Enzyme Engineering
Open Access
ISSN: 2329-6674
ISSN: 2329-6674
Research Article - (2013) Volume 2, Issue 1
Keywords: Fish waste, Pepsinogen, Extraction, Purification, Fractionation, Enzyme activity, Protein concentration, Recovery yield
Atlantic Canada has 15% (40,000 km) of the Canada’s coastline and 42% of the Canada’s fresh water (320,000 km2). About 80% of total fish landings come from the Atlantic fishery, while the Pacific fishery accounts for up to 16% [1]. In 2010, Atlantic Canada’s total sea fisheries landings were 788,599 t/y, with the total value of 1.3 billion dollars. A large portion of the fish (80%) landed in Atlantic Canada is processed [2].
There are three major common steps in fish processing: (a) removing the viscera, (b) removing the head, tail, fins and skin, and (c) removing the frame and producing fillets. A large fraction (30-80%) of fish (flesh, heads, bones, fins, skin, tails and viscera) is generated as waste during fish processing [3,4]. Fish wastes are usually disposed of in landfills or poured directly into the sea, which results in high disposal cost and causes environmental problems. About 56% of the fish landing in Canada is converted into waste; of which 13% is disposed in the ocean [5]. Conventional disposal of fish wastes underscores the need for a more reasonable utilization approach of fish wastes, as well as effective recovery of valuable ingredients from these wastes.
Fish wastes can be utilized as animal feed ingredients, as well as organic fertilizers [6,7]. The recovery of valuable biomolecules, such as collagen [8-11], ω-3fatty acids [12], trypsin [13,14], chymotrypsin [15-17], and elastase [18], have also been reported. Among the valuable products that can be recovered from fish, pepsin is one of the abundant and useful biomolecules that can be effectively recovered from fish viscera.
Pepsin is synthesized and secreted in the gastric membrane in an inactive state called Pepsinogen (PG) (molecular weight of 40 kDa). Pepsin is an important acidic protease, widely applied in the hydrolysis of proteins in the food and manufacturing industries. It can be used in collagen extraction [19-21], gelatin extraction [22], cheese making [23], and regulating digestibility [24]. Extraction of pepsins from fish viscera not only significantly reduces the capital costs of enzyme production, but also partially improves the economics of the fish processing industry, while minimizing the environmental impact of waste disposal [25,26].
Pepsin, as well as its zymogen, pepsinogen (PG), has been widely purified from several fish species, including arctic fish capelin (Mallotus villosus) [27], rainbow trout (Salmo gairdneri) [28], Atlantic cod (Gadus morhua) [29], bolti fish (Tilapia nilotica) [7], Antarctic rock cod (Trematomus bernacchii) [30], sea bream (Sparuslatus houttuyn) [31], African coelacanth (Latimeria chalumnae) [32], Mandarin fish (Siniperca chuatsi) [33], smooth hound (Mustelus mustelus) [34], orange-spotted grouper (Epinephelus coioides) [35], albacore tuna (Thunnus alalunga) [36], and European eel (Anguilla anguilla) [37]. During the purification of proteases, several conventional techniques such as Ammonium Sulfate Fractionation (ASF), Gel Filtration Chromatography (GFC) and ion exchange chromatography (IEC), are frequently performed. Conventional purification methods can give good enzyme purity, but are very complex, time-consuming and expensive. Therefore, efficient and economical methods for the purification of pepsin and PG that gives both high yield and high purity are needed. The aqueous two phase systems (ATPS) have proved to be effective method for purifying proteins. However, few investigations have been carried out to find out its efficacy and feasibility for pepsin and PG purification.
The aim of the present study was to optimize the ATPS purification using polyethylene glycol (PEG)-salt combinations, for purifying PG from fish processing waste. The specific objectives were: (a) to study the effect of the effects of PEG molecular weight (1000, 1500, 3000, 4000) and concentration (16%, 18%, 20%, 22%, 24%), on the total volume (TV), volume ratio (VR), specific activity (SA), purification fold (PF), partition coefficient (Kp) and recover yield (RY), and (b) to compare the efficiency of ATPS and ASF methods in purifying PG on the basis of SA, PF and RY.
Chemicals and reagents
Polyethylene glycol (PEG 1000, PEG 1500, PEG 3000 and PEG 4000) were obtained from Sigma-Aldrich Canada Ltd. (Oakville, Ontario, Canada). Hemoglobin, bovine serum albumin, Trichloroacetic acid (TCA) and Coomassie Brilliant Blue G-250 were purchased from Fisher Scientific Company (Ottawa, Ontario, Canada). Analytical grade salt of (NH4)2SO4 was procured from Fisher Scientific Company (Ottawa, Ontario, Canada). Reagents (50 mM sodium phosphate buffer, 100 mM phosphate-citrate buffer) were prepared [38].
Sample collection and preparation
Red perch (Sebastes marinus) were obtained from the Fisherman’s Market (607 Bedford Highway, Halifax, Nova Scotia, Canada). The fish were packed in polyethylene bag and transported in ice to the Biotechnology Laboratory, Department of Process Engineering and Applied Science, Dalhousie University, Halifax. Fish internal organs were separated, and the stomachs were collected. The undigested food in the stomach was removed and the stomach mucosa was rinsed with cold distilled water, then immediately stored at -20°C (to minimize autolysis of enzymes), until used in the experiments.
Experimental design
The optimum salt type and concentration 15% (NH4)2SO4) [39] were used, and the effects of PEG molecular weight at 4 levels (1000, 1500, 3000, 4000) and concentration at 5 levels (16%, 18%, 20%, 22%, 24%) were evaluated. After the optimal purification conditions of the ATPS were established, the ATPS and ASF methods were compared on the basis of SA, PF and RY.
Crude pepsinogen extraction
Frozen stomachs (35 g) were thawed using running water (4°C), until the core temperature reached -2 to 0°C. The samples were cut into pieces with a thickness of 1.0-1.5 cm, and homogenized in four volumes of 50 mM sodium phosphate buffer at pH 7.0. The homogenate was centrifuged in a refrigerated centrifuge (IEC Centra-MP4R refrigerated high speed table top centrifuge, International Equipment Company, Needham, Massachusetts, USA), at 15,000 g and 4°C for 20 min, to remove the tissue debris. The supernatant was collected and referred to as crude extract (crude PG). Crude extract was divided and stored in 4 ml vials and stored at -20°C.
Pepsinogen purification by Aqueous Two Phase Systems (ATPS)
The ATPS procedure used to purify pepsinogen (PG) and the parameters studied and their levels are shown in figure 1. ATPS were prepared in 15 ml centrifuge tubes by mixing PEG, salts and crude extract, according to the methods described [39,40]. ATPS were initially prepared at room temperature, but the PG extract had poor stability showing a rapid decrease in activity. Therefore, all experimental steps were performed at 4°C to reduce the autolysis or self-digestion of enzyme that resulted in drop of activity. The PEG optimization procedure is shown in figure 2. To study the effect of the polymer on PG purification, a 50% stock solution of PEG (selected from PEGs-PEG 1000, PEG 2000, PEG 3000 or PEG 4000) was mixed with the 15% (NH4)2SO4, to achieve the designated concentrations (16, 18, 20, 22 or 24%) in the aqueous system at room temperature. Approximately, 1 g crude PG extract (thawed overnight) was added into the cold salt and PEG mixture, and mixed by inversion several times. The cold mixture centrifuged for 5 min at 2000 g and 4°C (IEC Centra-MP4R, International Equipment Company, Needham, Massachusetts, USA). For each tube, the top phase (polymer phase) and bottom phase (salt phase) were carefully separated using a pre-chilled pipette, and the interface (≤ 0.05ml) was discarded. The volumes of the separated phases were measured using a 10 ml graduate cylinder. Based on literature, 60-95% PG was partitioned in the top phase [36,41]. Aliquots of the top phase were taken for determination of enzyme activity, and aliquots in both phases were taken for determination of protein content. Based on purity and yield, the PEG molecular weight and concentration which gave the highest RY were selected as the most effective purification PEG for further study.
Pepsinogen purification by Ammonium Sulphate Fractionation (ASF)
The ASF procedure used to purify PG is shown in figure 3. Approximately, 4 g PG crude extract were used for ASF purification. Ammonium sulfate powder was added slowly and the precipitates in the saturation range of 20-60% were collected. The solution was brought to 20% saturation first, and centrifuged in a refrigerated centrifuge at 10,000 g for 15 min (IEC Centra-MP4R, International Equipment Company, Needham, Massachusetts, USA). The supernatant was collected and volume was measured. Then, the supernatant was brought to 60% saturation and centrifuged in the same way. After centrifugation, the precipitate was collected. The purified protease was dialyzed against 50 mM sodium phosphate buffer with pH 7.0 overnight, during which time the buffer was changed three times. The enzymes were stored at 4°C for assay and comparison with ATPS.
Protease assay
The protease assay for the ATPS and ASF methods are different, as the two phases in ATPS account for more parameters in the assay process. For the ATPS method, the protease assay procedures determined total volume (TV), volume ratio (VR), enzyme activity (AE), protein content (CP), specific activity (SA), partition coefficient (KP), purification fold (PF), as well as recovery yield (RY). For the conventional method, only AE, CP, SA, PF and RY were assessed.
Determination of protein concentration (Cp)
Aliquots of both phases were taken for determination of CP. CP was measured by the method [42], using BSA as a standard. BSA solutions (0.1 ml) with concentrations of 0, 100, 200, 400, 600 and 800 μg/ml were prepared in test tubes by mixing stock BSA solution (1 mg/ml) with enzyme buffer. Sample containing protein (0.1 ml) was pipetted into the same test tube. Each tube containing BSA solutions and protein samples were added to 5 ml Bradford reagent, and mixed using a vortex mixer. The color reaction was conducted at room temperature for 5 min. The absorbance was measured at 595 nm, and a standard curve of absorbance of BSA solution against protein concentration was plotted in figure 4. A blank was conducted and three triplicates were performed. PEG mixed with the protein samples consistently caused a small reduction in absorbance. According to Barbosa et al. [43], this effect can be reduced if the PEG concentration is diluted below 10% (w/w). Therefore, this dilution was made for all samples, and the dilution fold was taken into account in the calculation of the original CP.
Determination of total enzyme activity (AE)
Aliquots in PEG-rich top phase of the ATPS were taken for determination of AE. Potential pepsin activity of PG was determined [44], with a minor modification. Crude or purified PG (0.5 ml) was added into 2.5 ml of 2% (w/v) hemoglobin in the phosphate-citrate buffer. The reaction was conducted at a pH of 2.5, and a temperature of 37°C for 10 min. To terminate the enzymatic reaction, 5 ml of 5% (w/v) Trichloroacetic acid (TCA) were added, and nonhydrolyzed substrates were filtered (P8 Grade filter paper, Fisher Scientific, Canada). The clear filtrate was collected, and the absorbance was measured at 280 nm. One unit was defined as an increase of 1.0 in absorbance at 280 nm per minute, at pH 2.5 and 37°C. A blank was conducted in a similar way and protease was added into the reaction mixture, after the addition of 5% TCA (w/v). The activity was assayed in triplicate. Aliquots from ASF purification were assayed in a similar way.
Determination of specific enzyme activity (SA)
The SA of recovered proteases in the top PEG phase was determined in units/mg protein as follows [36].
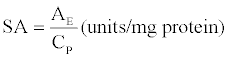 (1)
(1)
Where, AE is the enzyme activity in the top phase (U).
CP is the protein content in the top phase (mg).
Determination of purification fold (PF)
The PF (also called purification factor) of PG in the top phase was defined as follows [36].
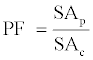 (2)
(2)
Where, SAp is the SA purified in the ATPS top phase (U/mg).
SAc is the SA of the crude PG extract (U/mg).
Determination of partition coefficient (KP)
The KP of recovered proteases for the ATPS was defined as follows [36]
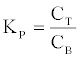 (3)
(3)
Where, CT is the CP in the top phase (mg).
CB is the CP in the bottom phase (mg).
Determination of volume ratio (VR)
The VR of recovered proteases for the ATPS was defined as follows [36].
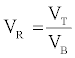 (4)
(4)
Where, VT is the volume of the top phase (ml).
VB is the volume of the bottom phase (ml).
Determination of total volume (TV)
The TV of recovered proteases in the ATPS was defined as follows [36].
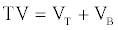 (5)
(5)
Where, VT is the volume of the top phase (ml).
VB is the volume of the bottom phase (ml).
Determination of recovery yield (RY)
The protease RY was calculated using the ratio of protease activities as follows [36].
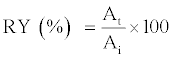 (6)
(6)
Where At is AE in the top phase (U).
Ai is the AE of the crude PG extract (U).
Statistical analysis
Analysis of variance (ANOVA) was performed on the data. Duncan multiple test was also performed on the data, to determine the differences among the levels of the salt types and concentrations. All the statistical analysis of data was conducted using Minitab statistics software 16 (Minitab Inc., State College, PA, USA).
Comparison between the ATPS and ASF methods
For the ATPS method, the crude and recovered proteases were investigated, and the AE and CE, as well as phase volumes were measured, and the SA, PF, KP, VR and RY were calculated. For the ASF method, the crude and recovered proteases were evaluated in a similar way; the AE and CE were measured, and the SA, PF and RY were calculated. The SA, PF and RY of ATPS and ASF were then compared to determine the feasibility of the ATPS method.
Crude pepsinogen extraction
The results of the extraction of crude PG from the stomach (35 g) of red perch are summarized in table 1. During the extraction process, the volumes of crude extract first decreased from 175 to 121 ml after centrifugation, due to the removal of the mucosa residues, and then increased to 152 ml after dialysis, because of the absorption of some water. The AE and CP decreased from 2154 to 1655 U, and from 3871 to 1595 mg, respectively. The SA and PF increased from 0.56 to 1.04 U/mg, and from 1.00 to 1.90, respectively. The RY decreased from 100.0 to 86.6% during extraction process.
| Extractionstep | Total extract volume (ml) | Total activity (U)* | Total protein (mg) | Specific activity (U/mg protein) | Purification fold | Recovery yield () |
|---|---|---|---|---|---|---|
| After homogenation | 175 | 2154 | 3871 | 0.56 | 1.00 | 100.0 |
| After centrifugation | 121 | 1969 | 2022 | 0.97 | 1.75 | 91.41 |
| After dialysis | 152 | 1655 | 1595 | 1.04 | 1.90 | 86.55 |
Sample size=35 g
Table 1: Profiles of PG extraction from red perch.
ATPS purification
The effects of PEG 1000, PEG 1500, PEG 3000 and PEG 4000 at concentrations of 16, 18, 20, 22 and 24% on ATPS purification parameters are presented in table 2 .The volumes of the top phase (VT) and bottom phase (VB), and the TV were measured, and the VR was calculated by dividing VT by VB. The AE, TA, SA, Cp, Kp, PF and RY were determined. The analysis of variance performed on the data in table 3 indicated that the effects of PEG molecular weight and concentration were significant at the 0.0001 level. A significant interaction between the PEG molecular weight and concentration was also observed at the 0.0001 level.
| PEG Molecular weight | PEG concentration (%, w/w) | Volume (ml) Top phase Bottom phase Total | VR | AE (U) | Cp (mg) Top phase Bottom phase Total | SA (U/mg) | KP | PF | RY (%) |
|---|---|---|---|---|---|---|---|---|---|
| 1000 | 16 | 4.44±0.01 4.06±0.01 8.50±0.01 | 1.09±0.01 | 7.32±0.18 | 1.78±0.03 0.42±0.02 2.21±0.05 | 4.11±0.17 | 4.58±0.17 | 3.96±0.16 | 66.3±1.67 |
| 18 | 4.41±0.01 3.86±0.01 8.27±0.02 | 1.14±0.01 | 7.81±0.16 | 1.81±0.03 0.38±0.03 2.19±0.07 | 4.36±0.14 | 4.71±0.30 | 4.20±0.13 | 71.7±1.48 | |
| 20 | 4.71±0.01 3.90±0.01 8.61±0.02 | 1.21±0.01 | 6.99±0.20 | 1.74±0.03 0.37±0.02 2.11±0.01 | 4.02±0.18 | 4.65±0.17 | 3.65±0.17 | 64.2±1.85 | |
| 22 | 4.73±0.01 3.64±0.02 8.37±0.02 | 1.30±0.01 | 5.46±0.17 | 1.55±0.03 0.35±0.03 1.90±0.05 | 3.53±0.16 | 4.48±0.28 | 3.40±0.15 | 50.2±1.57 | |
| 24 | 4.63±0.01 3.40±0.01 8.03±0.01 | 1.36±0.01 | 4.31±0.16 | 1.25±0.02 0.31±0.02 1.56±0.04 | 3.25±0.11 | 4.06±0.20 | 3.13±0.11 | 39.6±1.48 | |
| 2000 | 16 | 4.20±0.01 4.47±0.01 8.67±0.02 | 0.94±0.00 | 8.62±0.14 | 1.70±0.04 0.63±0.02 2.33±0.02 | 5.07±0.20 | 2.70±0.15 | 4.89±0.19 | 79.1±1.30 |
| 18 | 4.28±0.01 4.12±0.01 8.40±0.01 | 1.04±0.00 | 9.39±0.10 | 1.73±0.03 0.60±0.03 2.33±0.05 | 5.43±0.15 | 2.88±0.12 | 5.23±0.14 | 86.2±0.93 | |
| 20 | 4.67±0.01 4.17±0.01 8.84±0.01 | 1.12±0.01 | 8.20±0.12 | 1.64±0.04 0.59±0.02 2.23±0.02 | 5.01±0.10 | 2.76±0.16 | 4.83±0.10 | 75.3±1.11 | |
| 22 | 4.72±0.01 3.93±0.01 8.65±0.01 | 1.20±0.01 | 6.71±0.08 | 1.44±0.03 0.55±0.02 1.99±0.02 | 4.66±0.14 | 2.60±0.12 | 4.49±0.13 | 61.6±0.74 | |
| 24 | 4.61±0.01 3.74±0.01 8.35±0.02 | 1.24±0.01 | 5.19±0.15 | 1.15±0.02 0.49±0.03 1.64±0.05 | 4.50±0.13 | 2.37±0.13 | 4.35±0.13 | 47.7±1.39 | |
| 3000 | 16 | 3.89±0.01 4.87±0.01 8.76±0.01 | 0.80±0.00 | 5.52±0.10 | 1.55±0.02 0.82±0.03 2.37±0.03 | 3.56±0.11 | 1.89±0.10 | 3.16±0.11 | 50.7±0.93 |
| 18 | 3.98±0.01 4.45±0.01 8.43±0.02 | 0.89±0.00 | 6.31±0.06 | 1.57±0.05 0.78±0.02 2.35±0.06 | 4.02±0.17 | 2.01±0.06 | 3.88±0.16 | 58.0±0.56 | |
| 20 | 4.52±0.01 4.38±0.01 8.90±0.02 | 1.03±0.00 | 4.97±0.15 | 1.43±0.04 0.74±0.03 2.17±0.04 | 3.46±0.19 | 1.94±0.08 | 3.34±0.18 | 45.7±1.48 | |
| 22 | 4.55±0.02 4.15±0.01 8.70±0.03 | 1.10±0.00 | 3.90±0.18 | 1.18±0.03 0.69±0.02 1.87±0.01 | 3.30±0.13 | 1.70±0.09 | 3.18±0.13 | 35.8±1.67 | |
| 24 | 4.58±0.01 3.81±0.01 8.39±0.01 | 1.20±0.01 | 2.83±0.17 | 0.93±0.02 0.61±0.02 1.54±0.04 | 3.03±0.12 | 1.53±0.04 | 2.92±0.12 | 26.0±1.57 | |
| 4000 | 16 | 3.54±0.01 4.78±0.01 8.32±0.02 | 0.74±0.00 | 4.35±0.16 | 1.49±0.04 1.05±0.03 2.54±0.06 | 2.92±0.16 | 1.42±0.04 | 2.81±0.15 | 40.0±1.48 |
| 18 | 3.94±0.01 3.99±0.01 7.93±0.02 | 0.89±0.00 | 4.85±0.10 | 1.51±0.02 1.01±0.03 2.52±0.03 | 3.21±0.09 | 1.50±0.06 | 3.10±0.09 | 44.5±0.93 | |
| 20 | 4.10±0.01 4.28±0.01 8.38±0.02 | 0.96±0.00 | 3.72±0.19 | 1.34±0.03 0.95±0.03 2.30±0.05 | 2.77±0.19 | 1.41±0.04 | 2.67±0.18 | 34.2±1.85 | |
| 22 | 4.16±0.01 4.03±0.01 8.19±0.01 | 1.03±0.01 | 2.72±0.12 | 1.07±0.03 0.85±0.02 1.92±0.03 | 2.54±0.11 | 1.26±0.06 | 2.45±0.11 | 25.0±1.11 | |
| 24 | 4.27±0.01 3.63±0.01 7.89±0.02 | 1.18±0.00 | 1.95±0.09 | 0.86±0.02 0.75±0.02 1.61±0.04 | 2.28±0.14 | 1.15±0.03 | 2.20±0.13 | 17.9±0.83 |
Top phase: PEG phase
Bottom phase: Salt phase
VR: Volume ratio (the volume of top phase/the volume of bottom phase)
AE: Enzyme activity in the top phase
CP: Protein content in top and bottom phases
SA: Specific activity (AE/CP) in the top phase
KP: Partition coefficient (CP of top phase/CP of bottom phase) for the overall ATPS system
PF: Purification fold (SA of purified enzyme/SA of crude enzyme) in the top phase
RY: Recovery yield (AE of purified enzyme/AE of crude enzyme) in the top phase
Table 2: Effects of PEG molecular weight and concentration on partition of 1g PG, using 15% (NH4)2SO4.
| Parameters | Source | DF | SS | MS | F | P |
|---|---|---|---|---|---|---|
| TV | Total | 59 | 4.55702 | |||
| M | 3 | 2.29326 | 0.764419 | 2002.85 | 0.0001 | |
| C | 4 | 2.18153 | 0.545382 | 1428.95 | 0.0001 | |
| MC | 12 | 0.0697 | 0.005581 | 14.62 | 0.0001 | |
| Error | 40 | 0.01527 | 0.000382 | |||
| VR | Total | 59 | 1.65279 | |||
| M | 3 | 0.65469 | 0.218229 | 2785.90 | 0.0001 | |
| C | 4 | 0.95694 | 0.239236 | 3054.07 | 0.0001 | |
| MC | 12 | 0.03803 | 0.003169 | 40.46 | 0.0001 | |
| Error | 40 | 0.00313 | 0.000078 | |||
| AE | Source | DF | SS | MS | F | P |
| Total | 59 | 247.289 | ||||
| M | 3 | 147.699 | 49.2330 | 2285.66 | 0.0001 | |
| C | 4 | 96.757 | 24.1892 | 1122.99 | 0.0001 | |
| MC | 12 | 1.972 | 0.1643 | 7.63 | 0.0001 | |
| Cp | Total | 59 | 5.73174 | |||
| M | 3 | 0.25954 | 0.08651 | 41.25 | 0.0001 | |
| C | 4 | 5.22232 | 1.30558 | 622.53 | 0.0001 | |
| MC | 12 | 0.16599 | 0.01383 | 6.60 | 0.0001 | |
| Error | 40 | 0.08389 | 0.00210 | |||
| SA | Source | DF | SS | MS | F | P |
| Total | 59 | 45.6056 | ||||
| M | 3 | 37.4403 | 12.4801 | 582.05 | 0.0001 | |
| C | 4 | 7.0738 | 1.7684 | 82.48 | 0.0001 | |
| MC | 12 | 0.2339 | 0.0195 | 0.91 | 0.5470 | |
| Kp | Total | 59 | 89.4282 | |||
| M | 3 | 86.7354 | 28.9118 | 1515.96 | 0.0001 | |
| C | 4 | 1.7995 | 0.4499 | 23.59 | 0.0001 | |
| MC | 12 | 0.1304 | 0.0109 | 0.57 | 0.8530 | |
| Error | 40 | 0.7629 | 0.0191 | |||
| PF | Source | DF | SS | MS | F | P |
| Total | 59 | 42.0801 | ||||
| M | 3 | 34.7761 | 11.5920 | 532.19 | 0.0001 | |
| C | 4 | 6.2835 | 1.5709 | 72.12 | 0.0001 | |
| MC | 12 | 0.1492 | 0.0124 | 0.57 | 0.8520 | |
| RY | Source | DF | SS | MS | F | P |
| Total | 59 | 20653.1 | ||||
| M | 3 | 12362.4 | 4120.79 | 2267.28 | 0.0001 | |
| C | 4 | 8050.8 | 2012.69 | 1107.39 | 0.0001 | |
| MC | 12 | 167.2 | 13.93 | 7.67 | 0.0001 |
DF: Degrees of freedom
SS: Sum of squares
MS: Mean of squares
M: PEG molecular weight
C: PEG concentration
MC: Interaction of PEG molecular weight and concentration
R2 =0.99
Table 3: Results of the analysis of variance for the various parameters.
Total volume (TV)
The effects of PEG molecular weight and concentration on TV are presented in figure 5. All TV gave similarly shaped curves. The TV initially increased by 3.06-4.48%, when the PEG molecular weight increased from 1000 to 3000, and then decreased by 5.02-5.93%, when the PEG molecular weight increased from 3000 to 4000. On the other hand, all the TV decreased when the PEG concentration was increased from 16% to 18%, increased when the PEG concentration was increased from 18% to 20%, and then decreased when the PEG concentration was increased from 20% to 24%. The PEG 3000 with 20% concentration gave the highest TV (8.90 ml).
Volume ratio (VR)
The effects of PEG molecular weight and concentration on VR are shown in figure 6. The VR decreased with increasing PEG molecular weigh, and increased with increased PEG concentration. When PEG molecular weight increased from 1000 to 4000, the VR decreased by 13.24-32.11%, whereas when PEG concentration was increased from 16% to 24%, the VR increased by 24.77-59.46%. The PEG 1000 with 24% concentration gave the highest VR value (1.36).
Total enzyme activity (AE)
The effects of PEG molecular weight and concentration on AE are shown in figure 7. The AE increased by 17.31-22.67%, when PEG molecular weight was increased from 1000 to 1500, and then decreased by 48.35-62.43%, when PEG molecular weight was increased from 1500 to 4000.
The AE also increased by 6.69%-14.31%, when PEG concentration was increased from 16% to 18%, and then decreased by 44.73%-59.79%, when the PEG concentration was further increased from 18% to 24%. The PEG 1500 with 18% concentration gave the highest AE (9.39 U).
Specific Enzyme Activity (SA)
The effects of PEG molecular weight and concentration on SA are shown in figure 8. The SA increased by 23.36-38.46%, when PEG molecular weight was increased from 1000 to 1500, and then decreased by 40.88-49.33%, when PEG molecular weight was further increased from 1500 to 4000. The SA increased by 6.08-12.92%, when PEG concentration was increased from 16% to 18%, and then decreased by 17.1%-29.0%, when PEG concentration was further increased from 18% to 24%. The PEG 1500 with 18% concentration gave the highest SA (5.5 U/mg).
Protein Content (Cp)
The effects of PEG molecular weight and concentration on CP in top, bottom and total phases are shown in figures 9 and 10. In the top phase, the CP decreased with increased PEG molecular weight, but first increased slightly when the PEG concentration was increased from 16% to 18%, and then decreased with further increases in PEG concentration. The CP decreased by 16.29%-31.20%, when PEG molecular weight was increased from 1000 to 4000. The CP increased by 1.29%-1.76% when PEG concentration was increased from 16% to 18%, and then decreased by 30.94%-43.05% when PEG concentration was further increased from 18% to 24%. The PEG 1000 with 18% concentration gave the highest CP in the top phase (1.81 mg).
In the bottom phase, all CP increased with increased PEG molecular weight, and decreased with increased PEG concentration. The CP increased by 141.94%-156.76% when PEG molecular weight was increased from 1000 to 4000, and decreased by 22.22%-28.57%, when PEG concentration was increased from 16% to 24%. The PEG 4000 with 16% concentration gave the highest CP in the bottom phase (1.05 mg).
The total CP obtained with PEGs of various concentrations gave similarly shaped curves. The total CP first slightly increased when the PEG molecular weight was increased from 1000 to 1500, slightly decreased when the PEG molecular weight was increased from 1500 to 3000, and then slightly increased when the PEG molecular weight was increased from 3000 to 4000. Total CP decreased by 29.41%-36.61% when PEG concentration was increased from 16% to 24%. The PEG 4000 with 16% concentration gave the highest total CP (2.54 mg).
Partition Coefficient (KP)
The effects of PEG molecular weight and PEG concentration on KP are shown in figure 11. The KP decreased by 68.15%-71.67% when PEG molecular weight was increased from 1000 to 4000. The KP increased by 2.84%-6.67% when PEG concentration was increased from 16% to 18%, then decreased by 13.8%-23.3% when PEG concentration was further increased from 18% to 24%. The PEG 1000 with 18% concentration gave the highest KP (4.71).
Purification Fold (PF)
The effects of PEG molecular weight and concentration on PF are shown in figure 12. The PF increased by 23.48%-38.98% when PEG molecular weight was increased from 1000 to 1500, and then decreased by 40.73%-49.43% when PEG molecular weight was further increased from 1500 to 4000. The PF increased by 6.06%-22.78% when PEG concentration was increased from 16% to 18%, and then decreased by 16.83%-29.03% when PEG concentration was further increased from 18% to 24%. The PEG 1500 with 18% concentration gave the highest PF (5.23).
Recovery Yield (RY)
The effects of PEG molecular weight and PEG concentrations on RY are shown in figure 13. All the RY values increased initially, and then decreased with increased PEG molecular weight or PEG concentration. The RY increased by 17.3-22.7% when PEG molecular weight was increased from 1000 to 1500, and then decreased by 49.4-62.5% when PEG molecular weight was further increased from 1500 to 4000. The RY increased by 8.1%-14.4% when the PEG concentration was increased from 16 to 18%, and then decreased by 44.3%-59.8% when the PEG concentration was further increased from 18 to 24%. The PEG 1500 with 18% concentration gave the highest RY (86.2%).
Comparing ATPS and ASF
The comparison between the ATPS and the ASF methods is shown in table 4. The values of AE, CP, SA, PF and RY were 30.66 ± 1.84 U, 12.0 ± 0.32 mg, 2.55 ± 0.14 U/mg, 2.46b ± 0.14 and 70.4 ± 4.23% for ASF, and 37.67 ± 0.38 U, 6.98 ± 0.12 mg, 5.40 ± 0.09 U/mg, 5.20 ± 0.08, 86.6 ± 0.88 (%) for ATPS, respectively. All the parameters are significantly different at the 0.05 level.
| Purification Method | AE (U) | CP (mg) | SA (U/mg) | PF | RY (%) |
|---|---|---|---|---|---|
| ASF | 30.66 ± 1.84 | 12.02 ± 0.32 | 2.55 ± 0.14 | 2.46 ± 0.14 | 70.4 ± 4.23 |
| ATPS | 37.67 ± 0.38 | 6.98 ± 0.12 | 5.40 ± 0.09 | 5.20 ± 0.08 | 86.6 ± 0.88 |
| P-value | 0.023 | 0.002 | 0.000 | 0.000 | 0.023 |
Optimum condition of ATPS: 15% (NH4)2SO4-18% PEG 1500 at 4°C,
Conditions of ASF: 20-60% saturation at 4°C.
AE: Enzyme activity
CP: Protein content
SA: Specific activity (AE/CP)
KP: Partition coefficient (CP of top phase/CP of bottom phase)
PF: Purification factor (SA of purified enzyme/SA of crude enzyme)
RY: Recovery yield (AE of purified enzyme/AE of crude enzyme)
Table 4: ASF purification of 4 g of PG and comparison to ATPS (P-values were obtained from the t-test by comparing AE, CP, SA, PF and RY of the two methods, respectively).
Extraction of crude pepsinogen
The same PG extract and crude PG samples were used in ATPS and ASF purifications. AE, CP and RY decreased, while SA and PF increased during PG extraction. This indicated that some of the PG (proteins and small molecular peptides) was lost, but the portion remaining was concentrated, resulting in a higher purity. Lower RY was due to the destruction of enzyme structure and denaturation of PG caused by homogenization, centrifugation and dialysis. Zhou et al. [31] homogenized stomach samples using a homogenizer with phosphate buffer, centrifuged the homogenate to collect the crude enzymes from sea bream, and reported decreases in the AE, CP and RY of 35%, 55% and 36%, and increases in the SA and PF of 42% and 40% during extraction steps, respectively. Bougatef et al. [34] homogenized stomach samples of smooth hound (Mustelus mustelus), using a homogenizer with Tris buffer, centrifuged the homogenate to collect the crude enzymes, and reported AE, CP and RY decreases of 29%, 81% and 40%, and SA and PF increases of 272% and 271% during extraction steps, respectively.
Effects of PEG molecular weight and concentration
The results showed that biphasic systems occur at a critical PEG concentration, and a higher concentration is required at a low temperature. At 4°C, the two phase separation was achieved with all PEG molecular weights at a concentration of 16% or higher. Raghavarao et al. [45] reported two phase formation above 8-10% PEG at room temperature. Nitsawang et al. [46] reported two phase formation above 4-12% concentration of PEG 6000 with 15% (NH4)2SO4. Binodal curves with commonly used PEG (PEG 1000, 2000, 3000, 4000, 6000, 8000) at room temperature have been established as a reference [47-49], but data are quite limited at 4°C for salts.
A critical molecular weight of PEG is also required for phase separation. Tubío et al. [50] suggested that for ATPS formation, a minimum molecular weight of 600-3350 is required, which is consistent with our results with PEG molecular weight ≥ 1000.
In the present study, TV increased with increasing PEG molecular weight, and then decreased. Eliassi et al. [51] reported that the decrease in TV may be attributed to the changes of the density of the aqueous solution caused by PEG, which resulted in a change of volume upon mixing. Although the long chain of a large PEG molecule may be expected to occupy more space and increase the volume, a decrease in volume could occur if the PEG chains are coiled and twisted around each other, resulting in a decrease in the space occupied. With increasing PEG concentration, The TV showed an unusual pattern of a decrease, followed by an increase, and finally a last decrease in figure 5b. This was different from the expected trend of an increase, due to the enhanced space of more PEG molecules, followed by a decrease, resulting from the coiled PEG molecules at high concentration. The initial unexpected TV decrease observed in the present study could be explained through the formation of more ordered water structure, by increased hydrogen bonds at low PEG concentration. The consistency of the result for all the PEG molecular weights suggested that this result was not simply due to experimental error.
The result showed a decrease in VR with increased PEG molecular weight, and/or increased PEG concentration. These trends are consistent with those reported by Nalinanon et al. [36]. As with TV, the decrease in VR as a function of molecular weight may be related to the reduced volume of the top phase, resulting from the twisting of PEG chains. With an increased PEG concentration, more space was required to accommodate the PEG structures. Increasing PEG concentration may have caused a competition for water with the salt phase, resulting in an increased volume in the top phase, and decreased volume in the bottom phase [52].
The AE, SA, PF and RY initially increased with increased PEG molecular weight, and/or PEG concentration, and then decreased. Low PEG molecular weights (1000-1500) gave better partition than higher molecular weights. Similar results were reported by Nalinanon et al. [36] and Chaiwut et al. [53]. Partitioning is determined by a balancing of electrostatic interaction and the excluded volume effect of PEG. Xia et al. [54] suggested that an electrostatic interaction is formed between the protonated carboxyl groups of PG and the oxygen ether of PEG in figure 14, causing PG to transfer towards the PEG-rich phase. At low PEG molecular weight, the dominant electrostatic interaction helps to stabilize the enzyme. Increasing PEG molecular weight increased this interaction, and resulted in better hydration and higher solubility of PG in the PEG phase. Nalinanon et al. [36] and Bhat and Timasheff [55] stated that PEG steric exclusion driven by an entropic force, known as the excluded volume effect, occurs at large PEG molecular weights, and excludes the protein from the top phase. Knowles et al. [56] stated that at large PEG molecular weights, the excluded volume effect dominates over the electrostatic effect, creating an overall repulsion effect. At low PEG concentrations (16-18%), the electrostatic interaction is enhanced, which favors the partition of PG into PEG-top phase. However, high PEG concentrations lead to higher viscosities, and make the partition difficult. In addition, high PEG concentrations can result in denaturation and possible precipitation of PG [57].
KP characterized the protein distribution, and decreased with PEG molecular weight. The results are similar to those reported [36,58]. A sharp decrease was detected below PEG molecular weight of 1500. However, this decrease did not follow the rule that a lower KP usually gave a higher PF of enzyme. It was found that PEG 4000 had the lowest KP and PF, while PEG 1500 gave the highest PF. This may be because of the excluded volume effect associated with the PG interaction. It is estimated that with increased molecular weight, more PG was partitioned away from the PEG phase. In this study, 18% PEG 1500 gave the strongest electrostatic interaction, and gave the best partition (highest RY) and highest PF.
Comparing ATPS and ASF methods
ATPS and ASF are based on different separation principles. ATPS employs selective partitioning of the protein of interest in one aqueous phase, while other proteins remain in the other phase. In contrast, ASF purified protein by selective precipitation of the protein of interest within one saturation range based on protein solubility, while other proteins remain in the solution. The ATPS method gave a higher AE than that of the ASF method, while the ASF method gave a higher CP than that of the ATPS method, because ASF was found to be less selective in protein separation than ATPS, and therefore, it provided more proteins as impurities mixed with PG. The ATPS method gave much higher SA, PF and RY, compared to those of the ASF. There is no literature on the comparison of two methods, but the ASF using similar saturations for PG purification gave a SA of ~ 0.88-3.0 U, PF of ~ 1.1-3.7 and RY of ~ 64-75% [31,32,34,37]. The values of SA, PF and RY obtained in the present study were in these ranges. The AE, SA, PF and RY of the ATPS were higher by 23, 112, 111 and 11%, respectively. Therefore, ATPS showed better partition and higher effectiveness than ASF.
The partition of PG from red perch using ATPS at 4°C was investigated. The effects of PEG molecular weight (PEG 1000, 1500, 3000 and 4000) and concentration (16, 18, 20, 22 and 24%), on the total volume (VT), volume ratio (VR), total enzyme activity (AE), protein content (Cp), specific enzyme activity (SA), purification fold (PF), partition coefficient (Kp), and recover yield (RY), on the purification of chymotrypsin from red perch,were studied. PEG molecular weight and PEG concentration also had significant effects on each parameter. To form two phases, a critical PEG molecular weight and concentration were required. TV increased with increased PEG molecular weight and PEG concentration, while different patterns were found for VR, which was due to the volume change upon mixing and the competition of PEG with salt for water. AE, SA, PF and RY increased with increased PEG molecular weight and concentration, and then decreased. The PEG partition effect was determined by the balancing of electrostatic interaction, and the excluded volume effect. Low PEG molecular weight favored electrostatic interaction to yield a better stabilization, while high PEG molecular weight produced steric exclusion and brought a weakened partition effect. Low PEG concentration enhanced the hydrophobic interaction and helped partition PG, while high PEG concentration increased the viscosity and surface tension and obstructed partition. A low KP no longer gave a high purity of PG due to the excluded volume effect. PEG 1500 at 18% concentration gave the highest RY (86.2%), and was selected as the optimum PEG molecular weight and PEG concentration. (NH4)2SO4 at 15%, which gave the highest RY (71.7%), was selected as the optimum salt type and salt concentration. 15% (NH4)2SO4-18% PEG 1500 was the optimal ATPS combination, and presented a better partition (SA of 5.40 U/mg, PF of 5.20 and RY of 86.6%) than ASF (SA of 2.55 U/mg, PF of 2.46, RY of 70.4%). ATPS was proven as a feasible, effective and gentle way to purify PG.