Journal of Plant Biochemistry & Physiology
Open Access
ISSN: 2329-9029
ISSN: 2329-9029
Research Article - (2014) Volume 2, Issue 1
In the present study we investigated the performance of dark-stored wounded leaf discs and pieces (to some extent mimicking fresh-cut product) of Lactuca sativa L. in relation to the physiological maturity at harvest. We used two related genotypes, i.e. a green (cv. Troubadour) and a red butterhead (cv. Teodore) differing in their pigment levels. For both genotypes, senescence of the wounded (fresh-cut) tissue prepared from leaves of younger plants was significantly delayed compared to wounded tissue prepared from the more mature plants. Shelf-life (time to unacceptable quality) of fresh-cut was doubled when plants were harvested one week before the commercial harvesting date. To explain differences in shelf-life between fresh-cut products prepared from plants harvested at different age, a number of physiological and nutritional parameters were determined at harvest. The red lettuce contained about two times more chlorophyll, carotenoids, and polyphenolic antioxidants than the green lettuce, but the shelf-life of both genotypes was about similar. Increasing the amount of pigments and polyphenols through application of LED light (with high percentage blue) during cultivation did not affect the shelf life of the wounded leaf tissue. The content of chlorophyll, carotenoids, phenolic compounds, as well as total antioxidant capacity was not affected by age of the plants for either genotype. The content of ascorbic acid decreased with maturation in the green lettuce but it was not affected by maturity in the red lettuce. This shows that there are no obvious signs of leaf senescence with age and the differences in pigments and antioxidants show no relation to the fresh-cut shelf life and thus cannot explain the effect of plant age on senescence of the wounded tissue. The net photosynthesis rate and carbohydrate reserves in the red lettuce were about half of that in the green lettuce but the shelf-life of both genotypes was similar. The net photosynthesis rate was not influenced by plant maturity in the green lettuce, whereas it decreased with maturation in the red lettuce. A decrease in sucrose and starch, and therefore the total content of carbohydrates with aging was observed in both genotypes. This effect was more pronounced in the red than in the green lettuce. There was no apparent relationship between the absolute levels of the total carbohydrates and the shelf-life of the fresh-cut product showing that carbohydrate reserves in itself cannot explain the effect of plant age on senescence of the wounded tissue. The leaves from older plants apparently have a reduced capability to cope with the stress from wounding. No simple one to one relationship emerged between any of the measured nutritional parameters, their change during maturation and the eventual shelf-life of the fresh-cut produce.
Keywords: Lactuca sativa L; Antioxidants; Developmental stage; Fresh-cut; Senescence; Storage; Shelf-life; wounding.
The industry of ready-to-use fresh fruits and vegetables is constantly growing as a result of consumer awareness of the importance of a healthy diet [1-3] and the convenience of these products. Lettuce is an important agricultural commodity, which contains appreciable amounts of bioactive compounds [4-7], and appears to exert a diversity of beneficial health effects [4,8-10]. However, the quality of fresh-cut lettuce is still unpredictable and its shelf-life limited. Effective, noninvasive and non-chemical techniques for improving and maintaining quality are therefore needed.
In order to obtain high quality fresh-cut vegetables, it is necessary to start with high quality raw material [11]. To this respect, genetic background [12,13] and pre-harvest factors, such as cultivation conditions [14-22] and plant age at harvest [23-26] affect quality of fresh-cut vegetables. Among few reports on the influence of the plant age on the chemical composition and storability of leafy vegetables, only a small number has been devoted to lettuce, and its properties and quality as a fresh-cut product [23]. The significance of the plant maturity on lettuce quality was inconsistent between these studies, possibly due to the interaction of plant age with weather conditions during different growing times. The majority of these investigations also focused only on the effect of crop maturity on the content of antioxidants at harvest. Knowledge about age-dependent differences in the effect of wounding on the senescence of (fresh-cut) lettuce, as well as the mechanisms responsible for these differences, are still lacking, and were the subject of the present study. We investigated the senescence of fresh-cut lettuce in relation to the age of the plant at harvest. Fresh-cut prepared from laves of younger plants consistently showed a delayed senescence compared to fresh-cut prepared from leaves of more mature plants. The relation with nutritional and physiological features of the raw material is discussed.
Plant material, growth and storage conditions
Two related genotypes of Lactuca sativa L. i.e. green butterhead (GB) cv. Troubadour and red butterhead (RB) cv. Teodore (RijkZwaan, The Netherlands) were used in two experiments.
In experiment 1 the seeds were sown in boxes filled with vermiculite and, one week after germinating, the seedlings were transplanted to a hydroponic system (Hoagland’s solution, pH 5.9 ± 0.2; EC=1.2 mS cm-1) in a climate chamber located at Wageningen University, The Netherlands. Temperatures were maintained at 20°C during the day and at 15°C during the night, and the relative humidity was 70%. Two light sources, i.e. white fluorescent tubes (FL; TLD 50 W 840 HF, Philips, The Netherlands) or light emitting diodes (LEDs)were used to provide photosynthetically active radiation (PAR) at 250 μmol m-2s-1 (12 hours photoperiod). The LEDs arrays consisted of 30% of red (peak wavelength at 667 nm), and of 70% of blue (peak wavelength at 465 nm) LED modules (Green power LED modules HF, Philips, Eindhoven, The Netherlands). Thirty and 42 days after transplanting the plants were harvested in the morning, and leaves from the second outer whorl were used for the storage experiment.
For studying wound-induced senescence, leaf discs excised with a stainless steel cork borer (18 mm diameter) were used (mimicking fresh-cut product). The leaf discs were cut from the leaf lamina avoiding the inclusion of the midrib and major veins. This method of cutting was performed to keep extend of wounding similar for all samples and treatments. Randomly selected leaf discs and intact leaves were placed in plastic Petri dishes (25×100 mm), with vents, lined with wet filter papers (Whatman, #3) to prevent desiccation. The dishes were placed in temperature controlled storage units (Elbanton, Kerkdriel, The Netherlands) at 12°C in the dark, and relative humidity of 90%.
In experiment 2 the seeds of both genotypes were sown in boxes filled with vermiculite, and one week after germinating seedlings were transplanted to pots with standard pot soil under the climate conditions as described in experiment 1. In this experiment photosynthetically active radiation (PAR) at 250 μmol m-2s-1 was only provided by white fluorescent tubes. (TLD 50 W 840 HF, Philips, the Netherlands). Thirty five, 42 and 49 days after transplanting, plants were harvested in the morning, and second outer whorl leaves were used for the storage experiment.
In this experiment, leaf pieces (4 × 4 cm) were cut from the middle part of the leaf lamina with a sharp stainless steel knife avoiding the inclusion of the midrib and major veins. Randomly selected leaf pieces were placed in white plastic boxes (1300×1800 mm) lined with wet filter papers (Whatman, #3) to prevent desiccation. The boxes were equipped with a transparent plastic lid in which 16 small holes (approximately 0.5 mm diameter) were punched equally distributed over the lid. This allowed sufficient ventilation to prevent depletion of oxygen or accumulation of carbon dioxide or ethylene under the experimental conditions (data not shown). The boxes were placed in temperature controlled storage units as in experiment 1. The experimental conditions in the two experiments are summarized in Table 1.
| Experiment | Culture medium | Lighting conditions | Plant age | Fresh-cut storage |
|---|---|---|---|---|
| 1 | Hydroponics | White Fluorescenttubes and LED (30% red, 70% blue) | 30 and 42 days | 18 mm diameter leaf discs and whole leaves in petri dishes |
| 2 | Soil | White Fluorescent tubes | 35. 42 and 49 days | 4x4 cm leaf pieces in plastic boxes |
Table 1: Summary of the two experiments performed with the related genotypes of Lactuca sativa L.: green butterhead genotype (cv. Troubadour) and red butterhead genotype (cv.Teodore) (RijkZ waan, The Netherlands).
In experiment 1, at every sampling time, leaf discs were randomly selected from 3 different petridishes (replicates), weighted, and specific leaf weight (SLW, leaf FW/unit area) was calculated. In experiment 2, at every sampling time, leaf pieces were randomly selected from 3 different boxes (replicates), and from each leaf piece, 3 discs were excised. In each experiment, per each replicate, leaf discs were either used directly for determination of pigments, or discs were frozen in liquid nitrogen and stored at -80°C for later analysis of AsA. For determination of phenolics and ORAC, leaf discs were frozen in liquid nitrogen, dried using a freeze-dryer (Modulyo, Pirani 501, Edwards, UK) and ground into fine powder with a ball-mill (Retsch, Germany). Analysis were generally done on three independent biological samples (n=3).
Overall visual quality (OVQ) and shelf-life
Quality of both the discs and the leaf pieces was evaluated using overall visual quality (OVQ) ratings modified from Kader et al. [27]. The OVQ was scored on the basis of leaf characteristics such as color (yellowing, browning, and brightness), wilting, and presence or absence of defects such as dark brown (water soaked) lesions. Quality ratings were made on a scale from 9 to 1, where 9 = excellent, essentially free from defects, and 1 = extremely poor, not usable.
We modified the OVQ scores proposed by Kader et al. [27] to better suit our purpose by making the quality descriptions more explicit as follows:
9 = excellent, essentially free from defects
8 = very good, with minor changes in colour, and/or hardly visible browning
7 = good, with minor changes in colour and structure or slightly visible browning
6 = fair, with minor changes in colour and structure and slightly visible browning
5 = fair, clear changes in colour and structure and clearly visible browning, below the limit of sales appeal
3 = poor, with excessive changes in colour and structure, and very pronounced browning
1 = extremely poor, not usable
Although subjective in nature, the OVQ ratio is a good means to compare quality deterioration between different treatments. The OVQ rating integrates different types of quality defects that may occur in parallel during leaf ageing [28,29] and OVQ was found to correlate well with quality measured by a combination of instrumental measurements [30]. An OVQ rating of 6 was considered the lower limit of consumer acceptance. Shelf life is determined as the number of days till quality reached the lower limit of consumer acceptance (OVQ=6).
Determination of pigments
Pigments were extracted in dimethylformamide (DMF) in the dark at -20°C. The absorbance of the extract was measured in the range 400– 750 nm using a Cary 4000 spectrophotometer (Varian Instruments, Walnut Creek, CA, USA), and the pigment content was calculated using the equations provided by Wellburn [31].
Determination of ascorbate
Ascorbate (AsA) was measured following a procedure modified from Davey et al. [32]. Leaf discs that were previously stored at -80°C were ground in liquid nitrogen, and thawed on ice in the dark. These samples were mixed with 0.5 ml ice-cold 3.3% meta-phosphoric acid, and the mixture was placed in an ultrasonic bath at 0°C (melting ice) for 10 minutes in the dark. Total AsA was determined followed the reduction of DHA to AsA by DTT. 100 μl of the extract was added to 50 μl of 5 mM DTT in 400mM Tris base, resulting in a final pH of 6–6.8. Previous experiments showed that a DTT concentration of 5 mM was sufficient for this type of plant tissue. The reaction was stopped after 15 min at room temperature by acidification with a 50 μl of 8.5% orthophosphoric acid. Extracts were then analyzed using HPLC for total AsA. The Dionex ICS5000 HPLC was equipped with a Omnispher 5 C18 (150x3 mm; Varian) column that was eluted with 400 μl l-1, H3PO4, 2.5 ml l-1, MeOH and 0.1 mM EDTA in H2O followed by a wash step with 30% acetonitrile.
Quantification of polyphenolic compounds
Ten micrograms of powdered freeze-dried lettuce sample was mixed with 1.5 ml of 80% methanol, and the mixture was placed in an ultrasonic bath at 20°C for 4 minutes. After centrifugation, the supernatant was used for the determination of polyphenolic compounds.
The total amount of polyphenolic compounds in lettuce was determined using Folin-Ciocalteu’s reagent according to the method of Singleton et al. [33]. Three hundred microliters of the methanolic extract was mixed with 1.5 ml of 2M Folin-Ciocalteu reagent, and then the mixture was added to 1.20 ml of 20% Na2CO3. After incubation at room temperature for 30 minutes, the mixture was centrifuged, and the absorbance of the supernatant was measured at 735 nm. The standard curve was prepared using gallic acid (GA). The absorbance was converted to phenolic content in terms of milligrams of GA equivalent (GAE) per 100 gram of fresh weight (FW) of sample.
Oxygen radical absorbance capacity (ORAC)
The extraction of the antioxidant fraction and determination of antioxidant capacity in lettuce was performed using the oxygen radical absorbance capacity (ORAC) method modified from Huang et al. [34]. Fifteen micrograms of powdered freeze-dried lettuce sample was vortexed for 1 min with 1 ml of hexane. After centrifugation the supernatant was collected, and the pellet re-extracted in 1 ml of hexane. The combined hexane extracts were evaporated to dryness at 40°C using a vacuum evaporator (Savant SPD2010 SpeedVac, Thermo Scientific, Asheville, NC, USA). The pellet remaining after the hexane extraction was dried and re-extracted with 1.5 ml of acetone/water/acidic acid mixture (70:29.5:0.5; v/v/v) to obtain the hydrophilic fraction. For the lipophilic antioxidant assay, the dried hexane extract was re-dissolved in 250 μl of acetone and then diluted with 750 μl of a 7% randomly methylated b-cyclodextrin solution (RMCD; 50% acetone/50% water, v/v; Huang et al., 2002). Any further dilution was made with the 7% RMCD solution. The 7% RMCD solution was also used as a blank and to dissolve and dilute the Trolox standards for the lipophilic assay. For the hydrophilic assay, phosphate buffer (0.075 M, pH 7.4) was used as the solvent. This phosphate buffer was also used as the blank and the solvent for the Trolox standards in the hydrophilic assay. The fluorescence intensity measurement was performed using a Safire monochromatorbased microplate reader (Tecan USA, Research Triangle Park, NC) with the sample loaded on polystyrene, flat-bottom 96-well plate (Fluotrac, Greiner America, Inc.). The 20 μl of diluted sample was mixed with 100 μL fluorescein solution in a microplate and incubated at 37°C for 15 min, after which 40 μl of 2,20-Azobis (2-amidino propane) dihydrochloride (AAPH) (17.2 mg ml-1) was added to each well using a multi-channel pipette. Immediately following the addition of AAPH, the plate was agitated for 5 s prior to the first reading and for 2 s before each subsequent reading. Readings were done at 2 min intervals for 40 min. Excitation and emission filter wavelengths were set at 484 nm and 520 nm, respectively. Data were expressed as μmol Trolox equivalents (TE) per gram of lettuce on a fresh dry weight (FW) basis. The ORAC values were calculated by using a linear regression equation (Y = a + bX) between concentration (Y) (μM) and the net area under the fluorescence decay curve (X). Linear regression was used in the range of 6.25–50 μM Trolox. Blank, standard and sample were analyzed in triplicate to eliminate position effects.
The area under curve (AUC) was calculated as follows:
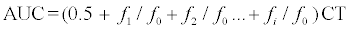
where f0 is the initial fluorescence reading at cycle 0, fi is the fluorescence reading at cycle i, and CT is the cycle time in minutes. The net area under the curve was obtained by subtracting the blank value from that of a sample or standard.
Determination of soluble sugars and starch
Ten micrograms of powdered freeze-dried lettuce sample was extracted in 5 ml of 80% ethanol for 20 minutes at 80°C. One milliliter of supernatant was vacuum dried (Savant SPD2010 SpeedVac, Thermo Scientific, Asheville, NC, USA), the residue was re-suspended in 2 ml of 0.01 MHCl, and resulting extracts were passed through an Extract CleanTM SCX column 100 mg, 1.5 ml-1 (Grace, Deerfield, IL, USA) activated with 5 ml 0.01 M HCl. The eluents were analyzed for soluble sugars on a Dionex ICS5000 HPLC equipped with a CarboPac1 (250×2mm) column eluted with a gradient from 16 to 45 mM NaOH. The remaining 4 ml of supernatant were discharged, the residue was centrifuged, and the pellet was washed three times with 80% ethanol before vacuum drying. The pellet was suspended in 1 mg m-1thermostable α-amylase (Serva 13452) in water at 90°C, and subsequently with amyloglucosidase (Fluka 10115) in 50 mM citrate buffer with pH=4.6 at 60°C to hydrolyze starch to glucose. The obtained starch extracts were analyzed on a Dionex ICS5000 HPLC equipped with a CarboPac1 (250×2mm) column eluted with 100 mM NaOH and 12.5 mM sodium acetate.
Net photosynthetic rate and dark respiration
Photosynthetic rates (Anet) of plants at harvest were measured with a portable gas analysis system (LI-6400 equipped with a leaf chamber fluorometer; Li-Cor Inc., Lincoln, NE). The leaf chamber temperature was set to 20°C, the air flow at 150 μmol s-1, the CO2 concentration during the irradiance-response measurements was 400 μmol mol-1 and the LED light source was set at 10% blue light. Water vapor concentration was similar to that in the ambient air (22.5 mmol mol-1; RH= 70%). The Anet were calculated as the mean value during a 120 second window following the establishment of a stable CO2 fixation rate. Dark respiration (RD) was measured after 10 min of dark adaptation in the leaf chamber.
Statistical analysis
Data were processed using the SPSS statistical package (IBM SPSS, release 19.0.0.1, 2010. SPSS Inc. and IBM Company, Chicago, IL, USA). Each experiment had three replicates per treatment. For initial levels of analyzed characteristics we assumed fixed effect for plant maturity stage and genotype, and random effect of the replicate. The interactions between maturity stage × genotype were studied by looking at maturity stage differences per genotype, and at genotype differences per maturity stage. The effect of cold storage was analyzed per genotype and for each treatment separately. Mean separations was performed using the LSD procedure, and significance was declared at P < 0.05. The trends in OVQ data were fitted in Excel (Microsoft Office 2010 for Windows) with polynomial (order 2) regression lines.
Effect of plant age on structural properties
The specific leaf weight (SLW) was similar for both genotypes (Figure 1). In general, the SLW did not greatly differ between maturity stages of plants in experiments 1 and 2. However, the SLW tended to be a little lower for younger plants, and this difference only appeared to be statistically significant comparing 30 and 42 days old plants of GB and RB grown under fluorescent light (FL) in experiment 1 (Figure 1A).
Figure 1: Initial levels at harvest of specific leaf weight (SLW) in two butter head genotypes (green, GB and red, RB). Plants in experiment 1 (A) were grown under different light conditions, i.e. white fluorescent tubes (FL) and 70B:30R light emitting diodes (LEDs) for 30 or 42 days. Plants in experiment 2 (B) were grown under FL for 35, 45 and 49 days. Error bars represent standard errors. Data points marked by different lower case letters for comparison between growth stages per genotype (a–b), are significantly different (P<0.05, n=9).
Effect of plant age on pigment concentration, polyphenolic compounds and oxygen radical absorbance capacity (ORAC)
The red butter head (RB) generally contained much higher amounts of pigments (Figure 2 A-D) and polyphenolic compounds (Figure 3A) than the green butter head (GB), at all growth stages. Total ORAC value was about twice as high in RB compared to GB (Figure 3 B, C). The chlorophyll, carotenoid and polyphenolic content, as well as total antioxidant capacity (ORAC total) and its lipophilic fraction (ORAC lipophilic) did not differ between maturity stages of plants of both genotypes in experiment 1 and 2 (Figures 2 and 3).
Figure 2: Initial levels at harvest of chlorophylland carotenoids in two butterhead genotypes (green, GB and red, RB) in two experiments. Plants in experiment 1 (A, B) were grown under differentlight conditions, i.e. white fluorescent tubes (FL) and 70B:30R light emitting diodes (LEDs) for 30 or 42 days. Plants in experiment 2 (C, D) were grown under FL for 35, 45 and 49 days. Error bars represent standard errors (n=3).
Figure 3: Initial levels at harvest of polyphenolic compounds (A), total oxygen radical absorbance capacity (ORAC) ( B), and lipophilic ORAC (C) in two butterhead genotypes (green, GB and red, RB). Plants were grown under different light conditions, i.e. white fluorescent tubes (FL) and 70B:30R light emitting diodes (LEDs) for 30 or 42 days in experiment 1. Error bars represent standard errors. Data points marked by different lower case letters for comparison between growth stages per genotype (a–b), are significantly different (P<0.05, n=3).
Effect of plant age on total ascorbate (AsA)
The content of total ascorbic acid (AsA) was about similar in both genotypes (Figure 4). The content of total AsA decreased with increasing plant maturity in GB, whereas a less pronounced (statistically not significant) trend was observed in RB (Figure 4).
Figure 4: Initial levels at harvest of total ascorbic acid (AsA) of two butterheadgenotypes (green, GB and red, RB) grown for 35, 45 and 49 days in experiment 2. Error bars represent standard errors. Data points marked by different lower case letters for comparison between growth stages per genotype (a–b), are significantly different (P<0.05, n=3).
Effect of plant age on soluble sugars and starch
In all growth stages RB had lower total soluble sugar content than GB (Figure 5E). Total carbohydrate content in RB was on average less than half of that in GB (Figure 5F). In RB all individual sugars and starch showed a clear decrease with increasing age of the plant (Figure 5A-D). In GB no consistent decrease in soluble sugars or starch was observed with age (Figure 5A-D). In RB total carbohydrates decreased more than 50% between 35 and 49 days of growth (Figure 5F); in 49 days old GB plants the amount of carbohydrate was higher than in 35 days old RB plants (Figure 5F).
Figure 5: Initial at harvest of sucrose (A), fructose (B), glucose (C), starch (D), sum of three soluble sugars (E), and total carbohydrate (F) in two butterheadgenotypes (green, GB and red, RB) plants grown for 35, 45 and 49 days in experiment 2.. Error bars represent standard errors. Data points marked by different lower case letters for comparison between growth stages per genotype(a–c), are significantly different (P<0.05. n =3).
Effect of plant age on net photosynthesis and dark respiration
In all growth stages, net photosynthesis rate (Figure 6A) was about 40-50% lower in RB compared to GB; the dark respiration rates (Figure 6B) of RB and GB were similar. Net photosynthesis rate and the ratio between net photosynthesis and dark respiration (Figure 5C) did not change with maturity of plants in GB. In contrast, a significant decrease in both parameters was observed with the increased maturity for RB, indicating that the plants of RB became photosynthetically less efficient with age.
Figure 6: Initial at harvest of net photosynthesis (A), dark respiration (B) and ratio of photosynthesis to dark respiration (C) in two butterhead genotypes (green, GB and red, RB) in experiment 2. Plants were grown for 35, 45 and 49 days. Error bars represent standard errors. Data points marked by different lower case letters for comparison between growth stages per genotype (a–c), are significantly different (P<0.05, n=5).
Effect of plant age on overall visual quality (OVQ) of freshcut product
OVQ ratings were mainly based on severity of senescence symptoms. In the lettuce leaf discs and the 4x4 cm leaf pieces of both GB and RB, quality decay was determined by the occurrence of edge browning, yellowing and by the later appearance of dark brown, water soaked areas and loss of turgescence. The shelf life was defined as the time to OVQ = 6 (unacceptable quality). The shelf-life of discs (in experiment 1) of both genotypes was approximately similar, and in both genotypes a comparable decrease in shelf-life was observed with increasing plant age (Table 2, Figure 7). The shelf-life of discs excised from older plants was shorter than that of discs from younger plants. This difference was more pronounced in GB (6-8 days depending on the light source) than in RB (3- 4 days).The shelf-life of the intact leaves from plants of different maturity stages was not different. Leaves of both genotypes grown under FL and LEDs, irrespectively of the age, had a shelf-life of 25-27 days (data not shown).
| Experiment 1 Plant age | Experiment 2 Plant age | |||||
|---|---|---|---|---|---|---|
| Genotype | Light source | 30 days | 42 days | 35 days | 42 days | 49 days |
| GB | FL | 24 | 18 | 14 | 10 | 4 |
| RB | FL | 24 | 21 | 16 | 12 | 6 |
| GB | LEDs | 24 | 16 | - | - | - |
| RB | LEDs | 24 | 20 | - | - | - |
Table 2: Shelf-life (number of days till Overall Visual Quality score drops below 6) of discs (experiment 1) and leaf pieces (experiment 2) excised from plants of different maturity stages.
Figure 7: Overall visual quality (OVQ) changes during storage of fresh-cuts of two butterhead genotypes (green, GB and red, RB) in experiment 1. Plants were grown under different light conditions, i.e. white fluorescent tubes (FL) and 70B:30R light emitting diodes (LEDs) for 30 (A, B) or 42 (C, D)days. Data points marked by different letters, i.e. upper case (A-E) for GB, and lower case for RB (a–e), for comparison between storage times are significantly different (P<0.05, n=9).
The shelf life of leaf pieces (in experiment 2), was about similar for both genotypes and a comparable decrease in shelf-life was observed with increasing plant age (Table 2, Figure 8). The difference in the shelflife between leaf pieces prepared from 35 days and 42 days old plants was 4 days; the difference between leaf pieces from 42 days and 49 days old plants was about 6 days.
Figure 8: Overall visual quality (OVQ) changes during storage of fresh-cuts of two butter head genotypes (green, GB (A) and red, RB (B)) in experiment 2. Plants were grown under white fluorescent tubes (FL) for 35, 45 and 49 days. Data points marked by different letters, i.e. upper case (A-E) for GB, and lower case for RB (a–e), for comparison between storage times are significantly different (P<0.05, n=9).
Experimental system to study wound-induced senescence in lettuce
The effect of plant age on the performance of wounded tissue, mimicking fresh-cut product, was studied. The experimental set-up that was chosen for this investigation differs from the conditions that prevail in fresh-cut in commercial practice. Instead of packing the fresh-cut product in a sealed bag with a rapidly developing modified atmosphere, we chose for a set-up where the fresh-cut produce is stored under atmospheric conditions. The advantage of such a system is that the effect of plant age can be studied without the interference with the uncontrolled and (plant age dependent) continuous changing headspace composition. In a sealed bag, depending on the initially applied concentration and the type of film used, the oxygen level rapidly decreases and will be near zero after 2 to 3 days; the carbon dioxide level will rise to 10 to 12 %. The low oxygen concentration will virtually block the rapid pink and brown coloration but, later it will lead to fermentation and tissue senescence. This merely uncontrolled change in gaseous conditions may easily obscure any effects of treatments or conditions applied prior to packaging. The experimental set-up used here allowed us to investigate the effects of genotype, growth lighting source and plant age on the performance of the fresh-cut product in an unbiased way. Commercial fresh-cut products often contain a heterogeneous mix of different cut sizes and tissue types (older and younger leaves, green leaf tissue and midribs and major veins) and this makes judgment of quality complex. Generally the wounded midrib tissue shows rapid pink and brown coloration whereas the green leaf tissue may show more classical signs of leaf senescence (yellowing, loss of turgescence, cell death). The quality judgment therefore is based on a plethora of symptoms arising from unrelated physiological processes. To study the coloration events in midrib tissue some authors have used model systems exclusively containing of wounded vein tissue (e.g. Saltveit, 2004) [35]. In the present investigation we focused on the effect of wounding on leaf senescence and therefore we chose to exclusively use leaf tissue from the leaf lamina excluding the midrib and the major veins. To assure that the extent of wounding was similar for all treatments discs or leaf pieces with a specified area were cut out of the leaf.
As plant material two related butter head genotypes were used, one being green, the other being dark-red colored due to increased levels of pigments. The LED light application with high % of blue during the growth phase was introduced as a mild stress factor to create even more variations in the levels of pigments. This allowed us to more closely study the relation between initial levels of pigments and performance of the wounded material.
Age-dependent shelf-life of fresh-cut lettuce
Fresh-cut lettuce prepared from younger plants (e.g. 35 days of growth) had a longer shelf-life than when prepared from older plants (e.g. 49 days of growth) (Figure 8). It should be noted that for both younger and older plants leaves from a similar position in the rosette were used for fresh-cut preparation; the leaves of older plants were therefore similar to the leaves of younger plants but more mature and more developed. These results are in agreement with results of Couture et al. [23], the only report on the age-dependent shelf-life of fresh-cut lettuce. Our study additionally showed that this phenomenon is independent of the genotype (GB vs. RB) and conditions during growth, i.e. light source (FL vs. LEDs). Apparently, younger tissue has a greater capacity to cope with the wounding.
Developmental leaf senescence at harvest
At harvest, plants of all ages were healthy, and visible signs of senescence were absent. We hypothesized that older plants may internally already show symptoms of senescence, e.g. chlorophyll degradation [28] that relate to the lesser resistance to wound-induced deterioration. However, chlorophyll and carotenoid levels were little affected by plant age (Figure 2). Buchanan-Wollaston et al. [36] have already suggested that the senescence is well underway by the time the leaf shows visible signs of senescence. Therefore, the retention of chlorophyll in older plants may not mean that they are not senescing. Also Múnne-Bosch et al. [37] reported that the chlorophyll degradation is an unreliable indicator for senescence.
Other initial measurable symptoms of senescence, i.e. a decreased photosynthesis and increased respiration [28,37,38] showed a genotypedependent behavior with advancing maturity in our experiments. The net photosynthesis rate and dark respiration were similar for different plant ages in GB, whereas the net photosynthesis rate significantly decreased in the aging tissue of RB (Figure 6). The latter might be due to the increased red pigmentation of RB with age allowing less light to penetrate the leaf and may therefore not be a sound marker of senescence. In line with the decrease in carbon fixation in aging RB, the concentration of fructose, glucose, sucrose and starch all decreased with plant age (Figure 5). In GB sugar levels were more stable, however, as in RB sucrose and starch tended to decrease with age.
We hypothesized that processes occurring during the later stages of developmental leaf senescence, such as lipid degradation, loss of antioxidants and loss of cellular integrity [28,37,38] may be the basis of the lesser performance of fresh-cut prepared from older leaves. Cell membrane disruption due to lipid peroxidation has been reported to correlate with chlorophyll loss [39,40]. As the chlorophyll content did not decrease with the age of the plant for either genotype (Figure 2) we assume that lipid peroxidation was also not present. At all plant ages, sugars were still ample available; therefore, it is rather unlikely that lipids were used as alternative energy source.
Carotenoids, phenolics and total antioxidant capacity in lettuce leaves did not change with plant age (Figures 2 and 3). This is in disagreement to a number of other studies [23-26]. For example, Bergquist et al. [25] found an increase of carotenoids with age of spinach leaves; Couture et al. [23] found lower levels of phenolic compounds in over-mature than in mature leaves of crisphead lettuce, whereas Pandjaitan et al. [24] reported higher levels of phenolic compounds, flavonoids and ORAC value in mid-mature than in immature and mature leaves of spinach. In addition, Zhao et al. [26] reported higher ORAC values in mature than in baby size spinach leaves, whereas no difference was reported in red leaf and romaine lettuce of different ages. The discrepancies in the effects of plant maturity on above mentioned characteristics between these studies might be explained by the interaction of plant age with e.g. weather conditions during different growing periods. It is likely that if the “maturity x weather condition” interaction is removed by growing plants under constant conditions, as we did, the plant age effect becomes more consistent.
In contrast to carotenoids and phenolic antioxidants that were stable during plant maturation in both genotypes, AsA levels in GB, but not (or to a lesser degree), in RB showed a clear decrease with maturation (Figure 4). This might have been due to developmental shifts in antioxidant requirements of both genotypes. The lack of agedependent decrease in AsA in the red tissue, which is inherently high in antioxidants [4,26,41,42] might have been due to the involvement of other antioxidants than AsA, i.e. carotenoids and anthocyanins in counteracting age-driven oxidative stress during growth. Carotenoids are well known for scavenging of ROS [43] whereas polyphenolic compounds have been suggested to act as a backup to the primary AsAdependent detoxification system [44], although their role in detoxifying ROS is often not sufficiently recognized. Polyphenolic compounds are often ascribed only to the epidermal cells, whereas it has been shown that they are also located in mesophyll cells where they act as antioxidants [45,46].
Together the observations do not clearly indicate that the leaves from older plants are already in an advanced state of senescence that may have affected the response to wounding.
Relationship between nutritional content and the (agerelated) shelf-life
In general, no simple one to one relationship emerged between any of the measured nutritional parameters, their change during plant maturation and the eventual shelf-life of the fresh-cut produce. This is in contrast to results presented by a number of researchers in the last years. For instance, it has been shown that high antioxidant content decreases the rate of senescence [47,48]. Following this rationale, Bergquist et al. [25] measured levels of AsA in spinach as affected by plant age, and showed that the content of AsA in the tissue can be used to predict the shelf-life. AsA is supposed to protect against oxidative stress and, therefore, to reduce the rate of senescence. Our results contradict these models. The levels of chlorophyll, carotenoids and phenolic compounds, as well as antioxidant capacity were about two times higher in RB than in GB, whereas the shelf-life of fresh-cut product from both genotypes was similar. In both genotypes these levels were not influenced by the maturation of the head, whereas the shelf-life was. A significant decrease in AsA was observed in GB with the maturation of the head, theoretically explaining the shortened shelf-life of fresh-cuts prepared from older plants. However, no, or a much smaller decrease in AsA was observed in RB plants, yet there was a pronounced decrease in shelf-life with age. Together this shows that the contents of AsA, carotenoids, phenolic compounds, as well as total antioxidant activity at harvest have no relation with either the age of the plant or the rate of senescence in the fresh-cut product.
Soluble sugars and starch are the primary energy reserves in the tissue. The levels of these energy reserves as well as the rate of their loss during storage have been suggested to define the storability of the product [49]. However, this relationship did not appear to be that simple in our study. The levels of soluble sugars and the total carbohydrates (soluble sugars + starch) were about two times higher in GB than in RB, whereas the shelf-life of fresh-cut product from both genotypes was similar. Carbohydrate levels in the older GB were about similar to those in the younger RB, whereas the shelf-life of freshcut prepared from the older GB was about 12 days shorter than that of the younger RB. However, the decrease in the levels of sucrose and starch, and therefore the level of the total carbohydrate with age did correlate to a certain extent with the maturation dependent decrease in the shelf-life within each individual genotype. It appears that the level of the carbohydrate reserves in the tissue required for good quality retention can be genotype dependent, and may be linked to the reserves of other respiratory substrates, e.g. lipids, proteins and organic acids in the tissue. Under conditions of severe depletion of primary respiratory reserves, these secondary respiratory substrates can be utilized [49].
Other possible plant age-related processes involved in freshcut performance
It has been suggested that the quality retention is worse in young tissue compared to older tissue owning to the higher metabolic activity and respiration rates in younger tissues [50]. In our study, this concept appeared to be invalid. Respiration rates of young and older leaves in both genotypes were similar, but the shelf life of fresh-cut from younger and older leaves greatly differed. Apart from the initial antioxidant and carbohydrate-reserve factors in young and older leaves measured by us, other factors influencing fresh-cut performance may be considered. This includes the possible difference in the initial levels of ROS and of plant hormone (like) substances. In relation to plant age, ROS accumulation has been shown to occur in older leaves in parallel with the decline in photosynthetic activity [51]. A possible increased level of ROS in older leaves may lie on the basis of the lesser fresh-cut performance. As the wounding presumably will also stimulate ROS production, the oxidative status of the tissue may become very unfavorable.
Ethylene is a known promoter of senescence and it has been found that ethylene has a more pronounced effect on older than on younger leaves [52]. The ethylene that is (presumably) produced due to the wounding in fresh-cut material may therefore have a more pronounced effect on older than on younger tissue. The role of ethylene in freshcut quality however is questionable as treatments with the ethylene blocker 1-methyl cyclopropene (1-MCP) generally do not improve the fresh-cut performance when no exogenous ethylene is added [53]. Jasmomate (JA) is another hormone-like substance thought to stimulate senescence. JA levels generally increase as the leaves age or senesce [54].
Among the plant hormones thought to counteract senescence, gibberellic acid (GA) and cytokinins (CK) are well known. Levels of active GA generally decrease in ageing leaves [55]. Active GA levels in romaine lettuce decline with the progression of senescence due to conversion into an inactive GA-glucoside [56]. CK levels generally decrease in aging or senescing leaves [57] and over expression of a CK biosynthesis gene suppresses senescence in leaves including lettuce [58]. Changes in the levels of these hormones may be responsible for the lesser performance of fresh-cut produce prepared from older leaves.
In conclusion, the inverse relationship between plant age and the shelf-life of the wounded leaf tissue could not be explained by the initial levels of antioxidants or carbohydrates. No clear signs of ongoing senescence were observed in the older plants, and the shelf-life of excised intact leaves was not affected by plant age. The older leaves apparently have lesser capability to cope with the stress from wounding. This may be related to differences in the oxidative status of the cells or in the levels of hormone (-like) substances such as ethylene, jasmonates, gibberellic acids or cytokinins, but these factors were not investigated in the current research.
This research was supported by The Greenery B.V., the Netherlands and by RijkZwaan B.V., the Netherlands. The help and advice of Arjen van de Peppel, Niovi Christodoulou and Najat Zenasni is greatly appreciated.