Journal of Oceanography and Marine Research
Open Access
ISSN: 2572-3103
ISSN: 2572-3103
Research Article - (2019)Volume 7, Issue 2
In recent decades, populations of many coral species have declined dramatically on reefs worldwide. A major factor in coral mortality has been photo-oxidative stress associated with both solar irradiance and elevated temperatures. While many studies have focused on species that have declined, fewer efforts have focused on the “survivor” species, those that have maintained relatively stable populations or even increased in abundance. The objective of this study was to assess temporal variability in photochemical efficiencies (Fv/Fm) as an indicator of potential photo-oxidative stress in the dinoflagellate symbionts in three species, the scleractinians Siderastrea siderea and Montastraea cavernosa, and the zoanthid Palythoa caribaeorum, whose populations have remained relatively stable along the Florida reef tract.
Coral colonies with no visual indication of disease or bleaching were assessed quarterly in 2012 and 2013, at sites at 6 or 18 m depths. Colonies were dark-acclimated prior to measurements using pulse-amplitude modulated fluorometry. The mean Fv/Fm values for P. caribaeorum colonies assessed at 6 m depth were consistently the lowest (0.59, 0.02 SE). Siderastrea siderea assessed at both 6 and 18 m revealed significantly lower Fv/Fm values (p=0.0006) for those living at 6 m (0.64, 0.02 SE) than for those living at 18 m (0.68, 0.01 SE) depths. The Fv/Fm values for colonies of M. cavernosa assessed at 18 m also averaged 0.68 (0.01 SE). Thus, photochemical efficiencies were lower in colonies living at 6 m than in colonies living at 18 m, while no significant seasonal differences were found in the mean Fv/Fm values in the examined species. This study provides the first known report of photochemical efficiency in P. caribaeorum.
Photo-oxidative stress; Dinoflagellate symbionts; Photosynthetic organisms
The loss in total coral cover and potential for reef accretion in recent decades has been observed nearly worldwide, and especially throughout the western Atlantic and Caribbean. With losses in coral cover after the 1997–98 El Niño bleaching event and the 2010 cold-water mortality event that devastated Orbicella populations, Montastraea cavernosa Linnaeus 1766 and Siderastrea siderea Ellis and Solander 1786 are now among the greatest contributors to total stony coral cover on the Florida reef tract [1]. Although reefs in the Florida Keys seem to be in a transition from hard-coral to octocoral domination, coral-population studies on this region show that benthic cover by M. cavernosa, S. siderea, and the common zoanthid, Palythoa caribaeorum Duchassaing and Michelotti 1860, has remained relatively stable [1,2]. With populations of the major reef-building species in rapid decline, research has tended to focus on the formerly dominant species of Acropora and Orbicella [3,4]. In this study, we focus instead upon three “survivor” taxa, M. cavernosa, S. siderea and P. caribaeorum, by assessing photochemical efficiencies in situ at two depths along the Florida reef tract.
Photosynthesis by symbiotic dinoflagellates (commonly known as zooxanthellae) provides corals with energy needed for metabolism, growth and reef building [5,6]. The taxonomic status of the zooxanthellae has recently been revised, with the establishment of the Family Symbiodiniaceae and assignment of generic names to clades [7]. Cunning et al. [8] found clades C (genus Cladocopium) and D1a (genus Durusdinium) in both M. cavernosa and S. siderea, while Kemp et al. [9] reported subclades C1 and D1a in P. caribaeorum. This zoanthid is abundant in shallow forereefs around the Caribbean, as well as those of both the North and South Atlantic [9-11]. Colonies of P. caribaeorum also are susceptible to bleaching under thermal and irradiance stresses [12].
The photosynthetic capacity of the symbionts can be affected by an array of environmental stressors, with solar irradiance and thermal stress being the most studied, as these can induce bleaching [13,14]. Pulse-amplitude modulated (PAM) fluorometery has become a widely used tool to study stress in photosynthetic organisms by using chlorophyll a fluorescence as proxy to assess the photochemical efficiency of photosystem II (PSII) of the symbiont [15]. Thermal and photic damage to PSII has been linked to symbiont loss in corals [16,17] and such damage can create a cascade of events resulting in an overall decrease in productivity of the symbiont and thus in less photosynthate available for the coral host [12].
Results from PAM fluorometry can provide insight into the photosynthetic capacity of PSII by measuring the ratio of variable fluorescence to maximum fluorescence (Fv/Fm = maximum quantum yield of PSII). For example, declines in Fv/Fm have been reported in corals with indications of bleaching, during diel fluctuations that correlate with the diurnal cycle of solar irradiance, and with seasonal changes in temperature and light [18-21].
The purpose of this study was to assess temporal variability in photochemical efficiencies of the symbionts in three “survivor” taxa. We examined how in situ maximum quantum yields of photosystem II of the anthozoans, M. cavernosa, S. siderea and P. caribaeorum, varied through the year and, for S. siderea, between 6 and 18 m depths. The working hypothesis was that photochemical efficiencies would change over the course of a year as irradiance and water temperature changed with seasons. Our results provide insight into why populations of these anthozoans in the Florida Keys have remained relatively stable over the past few decades.
Water temperature and solar irradiance data spanning the period of fieldwork were acquired from satellite-derived sources. Monthly means of sea-surface temperature data for 2012–13 were obtained from NOAA’s Advanced Very High Resolution Radiometer (AVHRR) at 1 km2 resolution. Data for ultraviolet (UVA, 380 nm) and visible (blue light, 480 nm) penetrating to 6 and 18 m depth were calculated using protocols established by Barnes et al. for Florida reef tract waters [22-24].
In situ fluorescence measurements were undertaken quarterly, as close as logistics permitted to the solstices and equinoxes, between June 2012 and October 2013, at Tennessee Reef on the Florida reef tract (Figure 1). Two sites were selected along this reef: a 6 m site (24.7453˚, –80.7818˚) and an 18 m site (24.7523˚, –80.7549˚). Colonies of Sidestrea siderea and Palythoa caribaeorum were assessed at the 6 m site, while S. siderea and Monstastraea cavernosa were assessed at the 18 m site.
Figure 1: Locations of the Tennessee Reef field sites on the Florida reef tract, USA; inset shows the general location relative to the Gulf of Mexico.
Each sampling event consisted of selecting three colonies of each species at each site. The colonies assessed during each sampling event were not always the same. Specimens selected for study exhibited healthy coloration and no visible signs of disease or bleaching. The colonies were dark-acclimated for at least 20 minutes by covering them under large, black plastic bags; those at 18 m were covered during an initial SCUBA dive and assessed during a subsequent dive, following an appropriate surface interval. The fluorescence measurements where taken while keeping the colonies shaded, therefore measurements (three per colony) were taken “blindly” across each colony to avoid intrusion of outside sunlight. A Walz DIVING PAM fluorometer was used to measure the parameters F0 (fluorescence after dark-acclimation) and Fm (fluorescence after a saturating flash of light). These measurements were then used to derive the mean maximum quantum yield of photosystem II [Fv/ Fm= (Fm–F0)/Fm] for each colony.
A non-parametric approach was used to test the null hypotheses of: a) no significant difference in Fv/Fm within species among sampling dates, and b) no significant difference in Fv/Fm between colonies of S. siderea at 6 m and 18 m. Permutation-based analysis of variance (NP-ANOVA) was carried out using the Fathom Toolbox for MATLAB [25,26].
Monthly means of sea-surface temperature and selected wavelengths of solar irradiance (380 and 488 nm) reaching the seafloor at Tennessee Reef for the years 2012 and 2013 [27] are shown in Figure 2. Average winter lows of ~25°C occurred in January each year, while summer highs averaging just under 30°C occurred each September (Figure 2A). Visible solar radiation reaching the seafloor at 6 m revealed a typical seasonal cycle, with winter lows (December-January) and summer highs in June (Figure 2B). Visible light reaching the seafloor at 18 m was more variable. Similarly, mean UVA radiation reaching the seafloor also varied, exhibiting not only winter lows but also July–August lows in 2012 and May– June lows in 2013 (Figure 2C).
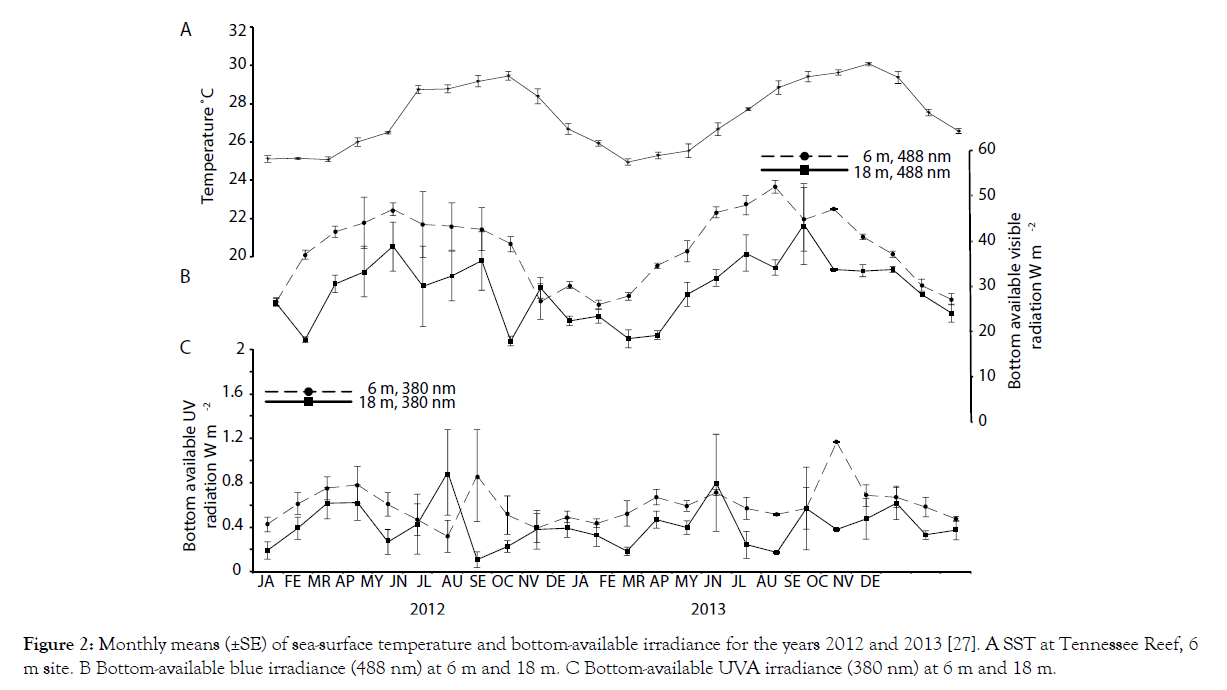
Figure 2: Monthly means (±SE) of sea-surface temperature and bottom-available irradiance for the years 2012 and 2013 [27]. A SST at Tennessee Reef, 6 m site. B Bottom-available blue irradiance (488 nm) at 6 m and 18 m. C Bottom-available UVA irradiance (380 nm) at 6 m and 18 m.
In situ data for maximum quantum yields for all taxa revealed Fv/Fm values in the range of 0.37–0.78. Based on dispersion analysis, the variability in Fv/Fm among the species did not differ significantly (p=0.08). The mean Fv/Fm values for Palythoa caribaeorum (0.59, 0.02 SE) were consistently the lowest (Figure 3), with no significant differences discerned over time (Table 1). The Fv/Fm values for Siderastrea siderea at neither the 6 m site (0.64, 0.02 SE) nor the 18 m site (0.68; 0.01 SE) differed significantly across sampling dates (Figure 3). The mean Fv/Fm values for colonies of S. siderea at 6 m were significantly lower than for those living at 18 m depth (Table 2). In colonies of M. cavernosa, no significant differences in Fv/Fm were seen over the course of the study (0.68; 0.01 SE; Table 1). Due to logistical issues, data from M. cavernosa colonies were not collected in July 2013.
| Species | Palythoa caribaeorum | Siderastrea siderea | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Depth | 6 m | 6 m | ||||||||
| Factor | Mean Fv/Fm | SE | df | F | p | Mean Fv/Fm | SE | df | F | p |
| Overall | 0.59 | 0.02 | 0.64 | 0.02 | ||||||
| Month | 5 | 1.4 | 0.28 | 5 | 0.75 | 0.59 | ||||
| Residual | 12 | 14 | ||||||||
| Total | 17 | 19 | ||||||||
| Species | Montastraea cavernosa | Siderastrea siderea | ||||||||
| Depth | 18 m | 18 m | ||||||||
| Factor | Mean Fv/Fm | SE | df | F | p | Mean Fv/Fm | SE | df | F | p |
| Overall | 0.68 | 0.01 | 0.68 | 0.01 | ||||||
| Month | 4 | 2.41 | 0.09 | 5 | 1.95 | 0.17 | ||||
| Residual | 16 | 12 | ||||||||
| Total | 20 | 17 | ||||||||
Table 1: One-way NP-ANOVA on the maximum quantum yield between sampling months for the zoanthid Palythoa caribaeorum, Siderastrea siderea at 6 and 18 m depths, and Montastraea cavernosa at 18 m depth. In each comparison, 5,000 permutations were run; a = 0.05.
| Factor | df | F | p |
|---|---|---|---|
| Depth | 1 | 13.31 | 0.0006 |
| Residual | 36 | ||
| Total | 37 |
Table 2: Two-way NP-ANOVA on the photochemical efficiency between 6 m and 18 m for colonies of Siderastrea siderea; 5,000 permutations; a = 0.05.
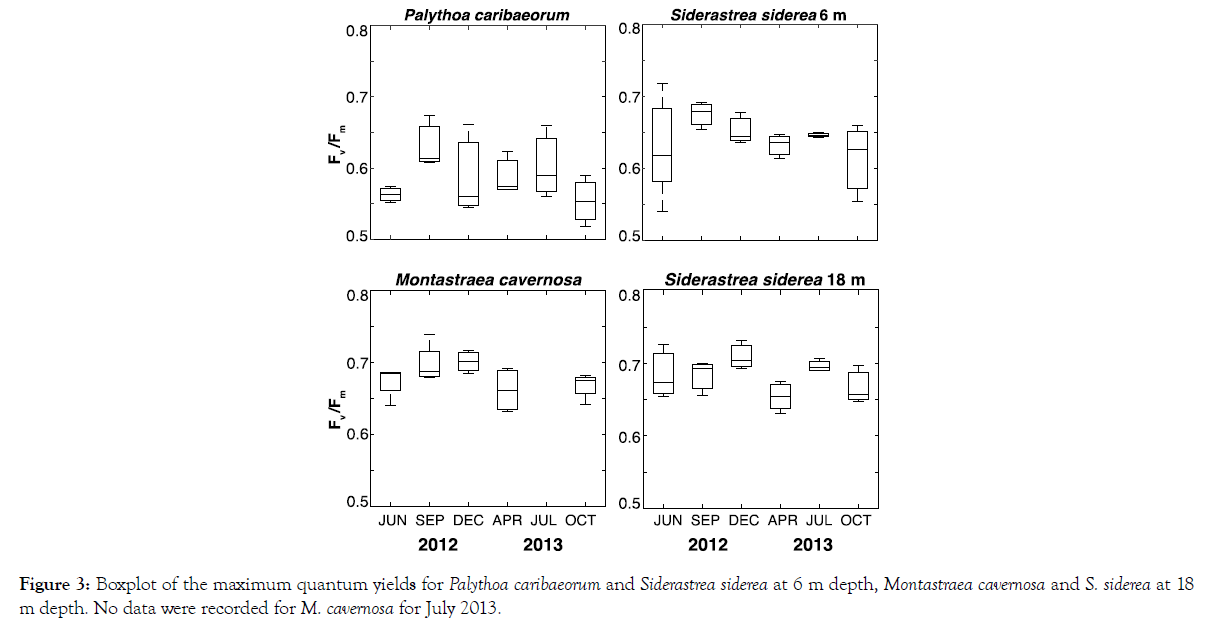
Figure 3: Boxplot of the maximum quantum yields for Palythoa caribaeorum and Siderastrea siderea at 6 m depth, Montastraea cavernosa and S. siderea at 18 m depth. No data were recorded for M. cavernosa for July 2013.
Considering the relatively strong seasonality in both temperature and solar irradiance experienced by corals living along the Florida reef tract, we anticipated finding seasonal changes for in situ maximum quantum yields in the taxa assessed. Warner et al, examining colonies of three species of Orbicella from the Bahamas, found the greatest fluctuations in Fv/Fm from corals in very shallow waters (1–2 m), whereas those from somewhat deeper waters (3–4 m and 14 m) produced consistently higher and less variable Fv/ Fm values [20]. These fluctuations in Fv/Fm were strongly correlated to seasonal patterns of water temperature and irradiance, with lower Fv/Fm consistently recorded during the summer months [20]. Significant reduction in densities of zooxanthellae in P. caribaeorum from Brazil was recorded during summer months [27,28]. In addition, photosynthesis and respiration studies on Orbicella (né Montastraea) faveolata colonies from the Florida Keys have also shown lower gross photosynthesis during the summer months [29].
The expected seasonal patterns of lower Fv/Fm during warmer months and higher during colder months were not observed in our study, consistent with those from sites >2 m [20], as noted above. Moreover, although we worked at the same sites each time, the colonies assessed where not always the same. Site depth, inherent variability among colonies, small sample sizes, and relatively mild winter and summer temperatures during our study could be reasons why seasonal trends were not readily evident.
On the other hand, given that fluctuations in Fv/Fm were quite minimal over time in all three species, these responses indicate that these species are well adapted to seasonal changes in temperature and irradiance, at least to those experienced during 2012–13. Moreover, the differences in Fv/Fm values between the 6 m (0.64 for S. siderea) and 18 m (0.68 for S. siderea and M. cavernosa) data are notably similar to with those reported by Lesser et al [30], for M. cavernosa from similar depths in the Bahamas.
The moderate temperature ranges recorded in 2012-13 likely played a role in the minimal fluctuations in Fv/Fm over the duration of our study. These temperature ranges are normal for the Keys reef tract and summer temperatures did not reach levels known to induce bleaching. The normal visible light cycle in which the highest light levels at 6 m depth were recorded in June, well before the September temperature peak, limited the interaction of high light compounding stress induced by elevated temperatures. Solar radiation reaching the seafloor at 18 m can be much more dependent upon water transparency, which can be influenced by plankton blooms, outflow of turbid water from Florida Bay, or frequency of storms [31].
This paper reports the first known assessment of photosynthetic efficiencies of the zoanthid Palythoa caribaeorum. This common species is widely studied for its production of palytoxin and other potential biochemicals of medical interest, but much less is known regarding its responses to photic stress [32]. Kemp et al. reported results of experiments on thermal tolerance, noting that this species in south Florida harbored two genetic types of zooxanthellae (C1 and D1a), though thermal tolerance did not seem to be influenced by the clade of zooxanthellae present in the host [9].
In situ analyses of photochemical efficiencies across seasons cannot provide a full picture of the photosynthetic performance of a symbiotic organism. Nevertheless, such analyses can contribute to understanding how anthozoan “survivor” species and their symbionts cope with environmental extremes. Future studies of symbiont heterogeneity, including during recovery from bleaching, predation or disease, in combination with in situ assessments of photochemical efficiencies, are needed to enhance understanding of how benthic zooxanthellate anthozoans respond to and recover from photo-oxidative stress.
Field work in Tennessee Reef, Florida reef tract, was carried out under the Florida Keys National Marine Sanctuary permit number FKNMS-2011-01. Fieldwork was supported in part by the Cushman Foundation for Foraminiferal Research and SRI International. The Walz® PAM fluorometer was purchased under the BP/Gulf of Mexico Research Initiative through the Florida Institute of Oceanography. We also thank the staff of the Keys Marine Laboratory for logistical support, and volunteer divers from the College of Marine Science at the University of South Florida who participated in the study: Andrea Schmidt, Benjamin Ross, Brian Barnes, Joshua Kilborn, María Vega-Rodríguez, and Tiffany Boisvert. Special thanks to the NSF FG-LSAMP Bridge to the Doctorate award (HRD #0929435), the Alfred P. Sloan Minority PhD program, the USF Mayor’s Advisory Council Fellowship, and the Johanna M. Resig Fellowship from the Cushman Foundation, for financial support of N. Méndez-Ferrer.
Citation: Mendez-Ferrer N, Hallock P (2019) Photochemical Efficiencies in Reef-Dwelling Anthozoans: Insights from “Survivor†Species. J Oceanogr Mar Res 7:193. doi: 10.35248/2572-3103.19.7.193
Received: 17-Apr-2019 Accepted: 28-May-2019 Published: 04-Jun-2019 , DOI: 10.35248/2572-3103.19.7.193
Copyright: © 2019 Mendez-Ferrer N, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.