Journal of Developing Drugs
Open Access
ISSN: 2329-6631
ISSN: 2329-6631
Research Article - (2020)Volume 9, Issue 2
Phytopharmaceuticals are healing the world from millions and billions of years even though their clinical validation is questioned by virtue of their impediments like low lipid solubility, poor stability, large size moiety and needless metabolism in gut. Phytosome technology has emerged as committed and promising targeting novel drug delivery with improved efficacy, quality and target ability of active plant constituents. Novel herbal formulation techniques have assured the researchers to deliver the plant based secondary metabolites to their systemic targets. This review highlights the unique properties of phytophospholipid complex along with their application in the novel natural drug delivery. Various methods employed in phytosomal preparation and characterization along with the phytosomal advantages over conventional herbal extracts is described in the present review. The prospectus of phytosome technique can suggest new directions and endless frontier as novel drug regimen.
Polydisparity index (PDI); Thin layer chromatography (TLC); Novel drug delivery system (NDDS)
Phytomedicine are accepted as natural healers in the whole world and were even used by lords in divine era. Advanced herbal drug delivery system such as phytosomes has demarcated the undefined bioavailability of lipid insoluble secondary metabolites [1]. Lipid insoluble herbal extracts can be redesigned into lipid compatible therapeutic candidate by chemically assimilating herbal extracts into phospholipids in specific ratio [2]. Cellular vesicles produced by phytosome technique prevent destruction of water soluble phytoconstituents such as terpenoids, glycosides, flavonoids and phenolics by gastric secretion and microflora of gut [3]. Numerous advantages of phytosome such as hepatoprotective action, reduced dose to produce desired therapeutic effect [2] improved stability due to chemical linkage, ability to permeate through skin [4,5] systematic targeting to transit from hydrophilic to lipophillic environment has revolutionized the phytomedicine industry. Nano size of Phytosomes has resolved the obstacles originating due to poor solubility and permeability of large size hydrophilic phytoconstituents across biological membranes [6]. The present study overlooked on phospholipids based drug administration can enlighten modern pathways in the formulation of novel herbal dosage forms.
Phytosomes prospects
Phytosomes represents advanced herbal drug technology that offers defined bioavailability of plant drugs over the herbal extract. Figure 1 represents the phytosome as lipid complex between a natural moieties and soy lecithin compounds such as phosphotidylcholine, phosphotidylethanolamine and phosphotidylserine in a stoichiometric ratio [6]. Reduced particle size, upturned rate of dissolution and absorption claims enhanced in vivo performance of the herbal extracts [7]. Structural elucidation reveals that the chemical interaction of phospholipids and herbal substrate involves the formation of hydrogen bonds between the polar side front of phospholipids and the polar functional group of the secondary metabolites generating a specific pattern [8]. Phytosomes show chemical reaction in solvents such as acetone, dioxane, methylene chloride, hexane and ethyl acetate and assumes a micelles shape network in water appearing liposomal-like cellular configuration in which the active polar moiety is docked to phospholipids behaving as integral part of the cell membrane [9,10]. There are numerous factors which govern the characteristic originality of phytosomes in physical state such as physical size, membrane permeability, entrapment ratio, chemical constitution as well as the quantity and purity of the precursor starting chemical ingredients [11].
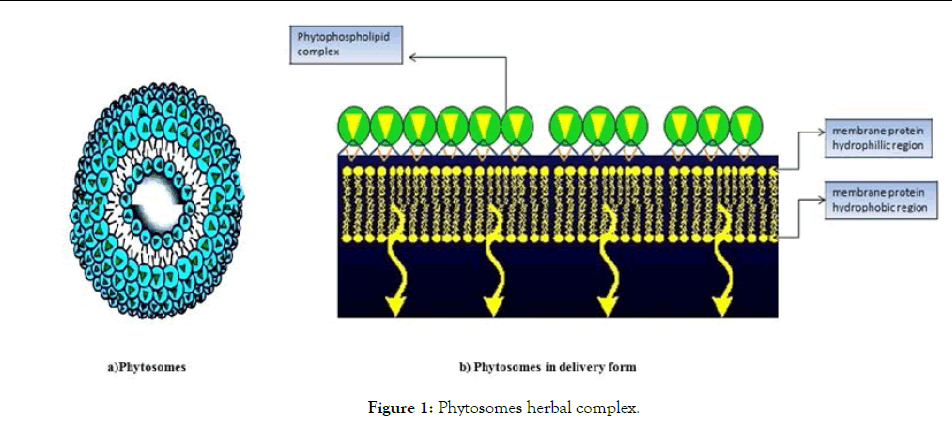
Figure 1. Phytosomes herbal complex.
Different methods of preparation
Non-conventional methods are usually employed in construction of phytosome complexes. Modernistic herbal complexes are formed by reaction between equimolar mixture of natural or synthetic phospholipid and active constituents or herbal extract in aprotic organic solvents [12,13]. Common stages in formulation of Phytosomes are depicted in Figures 2 and 3. Various methods of preparation are as follows:
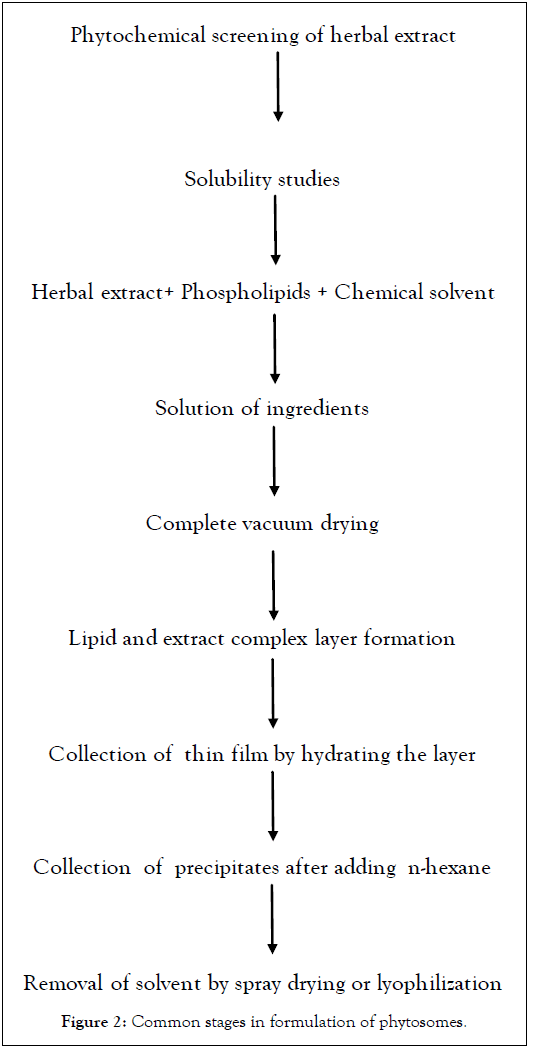
Figure 2. Common stages in formulation of phytosomes.
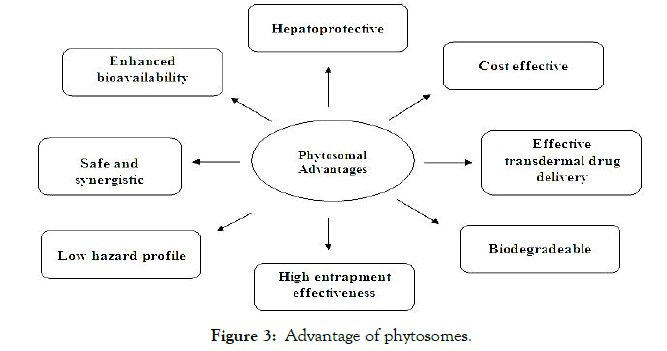
Figure 3. Advantage of phytosomes.
Anti-solvent precipitation process: Specific amount of herbal extract and phospholipids is refluxed with 20 ml of organic solvents such as acetone at specific experimental conditions below 50°C for 2-3 h. The reaction mixture is concentrated to minimum volume up to 10 ml and then on addition of solvent with low polarity such as n-hexane with stirring, precipitates are obtained. Filtered precipitates are stored in desiccators. The dried precipitates are pulverized and powdered complex are stored in dark amber colored glass bottle at room temperature [14].
Rotary evaporation process: Specific weight of herbal extract and phospholipids were mixed in 30 ml water miscible organic solvent such as acetone in round bottom glass container followed by stirring for 2 hours at a temperature less than 50°C in rota evaporator. Antisolvent such as n-hexane can be added to thin film which is obtained after uninterrupted stirring using a stirrer [15]. Precipitate of phytosomes so obtained can be stored in amber colored glass container at controlled temperature under specified humidity.
Solvent ether-injection process: This technique involves reaction of lipids dissolved in organic solvent with herbal extracts in aqueous phase. Phospholipids solubilised in diethyl ether are slowly injected drop wise in an aqueous solution of the phytoconstituents which is to be encapsulated. It results in the formation of cellular vesicles on subsequent solvent removal, leading to complex formation [16]. Structure of phytosomes depends upon concentration, amphiphiles in mono state are produced when the concentration is less, but variety of structures with different shapes viz. round, cylindrical, disc and cubic or hexagonal vesicles may be formed on increasing the concentration.
Novel methods: Novel methods for the phospholipid complexation include supercritical fluids which include gas solvent technique, compressed solvent process and supercritical solvent method [15].
Superiority of phytosomes over herbal extracts
Phytosomal technique was proposed as a drug carrier since 1989. Numerous phytosomal advantages as presented in fig 2, explains the increased validity and relevance of herbal extracts in bioevaluation and in-vitro studies [17,18]. They possess better metabolic profile than old conventional herbal extracts [19]. Various advantages of phytosomal technology are:-
Enhanced bioavailability: Phytophospholipid complex allows penetration of hydrophilic herbal extract from intestinal lumen for better absorption. There is remarkable improvement in the bioavailability of secondary metabolites on complexation with lipophilic head of phospholipids [20].
Safe and synergistic: Additives used in the phytosome formulation are approved ensuring it as safe and secure concept as phosphotidylcholine used in complexation is essential part of cell membrane. Synergistic effect has been observed on complexation with hepatoprotective drugs as phosphotidylcholine itself possess hepatoprotective action. Synergistic advantages are vividly seen in protecting the skin against exogenous or endogenous toxin in stressful environmental conditions. Phytosome concept assures increased duration of action at low dose with low risk profile due to upgraded absorption of the active constituent [21].
Low hazard profile: Toxicological outcome are negligible as seen in reported data moreover there is only small scale production.
Cost effectiveness: This technology provides economical delivery of phytoconstituents. Cellular vesicular system is submissive and is accessible for further instant development. It is comparatively easy to produce as no complicated technical investment is required and no complex practical speculation is essential for the manufacture of phytosomes [22].
Transdermal drug delivery: Herbal phytosomes can be also utilized to improve the diffusion of drug through the skin in transdermal drug delivery as they act as foretop for the delivery of huge assorted group of drugs such as peptides and protein [23].
Biodegradeable: Phosphatidylcholine utilized in phytosome formulation act as a carrier transporter and are integral portion of cell membrane so there is no obstacle with drug frame-up during formulation manufacturing [24].
High entrapment effectiveness: Drug entrapment effectiveness is very high furthermore no toxic metabolites are produced. Moreover the biomarker itself forms nano cellular vesicles on bonding with soya lipids and drug release can be predetermined [25].
Optimization and characterization techniques
Optimization and characterization of phytosomes can be carried out by estimating drug release, membrane permeability, vesicle shape and size distribution, percentage drug capture, entrapped concentration, chemical composition, quantity of material [18]. Stability of phospholipids complex depends upon various factors like drug to phospholipids ratio, experimental duration of time, temperature, solvent evaporation and type of drying method employed [26]. Physical parameters can be optimized statistically or by multiple evaluation techniques which can authenticate and validate its characteristics properties.
Visualization: Morphological studies are mostly used for observation of the particle size, entrapment behavior, surface attributes, and identifying the probable proportion of impurities on the particle surfaces [27]. High resolution images of phospholipid complexes can be resolved and visualized by scanning electron microscopy techniques. In SEM, posterior scattered electrons describe the atomic state and secondary electrons provide surface topography. However internal structure, crystallographic features, elemental composition of the phytosome complex can be evaluated through X-rays and TEM analysis [1].
Entrapment efficiency: The entrapment efficiency of herbal extract can be estimated by performing centrifugation. Centrifugation of solution containing weighed amount of phytophospholipid complex equivalent to quantity of encapsulated herbal extract in phosphate buffer of pH 6.8 can be carried out for 30 minutes at 5000 rpm. Stirred contents are allowed to remain undisturbed for one to two hours and finally absorbance of supernatant liquid collected by decantation is estimated by UV or HPLC [28]. The drug entrapment percentage (%) is calculated as:
Drug entrapment (%) = Actual amount determined/Theoretical amount present.
Crystallinity and polymorphism: X-ray diffraction studies are majorly accepted for characterization of crystallinity and polymorphism in phospholipid complex. DSC report explain the endothermic peaks, determination of transition temperature of the vesicular lipid complex, new peaks, peak shape, peak temperature, melting points and relative peak area [29]. The complete absence or reduction in the intensity of large diffraction peaks corresponding to its crystalline drug in phospholipid complex and polymorphism is characterized by XRD.
Vesicle stability: Particle size, polydisparity index (PDI) and zeta potential describes the vesicle stability. PDI determines width of a particle size distribution while zeta potential quantify the surface potential of cellular vesicles. Size of complex may vary from 50 nm to a few hundred μm but phytosomes with PDI value <0.5 are stable and indicating that the sample does have narrow size distribution and small particles in complex do not sediment in contrast to aggregates particles that may slowly settle down and sediment whereas samples with zeta potential > ± 30 mV are evaluated as stable complex [2,8].
Spectroscopic evaluation
The spectroscopic analysis of the designed phytosome can be validated by comparing spectrum of complex formed with soya lipid with the herbal extract. Spectroscopic analysis confirms the formation of lipid compatible complex [30,31]. Phytophospholipid complexation and molecular bonding interactions can be analyzed by employing different spectroscopic techniques like 1HNMR, 13CNMR, 31PNMR, and IR spectroscopy as follows:
1HNMR: Complex formation between the active phytoconstituents and phospholipid can be confirmed by NMR spectra. Marked alteration in signals emerging in 1HNMR evolving from atoms involved in the formation of complex describes the chemical bonding. The broad signals belonging to phytoconstituents and phospholipids and chemical shift corresponding to the N-methyl of choline confirm the formation of phytosomes [1,32].
13CNMR: The shift in signals corresponding to the fatty acids chains can be interpreted in 13CNMR of the phytoconstituents and the stoichiometric complex of phosphatidylcholine and herbal extract [1,5].
FTIR: Comparative spectrum study of phytosomes complex in solid form after lyophilization with that of micro dispersion in water at different times as seen in FTIR [6].
Retention Time: Retention factor in thin layer chromatography (TLC) is a simple method for characterization of phytosome. Retention time or retention factor varies for the phytoconstituents and phospholipids. The difference in retention time or retention factor indicates the generation of a new complex termed as phytosome [33-40].
Design of phytosomal formulation
Design of phytosomal formulation depends upon mode of delivery of the product either by topical or oral route [41-45]. With the advancement in pharmaceutical sciences, phytosomes finds applications in formulating various dosage forms as pharmaceuticals, nutraceuticals and cosmeceuticals. Several pharmaceutical companies involved in production of phytosome products are Indena, Jamieson natural resources, Thorne research, Natural factors and Nature herb [46-50]. Significant aspects as considered are:
Screening of herbal extractive: Phytosomes involve chemical interaction between hydrophilic herbals and lipophillic phospholipids moieties so aqueous extractive can be utilized for optimum bonding. Herbal extracts can be phytochemically screened for terpenoids, tannins, flavonoids as they show optimum bonding with phospholipids. Moreover drug release from phytosomal complex can be defined by optimizing various inherent properties of phytoconstituents. Fundamental inherent characteristics such as hydrophilicity, lipophillicity, cellular permeability, biodegradability, release characteristics and size of phytosome complex should be considered while selecting the phytoconstituents [51-55].
Selection of additives: Additives used in phytosomal formulation can be selected on the basis of dosage form and its mode of drug delivery. Phospholipids, solvent, dyes, buffering agents used in formulation of phytosomes are mentioned in Table 1.
| Chemical | Examples | Uses | References |
|---|---|---|---|
| Phospholipid | Soyaphosphatidylcholine, egg phosphatidyl choline, dipalmitoylphosphatidylcholine, distearylphosphatidylcholine |
Cellular vesicles generating component |
[39,40] |
| Solvent | Dioxane, acetone, methylene chloride | Aprotic solvent | [41] |
| Non- solvent | Aliphatic hydrocarbons or n-hexane | Complex precipitation | [41] |
| Alcohols | Ethanol, methanol | As a solvent | [42,43, 44] |
| Color and Dyes | Rhodamine 6G, DHPE-rhodamine, fluorescein, 6 carboxy fluorescence |
Cono focal scanning laser microscopy study | [45,46] |
| Buffering agent | Saline phosphate buffer (pH 6.5) 7 % v/v ethanol tris buffer (pH 6.5) | Hydrating medium | [44,43,47] |
Table 1: Recipients in phytosomes development.
Selection of dosage form: Phytosome complex can be formulated into oral and topical dosage form however relevant dosage form required for drug release can be selected on the basis on effectiveness and efficiency of biomarker compounds. Solubility is an important criterion to determine the stability of complex and selection of solvent can be done according to phytoconstituents whether hydrophilic or lipophilic [56-60]. Suspension formulated by dispersing phytosome in biocompatible edible or semi-synthetic oily vehicles [61-67]. Phytosomal capsule of herbal extract and lipid complex can be formulated manually or by auto filling method without compressing the complex. Phytosome complex can be chemically incorporated into prepared emulsion or ointment base. Different topical phytosomal formulations such as solution, emulsion, lotion are proposed after optimizing the solubility of ingredients and other pharmacokinetic parameters [68-73].
Phytosomes and liposomes both involve chemical interaction of active compounds and phospholipids but there is difference in chemical bonding, bioavailability and molecular arrangement. Phytosome are vesicular system in which each phytoconstituent molecule is surrounded and bounded by phospholipid molecule through chemical hydrogen bonds assuring enhanced bioavailability and permeability through cellular membranes, whereas liposomes are aggregate of many phospholipid molecules that encloses each chemical entity without specifically bonding to them possessing lesser bioavailability as compared to phytosome. In liposome, hundreds and thousands of phosphatidylcholine molecules surround the water soluble molecule but in phytosomes phospholipid and phytoconstituent interact in 1:1 or 2:1 ratio depending on chemical entity. Numerous patents of phytosomes have been granted in past 10 years mentioned in Table 2 explaining the future of phytopharmaceuticals. Phytosomes as novel herbal vesicular drug delivery systems assure to deliver the drug through the pathway channelizing the active phytoentity to the desired site of action. Cellular shaped nano herbal particles assure the mode of delivering the therapeutic agent to the tissues of need improving the therapeutic efficacy and reducing the other allied effects. Phytophospholipid complexation technique has evolved as an important revolution for herbal medicines which were not able to demonstrate a remarkable effect at in vivo and in vitro level. Commercially available registered phytosomal products by various manufactures as summarized in Table 3 claims safe and synergistic therapeutic benefits in various pathological conditions. Hence phytosome technology has emerged as safe and promising future of phytomedicne.
| Patent no. | Patent detail | References |
|---|---|---|
| EP/1844785 | Phytosomes of olive fruits | [51] |
| EP1813280 | Phytosomes containing Ginkgo biloba derivatives | [52,53] |
| EP1640041 | Phytosome containing cosmetic and dermatological preparation | [54] |
| US 7691422 | Oral phytosome for the treatment of cellulite | [55] |
| US/2007/001 5698 | Thymosin ß4 phytosome for skin and wound repair | [21] |
| WO/2004/045 541 | Soluble isoflavone phytosomal compositions | [56] |
| EP1214084 | Antioxidant phytosome formulation | [38] |
| EP2228062 A1 | Phospholipid-curcumin complex and piperine formulation | [57,58] |
| EPO283713 | Saponins with phospholipid phytosome | [59,60] |
Table 2: Patents of phytosomal dosage form.
| Biological Source | Synonym | Phytoconstituents Complexes |
Commercial product | Therapeutic indications | References |
|---|---|---|---|---|---|
| Aesculus hippocastanum | Horse Chestnut | Saponins | Escin ß sitosterol | Anti-oedema and vasoactive properties | [61] |
| PhytosomeTM | |||||
| Ammi visnaga | Khella | Visnadine | VisnadexTM | Improve microcirculation | [61] |
| Centella asiatica | Brahmi | Asiatic acid, | Centella triterpenoid | Skin disorders, antiulcer, wound healing, anti-hair loss agent | [61] |
| madecassic acid | PhytosomeTM | ||||
| Citrus aurantium | Bitter orange | Naringenin | Naringenin PhytosomeTM | Antioxidant | [61,30] |
| Crategus oxyacanthoides | Hawthron | Hyperin, quercitin | Hawthron PhytosomeTM | Nutraceutical, cardioprotective | [61] |
| and antihypertensive | |||||
| Cucurbita pepo | Pumpkin | Tocopherols, steroids, carotenoids | Cucurbita PhytosomeTM | Anti-inflammatory, Benign | |
| prostatic hyperplasia | |||||
| Fraxinus ornus | Flowering ash | Esculoside (Esculin) | Esculoside PhytosomeTM | Vasoactive, anticellulite | [61,62] |
| Gingko biloba | Maiden hair Tree |
Gingko flavonoids, Gingoic acids, ginkgo flavon glucosides, ginkgolides, bilobalide |
Gingkoselect PhytosomeTM | Cognition enhancer | [22, 61] |
| Gingko bilobaterpene | Raynaud’s disease, | ||||
| PhytosomeTM Gingko | Antiageing, anti-asthmic | ||||
| Bilobadimeric flavonoids PhytosomeTM | Antiamnestic, antidepressant, | ||||
| Cardioprotective, dermatitis, | |||||
| Anti-Inflammatory | |||||
| Glycine max | Soya | Genistein and daidzein | Soyselect PhytosomeTM | Antiangiogenic, anticancer, cardioprotective, immunostimulatory and hypocholesterolemic |
[61] |
| Glycyrrhiza glabra | Mulethi | Glycyrrhetinic acid | Glycyrrhetinic acid | Anti-inflammatory, | |
| PhytosomeTM | used in dermatitis | ||||
| Melilotus officinalis | Sweet clover | Melilotoside, flavanoids and terpenoids | LymphaselectTM | Anti-inflammatory, in oedema, thrombophlebitis | [22] |
| Olea europaea | Olive tree | Verbascoside, tyrosol, hydroxytyrosol | Oleaselect PhytosomeTM | Antioxidant, antihyperlipidimic, anticancer and anti-inflammatory. | [22] |
| Panax ginseng | Ginseng | Ginsenosides | Ginseng PhytosomeTM | Nutraceutical, immunomodulatory | [61] |
| Panicum miliaceum | Millet | Mineral salts, vitamins unsaturated fatty acids, aminoacids | Millet PhytosomeTM | Antistress, beauty food for skin, nails and hairs | [61] |
| Curcuma longa | Turmeric | Curcumin | Curcumin PhytosomeTM | Anti-inflammatory, osteoarthritis, anticancer | [63,64] |
| Curcuvet®(Meriva®) | |||||
| Camellia sinensis | Tea | Epigallocatechin, epicatechin-3-O-gallate, epigallo catechin-3-O- gallate, catechin | Green tea PhytosomeTM | Nutraceutical, anticancer, Antioxidant, atherosclerosis, hepatoprotective, antidiabetic, anti-inflammatory |
[61,65] |
| Echniacea angustifolia | Cone flower | Echinacosides and | Echniacea PhytosomeTM | Nutraceutical, immunomodulatory | [66] |
| Inulin | |||||
| Pinus maritime | Pine | Procyanidins | Pycnogenol PhytosomeTM | Anti-inflammatory, antiwrinkle, | [67] |
| Antiallergic | |||||
| Radix puerariae | Kudzu root | Puerarin | Puerarin and phospholipid complex | Antiinflammatory, cardiovascular diseases | [68,69] |
| Ruscusa culeatus | Butchers broom | Ruscogenin, neoruscogenin, | Ruscogenin PhytosomeTM | Anti-inflammatory, anti-ageing, Sunscreen agent |
[61] |
| Santalum album | Sandal wood | Ximenynic acid, ethyl ximenynate |
Ximilene and Ximenoil PhytosomeTM | Improve microcirculation | [61] |
| Serenoa repens | Saw palmetto | Phytosterols | Phytosterols | Noncancerous prostate Enlargement |
[22] |
| Silybium maranium | Milk Thistle | Silybin, silycristin, isosilbin |
Silybin PhytosomeTM (Siliphos®) |
Hepatoprotective, hepatitis, cirrhosis and inflammation |
[61,63,70] |
| Swertia alternifolia | Swertia | Xanthones 26 | Swertia PhytosomeTM | Antidiabetic | [71] |
| Syzygium cumini | Jamun | Tannins | Madeglucyl PhytosomeTM | Antihyperglycemic, anti-inflammatory, antioxidant |
[61] |
| Terminalia serica | Silver cluster | Sericoside | Sericoside | Anti-aging, skin restructuring | [61,72] |
| Vaccinium angustifolium | Blue berry | Anthocyanosidestocotrienol complex, | VitaBlue PhytosomeTM | Anti-oxidant, improves vision, memory enhancer | [61] |
| Vaccinum myrtillus | Bilberry | Anthocyanosides | Mirtoselect PhytosomeTM | Antioxidants, antiinflammatory, vasoprotective | [22,73] |
| Vitis vinifera | Grapes | Resveratrol, catechin. quercitin, epicatechin, | Biovin and leucoselect | Cardioprotective, systemic | [22,61] |
| Masquiliers PhytosomeTM | antioxidant, nutraceutical | ||||
| Zanthoxylum bungeanum | Tumburu | Hydroxy-a-sanshool | Zanthalene PhytosomeTM | Soothing and Anti-reddening | [61] |
Table 3: Commercial registered phytosome products.
Phytophospholipid complex technique has evolved as advanced frontier aspect in defining systemic absorption of herbal extracts. This technique has effectively resolved the irrational queries of plant based drugs. Aimed with predetermined lipid penetration at higher concentration with sustained and constant therapeutic levels in plasma, allows more quantity of active biomarkers to reach at desired site of action. However, it needs more emphasis including complete characterization with optimization, quantitative and qualitative exploration the lipid based system and its impact in different pathological states. However these novel complexes can act as reliable candidates for improved drug dosage therapy. As seen phytomedicines have been healing the world long back time and presently have major acceptance. Initially the phytosome complexes as used in cosmetics, but they are now widely utilized in therapies such as antioxidants, cardioprotective, antiinflammatory, liver protective, antitumor and anti-cancer. With this emerging formulation tool phytosomes has re-explained the relevance of herbals in modern drug targeting approaches.
No conflict of interest by authors.
We are thankful to Chairman, Sh. Parveen Garg; Director, Prof. GD Gupta and Pharmacognosy Department, ISF College of Pharmacy for providing support for this study.
Citation: Kumar S, Baldi A, Sharma DK (2020) Phytosomes: A Modernistic Approach for Novel Herbal Drug Delivery - Enhancing Bioavailability and Revealing Endless Frontier of Phytopharmaceuticals. J Develop Drugs 9:2.
Received: 20-May-2020 Accepted: 29-May-2020 Published: 05-Jun-2020 , DOI: 10.35248/2329-6631.20.9.195
Copyright: © 2020 Kumar S, et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.