 0.428, respectively. MPV values higher than 10.05 fLat PMA of 36 weeks could detect severe ROP with a sensitivity of 76.9%, specificity of 76.2%; PLT counts lower than 340 × 109/L at PMA of 36 weeks could detect severe ROP with a sensitivity of 92%, a specificity of 84.0% (Table 3).
| Variable | AUC | Sensitivity | Specificity | Youden’s index | Cutoff value |
|---|---|---|---|---|---|
| MPV (fL) | 0.840 | 0.769 | 0.762 | 0.531 | 10.05 |
| PLT (109/L) | 0.428 | 0.920 | 0.840 | 0.082 | 340.0 |
Table 3: Calculation of cutoff value of MPV and PLT for discriminating ROP status.
To our knowledge ROP is a preventable neovascular retinal disease with a great impact on vision. In our study the dynamic change of platelet parameters in premature infants was investigated. Platelet counts showed a physiological increase while MPV and PDW showed a decrease after birth. We found a persistent increase in PLT and a decrease of MPV and PDW during the first life of extremely premature infants, which was partly supported by the study from Henry et al. [7], who showed an increase in platelets during the first 3 months of life in newborn infants. When the metabolic demand of the developing retina increases, the retinal environment becomes hypoxic and these moves ROP in its second phase, in which hypoxia triggers the up-regulation of proangiogenic factors that, in turns, stimulate the growth of pathological blood vessels [8]. Chronic hypoxia might stimulate the generation of reticulocytes and reduce the number and total masses of megakaryocyte, as well as blunt the function of platelet [9].The newborn’s platelet count could be influenced by several factors. Infection and inflammation might increase platelets shortly (but followed with a sharp decrease?). Antibodies generated by maternal immune system might also enter the fetal circulation under some pathologic condition, attack the platelet and lead to decreased platelet count in neonate [10]. Platelets have the ability to rapidly adhere to the endothelium cells, become activated and secrete bioactive mediators, thus contributing to blood–brain barrier permeability, allowing the entry of white blood cells to cause? Cerebrovascular inflammation, which plays signifificant roles in neurons loss in neurodegeneration [11].
Specifically, MPV is a marker of average platelet size and PDW represents the heterogeneity of platelet size. MPV and PDW are widely used for measuring the function and activation of platelet in the blood [12]. Consequently, platelets change their shapes and these changes are reflected in the MPV [13].
Increased MPV and PDW levels indicate more active and larger platelets that release more thromboxane A2 and aggregate more readily in vitro [14]. Another study found that MPV and PDW attributed to an increase in the number of larger and wider platelets that appeared after the destruction of pro-inflammatory cytokines and endotoxins as part of a severe inflammatory response [15]. In this study, we found that higher MPV and PDW levels are correlated with severe ROP. The multiple linear regression analysis further confirmed that MPV at PMA of 36 weeks were independently associated with platelet parameters.
Thus, measurement of platelet parameters may be a useful tool to predict the development of severe ROP in extremely premature infants, which is expected to be a simple, convenient, and efficient approach to supplement ophthalmic examination in ROP. Moreover, considering that platelet activation appears to be important to retina damage, targeting the regulation of platelet function may offer novel therapeutic interventions in severe ROP.
However, this study has its limitations. Besides the retrospective nature, is the relatively limited sample size, especially in the severe ROP groups? Therefore, our data should be interpreted with care. Furthermore, an inclusion bias in our study has incurred because we excluded the infants who died before the diagnosis of ROP was made. These infants may be also at increased risk of moderate or severe ROP.
The data used to support the findings of this study are available from the corresponding author upon request.
The sponsors had no role in the design, execution, interpretation, or writing of the study. The funders were not involved in the study design, data collection, analysis, interpretation, or manuscript preparation.
The authors declare that they have no competing interests.
C.C, C.Y, and X.X. are involved in the conceptualization of this study; Z.H. and Z.J. in the methodology; M.W in the validation; W.X.in the investigation; B.Y in the data curation;C.C. in writing and original draft preparation; C.C. and X.X. in the writing, review, and editing; C.Y. in the supervision; and C.C. and C.Y. in the funding acquisition. All authors read and approved the fifinal manuscript.
This study is supported by Shenzhen Fund for Guangdong Provincial High level Clinical Key Specialties (SZGSP009).
[Cross ref] [Google Scholar] [PubMed]
[Cross ref] [Google Scholar] [PubMed]
[Cross ref] [Google Scholar] [PubMed]
[Cross ref] [Google Scholar] [PubMed]
[Cross ref] [Google Scholar] [PubMed]
[Cross ref] [Google Scholar] [PubMed]
[Cross ref] [Google Scholar] [PubMed]
[Cross ref] [Google Scholar] [PubMed]
[Cross ref] [Google Scholar] [PubMed]
[Cross ref] [Google Scholar] [PubMed]
[Cross ref] [Google Scholar] [PubMed]
Citation: Chen C, Huang Z, Yang B, Wang X, Wang M, Yang C, et al. (2023) Platelet Parameters and Their Relationships with Severe Retinopathy of Prematurity in Extremely Premature Infants. Clin Pediatr. 8:231.
Received: 01-Feb-2023, Manuscript No. CPOA-22-19287; Editor assigned: 03-Feb-2023, Pre QC No. CPOA-22-19287 (PQ); Reviewed: 17-Feb-2023, QC No. CPOA-22-19287; Revised: 24-Feb-2023, Manuscript No. CPOA-22-19287 (R); Published: 06-Mar-2023 , DOI: 10.35248/2572-0775.23.8.231
Copyright: © 2023 Chen C, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.