
Biochemistry & Pharmacology: Open Access
Open Access
ISSN: 2167-0501

ISSN: 2167-0501
Research Article - (2015) Volume 4, Issue 5
Resvertrol (Resv) is an extensively studied molecule – as of 2015 PubMed held more than 7100 publications on the subject. The First International Resveratrol Conference in 2010 found insufficient evidence to justify recommending chronic administration of Resv in humans, a finding in stark contrast with the claims of its therapeutic effects often made by the media, based on its supposed role in the beneficial properties of red wine and in the so-called French Paradox. However, pharmacological studies carried out on different formulations of Resv from 2010 onwards suggest that these recommendations should be reviewed. Pharmacokinetic Resv is characterized by high inter-individual variability within pharmacokinetic parameters. Resv exhibits a rapid absorption rate, with extensive pre-systemic metabolism by human cytochrome P 450 and intestinal microbiota. Its metabolism leads mainly to conjugation products, the biological activity of which is still under discussion. It is also rapidly cleared by the kidneys. Finally, the estimated bioavailability of Resv is around 1% of orally-administered doses. Clinical trials have shown that Resv seems to exert a therapeutic effect on endothelial dysfunction consistent with in vitro observations demonstrating that Resv stimulates the eNOS enzyme. Inflammatory markers and CRP reductions obtained from doses of Resv equal to or less than 20 mg/day are not observed in larger doses, which imply hermetic behavior. Resv has also been shown to reduce the atherogenic potential of LDL cholesterol by reducing oxidized LDL and ApoB levels, which would in turn reduce atherogenesis. Resv is a well-tolerated compound; short-term clinical trials have shown frequent gastrointestinal discomfort or spontaneously resolving diarrhea only with the administration of high doses.
<Keywords: Resveratrol; Pharmacology; Cardiovascular
Resveratrol (Resv) or 3,5,4’-stilbenotriol (Figure 1), is a secondary metabolite present in around 70 plant species, which was isolated for the first time in 1940 from white hellebore (Veratrum grandiflorum) root extract [1]. It is a phytoalexin, that is, a compound synthesized by plants in response to stress and infections. Structurally, it is a nonflavonoid polyphenol from the stilbene family present in a number of regularly consumed plant species such as berries, peanuts, and the epidermis of grapes, although its highest concentration is in Polygonum cuspidatum roots, a plant mentioned in traditional Chinese and Japanese pharmacopoeias [2], and currently used for commercial extraction. Even when biosynthesized in both its cis and trans forms, a wide consensus considers the trans form as more biologically active, besides being the most stable isomer [1,3].
Scientific interest in Resv grew significantly fifty years later, when work was published in The Lancet [4] on the low incidence of cardiovascular disease in the French population despite its high intake of saturated fats, possibly due to moderate and habitual consumption of red wine, which contains Resv among other polyphenols, a contradiction that soon became known as the so-called French Paradox. Later, it was shown that Resv had a preventive effect on the initiation, promotion and progression phases of in vitro and in vivo models of carcinogenesis [5]. While the French Paradox suggested the effectiveness of Resv at dietary concentrations by consuming food containing Resv, it was soon realized that the results in cancer research were in fact the reflection of an exponentially growing number of in vitro experiments that, at least in the first years, used concentrations far above those attainable in vivo [6,7]. Consequently, the initially high expectations arising from these experiments were soon tempered in the light of results from studies in animal models [8]. Different mechanisms have been proposed to explain the observed effects of Resv, especially in therapeutic areas as dissimilar as cancer [9], obesity, metabolic syndrome and diabetes [10], cardiovascular health [11] and neurodegenerative diseases [4,12-14].
This article reviews aspects regarding the pharmacokinetics and safety of resveratrol in animal and human models, and discusses evidence that could support its possible use as a therapeutic agent in cardiovascular health.
A range of assays have been carried out to determine whether Resv can reach the proposed sites of action after oral administration in humans, as the different pharmacokinetic stages may necessitate in vivo Resv concentrations that greatly differ from those used in vitro.
Absorption
Six healthy volunteers were administered 14C-resveratrol in 25 g oral doses [15]. Absorption was estimated to be equivalent to 70% of the dose, however, in plasma; Resv was only detected in quantities slightly above the detection limit (5 ng/ml). The majority of the dose was detected in urine, both for Resv and its three metabolites. In another study, 40 healthy volunteers were exposed to single oral doses of 0.5 g, 1.0 g, 2.5 g, or 5.0 g [16], and the time taken to establish the maximum plasma concentration (tmax) was estimated at between 0.83 and 1.5 h. This estimate was corroborated by a study administering trans-resveratrol in doses of 25, 50, 100 and 150 mg to eight healthy volunteers, in whom average tmax levels between 0.8 and 1.5 h were subsequently observed, depending on the dose received [17].
The passing mechanism for oral Resv would seem to be passive diffusion through the apical membrane of the enterocytes, which may be inferred from an in vitro study carried out on a monolayer of Caco- 2 cells [18], consistent with its low water solubility estimated at less than 0.05 mg/ml [19]. Nonetheless, enterocytes express ATP-Binding Cassette (ABC) type transporters, which have been demonstrated to actively secrete Resv in the opposite direction, from the cytosol to the lumen, thus limiting the concentration of Resv passing into portal circulation.
After administering a single dose of 5.0 g of Resv to 10 healthy volunteers, its maximum plasma concentration (Cmax) reached 539 ng/ ml ± 384 ng/ml [16]. Another pharmacokinetic study administered chemically synthetized Resv per os to 10 healthy volunteers, with a daily dose per group of 0.5, 1.0, 2.5 and 5.0 g, for 29 days [20]. Its accumulated plasma concentration was determined between days 21 and 28, and showed an average Cmax of 967 ng/ml. In order to increase Resv absorption, another study used micronized Resv (SRT501) and determined its pharmacokinetic parameters [21]. 5.0 g of SRT501 was administered daily for 14 days to six patients with rectal colon cancer and hepatic metastasis; the Cmax after the single administration of SRT501 was 1942 ng/ml ± 1422 ng/ml, a value two to four times higher than the value reported for non-micronized forms [16,20]. The average tmax was 2.8 h, far above that reported in other pharmacokinetic assays for Resv [16,17]. Considering the molecular weight of Resv (228.25), none of the pharmacokinetic studies in vivo in humans have shown plasma concentrations greater than 10 μM, far below the concentrations of up to 200 μM observed in in vitro studies.
It is widely accepted in pharmacologythat the absorption and bioavailability stages of drugs administered by mouth can be modified by the presence of food and in certain cases it is decided to administer the drug on an empty stomach. Along these lines, one study administered trans-Resv to healthy volunteers in red wine in three different dietary conditions: while fasting (1920 μg of trans-Resv in 600 ml of red wine, n=5), after a standard meal (246 μg of trans-Resv in 300 ml of red wine, n=10) and after consuming a meal rich in fats (480 μg de trans-Resv in 600 ml of red wine, n=10). According to the authors of the study [22], trans-Resv bioavailability was not related with dietary status or lipid content, subsequently confirmed by another study in humans [23]. However, it should be noted that trans-Resv metabolites 3’- and 4’-glucuronate were detected, and that the pure compound was present only in trace amounts below the detection limit. Therefore, caution should be exercised with regards to the beneficial effects of dietary consumption of Resv [23], as the benefits associated with red wine consumption and its role in the French Paradox, are probably due to the total antioxidant content in red wine. The quantity of trans-resveratrol in red wine fluctuates from undetectable to 14.3 mg/l, with an average value of 1.9 ± 1.7 mg/l (8.2 ± 7.5 μM); however, the quantity of the trans- and cis-resveratrol-glucoside conjugate (trans- and cis-piceid) can be up to three times greater than Resv and, although it is absorbed in lower amounts than Resv, the Resv isomer can be produced by metabolization [24].
Metabolism
After oral administration, Resv undergoes intense metabolism by the bacterial flora in the human intestine [25]. Twelve healthy volunteers were orally administered a single dose of 0.5 mg/kg body weight of trans-Resv (Vineatrol®, 7.7% trans-Resv and a range of stilbenes). In addition to the aforementioned dihydro-resveratrol metabolite [8], two new trans-Resv metabolites were reported due to bacterial metabolism, probably by the Slackia equolifaciens and Adlercreutzia equolifaciens strains of 3,4’-dihidroxi-trans-stilbeno and lunularin (3,4’dihidroxibibenzil). Bacterial metabolism was up to 62.7% of administered doses. On the other hand, the bacteria responsible for the dehydroxylation reactions have not been identified [25].
The most important metabolites arising from the human metabolism of Resv are created by phase II reactions. Mono- and di-glucuronide, mono- y di-sulfate and glucuronide sulfate metabolites have been described, of which Resv-3-O-sulfate is consistently identified as the most abundant [16,20,26]. The concentrations reached by these metabolites in plasma have been shown to be greater than those reached by pure trans-resv, exhibiting an AUC 20 [20] to 23 [16] times greater than those of the original molecule in the case of the majority metabolite. The intense metabolism of Resv explains why, despite its quick absorption, its bioavailability remains around 1% [8].
Based on in vitro experiments, it has been suggested that the metabolites are biologically active, as ubiquitous enzymes such as β-glucuronidase could convert these metabolites to the original Resv molecule either locally or systematically [3,27]. Sulfate metabolites [3] and dihydro-resveratrol [25] have also been shown to be active in vivo.
When plotting Cmax using escalated doses from 25 to 5,000 mg, near linear behavior is seen in Resv plasma concentration, which would prove non-saturation of the metabolism [8]. However, some studies show that sulfate-conjugated Resv metabolites can be found in higher amounts as long as the highest doses of Resv are administered [16,28]. It should be noted that these results could indicate possible saturation of glucuronosyltransferase enzymes at high Resv doses, contrasting with observations of the sulfatation route, as the latter present’s non-competitive substrate inhibition [29]. Such saturation in Resv metabolism implies a change in the route from glucuronidation to sulfatation [30], which would explain the high amount of sulfate metabolites in reports of studies using high doses of dietary Resv [16,28].
In a clinical study, the metabolic interaction of Resv with the activity of enzymes from the CYP-P450 family and phase II detoxification enzymes was quantified in 40 healthy volunteers subjected to 1.0/g doses of Resv for four weeks [26]. Resv was shown to inhibit the phenotypical index of CYP3A4, CYP2D6 and CYP2C9 and was able to induce the phenotypical index of CYP1A2. On the other hand, in phase II enzymes, GST and UGT1A1 activity was minimally affected, although in volunteers with low base levels of enzyme activity, induction of GST-π was observed. Drugs such as caffeine, dextrometorphan, losartan and buspirone, could potentially increase dose-dependent adverse reactions or alter the efficacy of Resv co-administration [26].
Table 1 shows main resveratrol metabolites, metabolic route/agent, in vivo metabolite activity, and molecular structure.
| Source | Metabolization | Metabolite | Activity | Molecular structure |
|---|---|---|---|---|
trans-resveratrol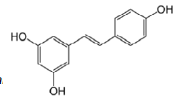 |
bacterial flora | dihydro-resveratrol | Active | 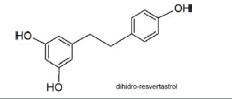 |
| 3,4’-dihydroxy-trans-stilbeno | Non active | 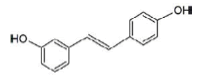 |
||
| 3,4’-dihydroxybibenzyl (lunularin) | Non active | 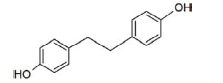 |
||
| phase II glucuronosyl-transferase route | resveratrol-3-O-glucuronide | active | 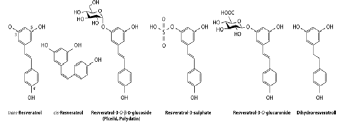 |
|
| phase II sulfation route | resveratrol-3-O-sulfate | active | 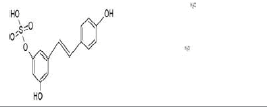 |
|
| resveratrol-3,4’-disulfate | active | 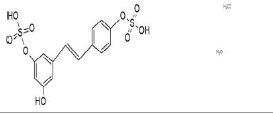 |
||
| phase II mixed route | resveratrol-3-sulfate-4’-glucuronide | active |  |
Table 1: Main resveratrol metabolites and activity (see text).
Distribution
In vitro studies have determined that Resv is reversibly bonded to bovine serum albumin at a bonding constant of 2.52 ± 0.50 x 104 M3-1, in addition to likely partial protein cleavage [31]. Resv metabolites, sulfates, disulfates and resveratrol-C/O-diglucoronide are noncovalently bonded to plasma proteins [28].
In dried colon tissues from 20 patients treated with Resv SRT501 in daily doses of 500 mg or 1,000 mg per 29 days, it was observed that non-conjugated Resv was distributed in greater concentrations to the colon tissue than to plasma [21]. In any case, this organ forms part of the administration route, which is also exposed to the entire non absorbed fraction. The Resv concentrations in tissue from dried liver with metastasis and healthy liver in 5 of 6 patients were 4.81 nmol/g and 1.84 nmol/g, respectively [21]. In a preclinical study administering 100 Resv mg/kg body weight to 15 rats, tissue concentrations between 5 to 10 times greater than those found in liver, heart, lung, spleen and kidney tissue were detected in the stomach and the small intestine [32].
The estimated apparent distribution volume of Resv ranges from 16.07 liters for a 500 mg dose [20] to 66.99 liters for a dose ten times greater [17].
Excretion
Little information is available on the excretion of Resv. It is estimated that the total clearance of Resv ranges between 2.5 to 3.0 l/h (41.7 ml/min to 50 ml/min) [16,20]. The elimination half-life (t½) has been calculated at 1.1 h after a dose of 100 mg Resv, while after 1.0 g/ day administration for 21 days, the t½ is 9.7 h [17]. Resv is eliminated relatively quickly and it has been reported that up to 77% of the dose is eliminated within the first four hours post-administration [33].
Resv plasma level curves consistently show a second peak after Cmax, which has been interpreted as proof of enterohepatic recirculation. Conjugated forms of Resv metabolites excreted via biliary routes may be metabolized by bacterial hydroxylase enzymes in the small intestine, facilitating their reabsorption [33], which has been demonstrated in animal models [34]. Fecal excretion is highly variable, between 0.3% and 23% of the administered dose, with an average of 12.7% ± 6.1% [15].
Chronopharmacology and variability
The pharmacokinetics of Resv show two interesting characteristics worth consideration, however, these require further study for corroboration. Firstly, the administration of Resv is circadiandependent, as the AUC in a plasma level graph is larger after morning than after afternoon oral administration [17]. Consequently, Resv bioavailability would be higher if administered in the morning. Secondly, as mentioned, the pharmacokinetic parameters have high interindividual variability, with variance coefficients of around 40%, in which the impact of patient gender or age is insignificant, as shown in comparisons of groups of men and women, young and older people [35].
Cardiovascular therapeutic potential
Resv has hormetic effects, that is, it is active at low doses and less responsive or non-responsive at high doses. This characteristic has been observed in preclinical studies on Resv and at least six types of human cell tumors in the breast, prostate, leukemia, colon, uterus, and lung [36]. In another in vitro assay of ischemia-reperfusion in Sprague-Dawley rats that were administered Resv in escalated doses of 2.5 and 5.0 mg/kg body weight for 14 days, after which the size of the infarction and the cardiomyocyte apoptosis were compared [37]. In comparison with the control group, both the size of the infarction and the cardiomyocyte apoptosis recorded were significantly less for the 2.5 and 5.0 mg/kg dose of body weight, while these variables increased in comparison with the control in groups subject to doses above or equal to 25 mg/kg body weight.
Consequently, the choice of a therapeutic dose of Resv must additionally include this non-linear behavior in dose-effect curves. This is particularly true for clinical trials in humans which, nonetheless, have not always been included.
Table 2 shows a summary of clinical trials with Resv in humans to determine its efficacy in cardiovascular problems. These seven clinical trials included a total of 426 patients administered Resv in different formulations including nutraceuticals from Vitis vinifera extracts (with other compounds such as polyphenols and vitamins) and red wine. If only those administered solely Resv are counted, this number drops to 175 patients.
| Number and health of subjects | Study characteristics | Form of administration | Dose, administration intervals and duration | Variables studied (outcomes) | Noteworthy results | Ref | |
|---|---|---|---|---|---|---|---|
| 40 subjects who have previously suffered heart attacks | Randomized, double blind, controlled by placebo | RESV Capsules | 10 mg of pure RESV daily for 3 months | Endothelial function, measured by the Flow Mediated Dilatation technique (FMD), diastolic function of the left ventricle | Increased FMD and diastolic function of the left ventricle | 26 | |
| 19 overweight or obese subjects and/or with high values of untreated AP (up to 160 mmHg SAP or 100 mmHg DAP) | Randomized, double-blind, controlled by placebo, cross-over | ResVidaTM capsules containing RESV | Three doses of 30, 90 or 270 mg RESV in an interval of one per week. Study of acute effects | Endothelial function, measured by FMD | Significant increase of FMD in all dose groups. A linear relationship is observed between this variable and the vulgar dose logarithm. | 54 | |
| 116 overweight subjects suffering from stable angina | Randomized, double blind, controlled by placebo, parallel arms | Capsules of pure RESV and RESV with Calcium Fructoborate (CFB) | 20 mg daily for 60 days | Plasma levels of PCR-as, NT-proBNP, LDL, HDL and triglycerides. Quality of life (1) | Significant reductions in PCR-as levels in all groups; greater in those consuming only CFB. The group receiving RESV obtained better results in NT-proBNP, total cholesterol, triglyceridemia reductions; and also 50% reductions in weekly angina episodes | 28 | |
| 67 diabetic subjects or subjects with 3 or more risk factors (2) | Randomized, blind, controlled (with ingestion of an alcoholic beverage without polyphenols), cross-over | Red wine (RW) and alcohol-free red wine (AFRW) with 5.26 + 0.83 mg/L and 5.01 + 0.86 mg/L total RESV (5) and 2.92 + 0.36 mg/L and 2.73 + 0.23 mg/L t-RESV, respectively | 272 mL daily (0.79 and 0.74 mg pure RESV for RW and AFRW respectively); for 28 days | Expression of soluble cellular adhesion molecules and in leukocytes; expression of pro-inflammatory citoquins | In groups consuming wine a reduction was observed in serum ICAM-1 and IL-6, concentrations and in the expression of T lymphocyte and monocyte adhesion in molecule membranes. | 13 | |
| 75 patients with diabetes or hypercholesterolemia, in treatment with statins, and one or more CV risk factors (4) | Randomized, triple blind, controlled with grape extract capsules without RESV and placebo, parallel arms | Stilvid® capsules containing 8 mg RESV and standardized grape extract | 8 mg daily for 6 months | Lipid markers: triglyceridemia, total cholesterolemia, plasma HDL, LDL, non-HDL cholesterol, apolipoprotein B (ApoB) and oxidated LDL (LDLox) concentrations | Significant reduction of plasma LDLox and ApoB concentrations only in the group consuming capsules with RESV. The ApoB values for this group reached the optimums proposed by the Canadian Cardiovascular Society (<90 mg/dL) | 45 | |
| 75 patients with diabetes or hypercholesterolemia, in treatment with statins, and one or more than three CV risk factors (4) | Randomized, triple blind, controlled with grape extract capsules without RESV and placebo, parallel arms | Stilvid® capsules containing 8 mg of RESV and standardized grape extract | 8 mg daily for 12 months | Inflammation markers: soluble ICAM-1, Interleukins 6, 10 and 18; TNF-a, HS-RCP y PAI-1 | Significant reduction HS-RCP, TNF-a and PAI-1 only in the group consuming capsules containing RESV. Reductions of "marginal significance" of soluble ICAM-1 and absence of changes in IL-6 | 44 | |
| 34 subjects diagnosed with Metabolic Syndrome (3) | Randomized, not blind, controlled, cross-over | Longevinex® Capsules containing pure RESV (100 mg), quercetin, vitamin D3 and rice bran phytic acid | 100 mg daily for three months | Anthropometric measurements and indexes, arterial pressure, fasting glycemia, fasting insulinemia, HbA1c, plasma levels of HDL, LDL, IL-6 and HS-RCP. Entothelial function measured by FMD | Significant rise of FMD; no significant changes in any of the other study variables | 20 | |
(1) Operationalized as the classification of angina according to the Canadian Cardiovascular Society and number of angina episodes per week
(2) Smokers, hypertension, dyslipidemic, overweight or obese patients, or with a family history of premature coronary disease
(3) Abdominal obesity and 2 or more of the following: triglyceridemia >150 mg/dL, [HDL] <40 mg/dL, SAP >130 mmHg, DAP >85 mmHg or fasting glycemia >110 mg/dL
(4) Smokers, hypertensive or obese patients
(5) Includes isomer and piceid forms
HS-RCP High Sensitivity Reactive C Protein, NTproBNP N-terminal prohormone brain natriuretic peptide, SAP Systolic Arterial Pressure, DAP Diastolic Arterial Pressure
Table 2: Clinical trials with resveratrol to determine cardiovascular health issues as an outcome. In the first three trials (above the dotted line) pure resveratrol was administered. In the last four trials (below the dotted line) resveratrol was administered as part of a nutraceutical formulation.
Clinical trials have focused on the efficacy of Resv in the primary prevention of cardiovascular events in sub-populations with different factors of CV risk, such as arterial hypertension, diabetes, dyslipidemia, active smoking, or a family history of early cardiovascular disease. Resv was administered concomitantly with each patient’s base medication. The most dose of Resv administered was 270 mg per day [38], in the form of supplements for hypertense obese patients. The lowest dose of Resv is 0.74 mg daily in 272 ml of red wine [39]. The longest study of the administration of Resv to humans in a nutraceutical formulation has been for 12 months, at a dose equivalent to 8 mg/day [40].
The effect of Resv on endothelial function has been determined by ultrasound as the percentage variance of flow measured dilatation (FMD) in the brachial arteria. In a study of 19 overweight patients and/ or patients with elevated values of arterial pressure given single doses of a Vitis vinifera nutraceutical equivalent to 30, 90 y 270 mg of Resv, a significant increase in the FMD percentage was recorded in all dose groups in contrast with the control group [40], demonstrating linear behavior between the logarithm of the dose used and percentage FMD values. Two additional studies corroborated this finding. In 34 patients diagnosed with metabolic syndrome (see criteria in Table 1), a significant increase in the FMD percentage was observed after three months of administering 100 mg/day of Resv in a preparation that also included quercetin, vitamin D3 and rice bran phytic acid controlled against a placebo [41]. Similar results were observed in a clinical trial with 40 patients who showed significant increases both in the FMD percentage and diastolic function of the left ventricle in groups given 10 mg Resv daily for three months [42, 43]. These results show that Resv supplementation improves endothelial function, and thus reduces cardiovascular risk. However, the results should be interpreted carefully, due to the fact that the base diameter of the brachial artery conditions the flow increase percentage, especially in patients with developing atherosclerotic injuries, which could bias the conclusions, therefore studies based on this index are not recommended for assigning cardiovascular risk in asymptomatic adults [44].
Given that atherogenesis is an inflammatory process, different serum markers have been used to determine inflammation in the system, such as clinically significant C reactive protein levels (CRP), due to their prognostic value in patients with acute coronary syndrome. In a one year study administering a Vitis vinifera nutraceutical equivalent to 8 mg/day of Resv, significant and clinically relevant drops were reported in CRP levels in both hypercholesterolemic and diabetic patients undergoing statin treatment [40]. Similar results were reported in a study administering a 20 mg Resv supplement and calcium fructoborate daily for two months to overweight patients with stable angina [45]. However, not all CRP reduction can be attributed to Resv, as it has been shown that pharmacotherapy with statins per se induces statistically significant reduction in CRP levels [46].
Conversely, in a clinical trial administering Resv daily for three months as a nutritional supplement (Longevinex®) equivalent to 100 mg of Resv, no changes of any type were registered in CRP levels [41]. Given that lower doses of Resv did have an effect, the authors consider that this result was due to the hormetic behavior of Resv when acting on CRP levels.
The effect of Resv on plasminogen-1 activator inhibitor levels has also been determined in a 12 month clinical trial, reporting clinically and statistically significant reductions in groups given Resv in comparison with the placebo group [41]. Nonetheless, no change in pro-inflammatory interleukin IL-6 levels was recorded in this trial. Similar results were shown in a study comparing the effect of ethanol with the phenolic compounds of red wine on the expression of inflammation markers related with atherosclerosis due to cardiovascular disease [39]. The results suggest that the phenolic compounds of dealcoholized red wine can modify leukocyte adhesion molecules, whereas the ethanol and polyphenols present in red wine may modulate soluble inflammation mediators, however the effects cannot be attributed exclusively to Resv, as it is probable that the other polyphenol molecules present in red wine could be responsible for the effect observed [39].
Several clinical trials show that administering Resv does not modify plasma HDL- or LDL-cholesterol levels [41,45,47], classical markers in evaluating cardiovascular risk. Nonetheless in a six-month study administering a Vitis vinifera nutraceutical equivalent to 8 mg of Resv to patients with cardiovascular risk showed significant reductions both in oxidated LDL and Apolipoprotein B (ApoB) concentrations, although LDL or total-cholesterol were not modified [47]. This finding, still unverified in additional clinical studies, is interesting and relevant, as oxidation of these particles is a recognized progression factor of atherosclerotic lesions, given that it facilitates their precipitation. ApoB, has become known as a valuable prognostic marker of the capacity for interaction of apolipoprotein as a specific cellular receptor [48]. Figure 2 shows a flow chart that summarized in vitro and in vivo resveratrol mechanism of action.
Toxicity in animal models
The toxicity of Resv in different animal models has already been discussed in extenso, including its acute, subchronic and chronic toxicity [49].
The acute toxicity of a commercial product (resVida®) containing pure 99% trans-Resv was examined in three classic tests: dermic irritation, ocular irritation and cutaneous sensitization. In the dermic irritation test, semi-occlusive resVida® dermic patches equivalent to 500 mg of Resv were applied to the flanks of New Zealand rabbits for four hours; no adverse reaction was observed for 72 hours postadministration [49].
For the ocular irritation test, resVida®, equivalent to 100 mg of undiluted Resv, was administered to corneas of New Zealand rabbits, which exhibited slight to moderate redness that disappeared 72 hours later, without any other adverse effect [49].
The cutaneous sensitization test (local lymph node assay) consisted of topical administration of the assayed drug to the earlobes of the rabbits for three consecutive days. Resv at 6.25%, 12.5%, 25% weight/ volume was used, and compared with a control group. Five days after the study began, radio-marked thymidine was injected, and incubated for five hours, after which animals were culled to examine auricular lymph node cells. The calculated sensitization index was <3, below the threshold that characterizes a sensitizing substance [49].
Subchronic and chronic Resv toxicity has been tested in numerous animal models, such as Wistar and Sprague-Dawley rats [49-51], rabbits and dogs [52]. Crowell [50], administered Resv in 300, 1000 y 3000 mg/kg body weight doses for 28 days to Sprague-Dawley rats through a gastric tube. The toxic effects observed in the group that was administered 3000 mg/kg were severe, and generally attributed to a process of nephrotoxicity due to Resv. On the other hand, the group that was administered 1000 mg/kg manifested effects depending on the sex of the animals; the males had increased white cell count, while the females did experience blood count alterations, but gained significantly less weight during the study compared with the control group. The lower female body weight gain was attributed to dehydration and not reduction in food consumption. The group that was administered the smaller 300 mg/kg dose did not show toxicity or any other adverse effect.
Based on these results, a study of the same duration in Wistar rats [49] used doses of up to 500 mg/kg Resv incorporated into the standard diet. Although weight loss was not verified in any of the groups, variations in food consumption were observed, which the authors judged as irrelevant without providing more details. Alterations were also reported in partial thromboplastin time, with a shorter average partial thromboplastin time in the group that was administered 50 mg/ kg, while it increased in the group administered 500 mg/kg. In contrast to what was observed regarding partial thromboplastin time, other animal studies did not reveal changes in coagulation parameters [51].
In a 13 week study incorporating doses of Resv of up to 750 mg/kg into the diet of rats, the group of Wisar rats that was administered the higher dose of Resv exhibited a range of sex-dependent alterations in biochemical markers; the males had increased plasma levels of inorganic phosphate and albuminemia, and the latter was also observed in the groups of males administered even the lowest doses of 300 mg/kg [49]. The females exhibited only an increase in plasma alkaline phosphatase levels. As these findings did not follow a dose dependent pattern and as any macroscopic or hystopathological findings explaining them were also absent, they were judged to be toxicologically non-significant [49]. Other studies have not reported pathological changes in any organ within this dosage range of Resv [50].
In another toxicological study, Sprague-Dawley rats were administered Resv in doses of 200, 400 y 1000 mg/kg via gastric tube for 13 weeks. Even when a lower dose-dependent weight gain was observed in females in comparison with the control group, only the group administered the higher dose showed significantly lower body weight at the end of the study, without varying either group’s food consumption [51]. In the group administered the highest dose increased average bilirubinemia was observed.
The administration of Resv to Beagle dogs over three months [51] led to lower body weight gain only in the higher dose group (1200 mg/ kg), which was observed in both sexes and which may reflect lower dietary intake. At lower doses no adverse effects were observed. Dogs treated with Resv underwent cardiovascular monitoring, including ECG, but no alterations were detected throughout the range of the tested dose.
Another preclinical study was carried out to confirm the efficacy of Resv on the survival of mice consuming a diet rich in calories for more than 20 months [53]. Despite administering Resv in daily doses of up to 22.4 ± 0.4 mg/kg, no significant adverse effect was reported.
Adverse reactions and toxicity in human beings
A recent review addresses adverse reactions to Resv and Resv toxicity in human clinical trials [54] In these studies, Resv was used either in pure form or as part of nutraceutical formulations including polyphenols or other chemical compounds.
Tomé-Carneiro [40] carried out a 12-month clinical study divided into six-month phases. 25 healthy volunteers were administered capsules of a Vitis vinifera nutraceutical equivalent to 8 mg of Resv. Throughout the clinical trial, no adverse reactions or hypersensitivity were reported. Aminotransferase, thyroid hormone, albuminemia and creatininemia levels were determined, which did not reveal clinically or statistically significant evidence of changes.
Timmers [55] administered 150 mg of pure Resv to 11 obese subjects for 30 days. No alterations were observed in hematological, coagulation, general biochemical, or electrocardiographic parameters. However, statistically significant reductions were observed in plasma alanineaminotransferase levels, white blood cell count and inflammatory parameters such as plasma IL-6 or TNF- concentrations in the treatment group. In contrast, another study carried out one year later with a 500 mg/day dose of Resv did not result in altered aminotransferase levels or inflammation factors [56]. The latter study, lasting only 28 days and involving 12 obese patients, was free of adverse events, except for the development of a generalized rash in one patient from the vero group during the first week of the clinical trial. The patient concerned subsequently left the study [56].
In a clinical study of 20 cancer patients, subjects were administered Resv in 500 or 1000 mg/day doses for 8 days before a colectomy [57]. In this short study, no adverse treatment-related events were observed, and both doses were well-tolerated.
Chow [26] administered 1000 mg/day of Resv to 24 healthy volunteers for 28 days, without reporting clinically significant alterations in biochemical or hematological parameters. Two volunteers prematurely abandoned the study, one due to a case of diarrhea after the first dose and the second due to the appearance of peri-menopausal symptoms – hot flushes – in a postmenopausal woman.
Coincidentally, in another study carried out on eight healthy volunteers who were administered 2 g/day doses of Resv daily for eight days [58], episodic diarrhea was reported in six of the eight volunteers undergoing treatment. Furthermore, a reaction of rashtype hypersensitivity was reported in one volunteer, which resolved spontaneously.
Howells [21] used a dose of 5 g/day of micronized Resv SRT501 in patients with hepatic metastasis. In five of the six patients undergoing treatment, episodes of light diarrhea were recorded; a second patient also presented nausea and anal pruritus, and a third developed symptoms of hypersensitivity.
A phase II clinical trial using micronized Resv SRT501 coadministered with bortezomib to patients with multiple refractory myelomas was terminated early as many patients developed chronic renal insufficiency during the assay [59]. The close causal relationship between multiple myeloma and deterioration of renal function prevents clearly associating these events with a toxic Resv effect.
The evidence would seem to show that adverse reactions to Resv in doses of less than 1,000 mg/day are scarce and mild, meaning that a cause-effect relationship cannot always be established. At higher doses, the most frequently reported adverse reactions are mild and spontaneously resolving diarrhea. Based on this data and the results of metabolic interactions with cytochrome P-450 complex enzymes at doses higher than 1,000 mg/day, it has been suggested that this dose is the upper limit for clinical trials [43,59].
A large amount of information is available on Resv. A simple search for the keyword “resveratrol” in PubMed carried out in January 2015 delivered 7,130 results, with an exponential increase in publication of articles on this subject from 1999 to the present. This has led the scientific community to comment on recommendations of its use in humans. The recommendations proposed at the First International Resveratrol Conference, Resveratrol 2010, in Denmark, found insufficient evidence to justify recommending chronic administration of Resv in humans [43], in contrast to the therapeutic effects reported in commercial material and the media, based on the supposedly beneficial properties of red wine and as a potential explanation for the so-called French Paradox [60]. However, results from clinical assays carried out with different formulations of Resv from 2010 onwards suggest that these recommendations should be reviewed.
Pharmacokinetically, Resv is characterized as a rapidly absorbed compound, but which undergoes extensive pre-systemic metabolism by human cytochrome P-450 and intestinal microbiota [17,25,61]. Its metabolism mainly leads to conjugation products, the biological activity of which is still under discussion [28]. It is also rapidly cleared. All of this would explain the bioavailability estimated at around 1% of orally-administered doses [16,61]. Another distinctive feature of Resv is its high inter-individual variability in pharmacokinetic parameters [54].
Resv seems to exert a therapeutic effect on endothelial dysfunction that, although measured with a currently questioned technique [44], is consistent with the in vitro observation that Resv stimulates the endothelial nitric oxide synthase enzyme [11]. The inflammatory markers and CRP reductions obtained from doses of Resv equal to or less than 20 mg/day are not observed in larger doses, which could imply that the effects of Resv are hermetic [36]. On the other hand, Resv has been shown to reduce the atherogenic potential of LDL-cholesterol by reducing oxidized LDL and ApoB levels, which are important lipid markers for atherogenesis [47,48].
Analysis of the safety of Resv shows that it is well-tolerated. Shortterm (29 days) human studies have shown frequent gastrointestinal discomfort or spontaneously resolving diarrhea only with the administration of high doses (2.5 g to 5 g per day). Only minor and inconsistent adverse effects have been observed in other short acute studies [60]. In animal models, toxic doses produce physiopathological processes in the kidney [40,49].
The published evidence suggests that Resv reduces cardiovascular risk, whether prophylactically or therapeutically. It reduces the incidence of arterial hypertension, heart failure and ischemic cardiac disease in different animal models. Similarly, there is sufficient evidence to suggest that Resv improves insulin sensitivity and reduces the plasma glycemia levels and obesity caused by high-fat diets in rodent models.
The data obtained from animal models is promising and justifies the need for more clinical trials in humans to show the cardiovascular therapeutic potential of Resv, whether administered in pure form or jointly with other formulated or nutraceutical natural compounds.
However it should be reiterated that the current published evidence is not sufficiently strong to recommend the chronic administration of Resv to human beings beyond doses able to be obtained from dietary sources. The administration of Resv in chronic doses, above the concentrations found in food should be considered experimental, until longer clinical trials can take place in humans to ensure the efficacy and safety of Resv.
The authors are grateful to the Dr. Tulio Nuñez (University of Chile) for his observations and suggestions.
The authors declare have no conflict of interest.