Immunome Research
Open Access
ISSN: 1745-7580
ISSN: 1745-7580
Research Article - (2021)Volume 17, Issue 1
In wildlife, the natural reservoir host for most animal viruses, overt clinical infections are generally absent. In this paper it is hypothesized that for hundreds of millions of years viruses co-evolved with arthropods, fishes, amphibians, reptiles, birds, and mammals, in a friendly manner, by minimizing virus replication costs and host damage. This virus-host mutualism hypothesis may be viewed as diametrically opposite to the virulence transmission hypothesis. Building on previous work, the transmission ecologies of 36 of the world main livestock viruses are examined in more detail. Viruses and organ systems are aligned on ecological grounds, in an outer to inner-body fashion. The virus-host interplay changes from acute to persistent infection to ever more virus-host intimacy. From outer to inner-body virus-host mutualism is on the increase. Virus-host antagonism increases from inner to outer-body. Also explored is the role of the host domain in mutualism-antagonism evolution. For this, the virus evolution trajectory upon a host shift from wildlife to humans or livestock is examined. Again, epithelial viruses are contrasted to non- epithelial, fully internalized viruses. It is found that a-clinical enteric viruses of wild birds and bats, in humans and livestock evolve as respiratory or also enteric pathogens. Viral virulence is most prominent among respiratory and enteric poultry and pig viruses. Regarding the internalized viruses, it is found that upon a transfer from wildlife to humans or livestock as host, the virus evolves to return to the sylvatic trait profile, very gradually so. Collectively, the results of this study corroborate the notion that in natural ecosystems friendly virus-host relationships tend to be selected for. Evolution of viral virulence and other forms of pathogenic fitness, including immune-suppression, primarily occur in animal smass rearing and so are anthropogenic in nature. The crowd agents circulating in humans as host take an intermediary position.
Virus-host co-evolution; Mutualism; Antagonism; Immune-tolerance; Virus organ system tropism
New viral diseases continue to emerge in humans and in livestock. Among the pandemic viruses that over the past decades emerged in humans are HIV-1, SARS coronavirus, pandemic H1N1 plus other influenza viruses, Ebola viruses, Zika virus, and SARS- CoV-2. Panzootic viruses that have shown up in livestock comprise African Swine Fever Viruses (ASFV), Porcine Epidemic Diarrhea Virus (PEDV), two distinct Porcine Reproductive and Respiratory Syndrome Viruses (PRRSV), H5N1 plus other Highly Pathogenic Avian Influenza Viruses (HPAIV), and the ruminant Schmallenberg Virus (SBV) [1-11].
Most of the above viruses are not truly novel and were already circulating, and continue to do so, in wildlife, the natural reservoir host, in a friendly manner, causing no harm. A-clinical virus transmission typically escapes the attention and, by implication, the virus ecology and evolution remain poorly understood. In this paper, it is hypothesized that for hundreds of millions of years viruses co-evolved with arthropods, fishes, amphibians, reptiles, birds, and mammals, towards virus-host mutualism, by minimizing both host damage and virus replication costs.
The suggestion that animal and plant viruses may evolve a mutualistic relationship with their host is not new [12-14]. Villarreal in 2000 contrasted acute to persistent animal viruses, as two opposing life history strategies shaped by natural selection [15]. As expanded upon by Van Blerkom in 2003, an acute virus is increasing fitness by increasing its reproductive rate [16]. Often disease-associated, the strategy is characteristic of viruses with high mutation rates and the ability to infect multiple host species. Viral transmission tends to be horizontal and dependent on host population structure and density, more so than it is for viruses using a persistent life strategy. The latter virus increases fitness by increasing the survival time of its offspring, thus enhancing persistence in an individual host and the probability of transmission over time. The persistent virus may transmit vertically or sexually. Consistent with the above, Hicks and Duffy established in 2014 that epithelial RNA viruses were highly evolvable, featuring higher long-term nucleotide substitution rates than internalized viruses [17].
The vertebrate host-body plan provides for a restricted number of viral habitats. In a previous study I explored, for livestock viruses, how virus ecology and evolution are shaped by the virus host-body infiltration and colonization patterns [18]. For this, 36 of the world main livestock viruses were aligned with the main organ systems, in an outer to inner-body fashion. Respiratory and enteric viruses were found to be associated with poultry and pig mass rearing. Ruminant and equine viruses were found more deep-rooted and host-specific, also establishing themselves in the vital inner-body systems.
The present study seeks to build on the above findings. It is first recalled, for the above 36 livestock viruses, how from outer to inner-body the infection changes from acute to persistent and how the virus-host relationship becomes ever more intimate. As a next step, the evidence for evolution towards virus-host mutualism is examined, in general, for livestock, human and wildlife viruses, again from outer to inner-body, organ system by organ system. Likewise, the evidence for virus-host antagonism is explored. For this, 20 viruses that have newly shown up in livestock over the past 150 years, a period of progressive increase in the world livestock populations, are considered in more detail [19].
Next, it is established how the host domain effects mutualism- antagonism evolution. For this, the virus evolution trajectory upon a host shift from wildlife to humans or livestock as host is examined. Again, epithelial viruses are contrasted to non-epithelial, fully internalized viruses.
The collective results serve to establish the outer to inner-body shifts in the virus-host relationship for the three main animal host domains of the world today; wildlife, humans and livestock.
The analysis entails an iterative process. Building on previous work [data from 18], 36 of the world main livestock viruses are aligned with the host-body organ systems, in an outer to inner body fashion, as per the organ system tropism of the individual viruses. A distinction is created between epithelial viruses and viruses colonizing the inner-body, in part or in full. The epithelial viruses are lined up so that the duration of infection and shedding as well as the virus environmental durability increase, from respiratory tract to alimentary tract to skin. Inner-body viruses are lined up so that the virus becomes ever more deep-rooted, shifting to non-epithelial transmission modes. With it, the virus environmental durability decreases. The resulting line-up runs from the peripheral nerves and ganglia to the reproductive organs system to the immune and circulatory systems to just the blood circulation.
Also established is an outer to inner-body line-up of the respective virus families. In addition, common human viruses belonging to these same virus families, 19 in total, are identified with the aim to establish also a line-up of human virus families.
As a next step, the evidence for evolution towards virus-host mutualism is examined, from the infection-shedding-transmission characteristics of the above livestock and human viruses, and where possible also from the virus ecology in the sylvatic cycle, again from outer to inner-body. Virus-host mutualism is defined in terms of minimal virus replication costs coupled to minimal host damage. As above established, from outer to inner-body the virus-host interplay shifts from acute to persistent infection to ever more deep-rootedness and virus-host intimacy.
The acute respiratory infection may be localized, peripheral, and transient. There may be virus shedding already during the incubation period. The virus is swiftly transmitted to the next host. A more persistent, enteric virus may still be shed after the cessation of the clinical signs. Skin viruses are expected to be utmost persistent, both on the host-body and in the environment. As a result, the skin virus may slowly propagate through a herd or flock, with few animals being clinically affected at any given point in time. In the inner-body environment, virus-host intimacy may evolve to the point that the divide between virus and host becomes blurred. Viruses colonizing peripheral nerves and ganglia tend to persist in the individual host and so may turn host specific. Viruses of the reproductive organs may transmit vertically, bringing virus and host ever more together, from one host generation to the next. Viruses of the immune and circulatory systems may evolve immune-tolerance. Immune-tolerant viruses may circulate in the bloodstream for extended periods of time. The virus environmental durability is expected to change with the shift from acute to persistent to ever more intimacy.
Likewise, the evidence for virus-host antagonism is explored, again from outer to inner-body. Antagonism is defined in terms of the presence of overt clinical signs contributing to the virus transmission success. For this, the infection-shedding-transmission characteristics of 20 viruses that have newly emerged in livestock over the past 150 years are examined in more detail.
Acute respiratory and enteric viruses may cause a generalized mucosal tract infection translating in major, profuse virus shedding. These viruses may cause boom-and-bust disease dynamics. Instead, viruses causing a generalized skin condition are expected to support more sustained virus shedding and transmission, resulting in enzootic forms of disease. In the inner-body, pathogenic fitness may take the form of immune-suppression, indirectly enhancing virus shedding and transmission. The latter may also be observed for pathogens of the reproductive organs system, via abortion and birth products. Not further considered are the clinical signs not contributing to virus transmission. Again, the virus environmental durability is expected to change with the shift from acute to persistent to ever more intimacy.
Also obtained for the above 20 viruses is a measure of virus opportunism. For this, the viruses are ranked in order of first appearance as livestock pathogen, as are the respective virus families. In addition, the host category is established, contrasting equines and ruminants to poultry and pigs as host.
Finally, the role of the host domain in mutualism-antagonism evolution is examined. For this, the virus evolution trajectory is explored upon a host shift from wildlife to humans or livestock as host. Again, epithelial viruses are contrasted to non-epithelial, fully internalized viruses. The inventory of sylvatic epithelial viruses comprises Avian Influenza Virus (AIV), Avian Paramyxovirus Type 1 (APMV-1), the avian gamma-coronavirus Infectious Bronchitis Virus (IBV), and SARS-like plus MERS-like coronaviruses of bats. The inventory of internalized viruses includes five arboviruses, including the ruminant Bluetongue (BTV) and Schmallenberg (SBV) viruses, plus the non-human primate origin dengue (DENV), Chikungunya (CHIKV), and Zika (ZIKV) viruses. Also added is the Simian Immunodeficiency Virus (SIV). Inferred for each virus is the eventually evolving, stable virus-host relationship, in terms of mutualism-antagonism evolution and, with it, virus organ system tropism. Also checked is whether or not the new host is genetically related to the ancestral host.
The collective results above permit a recap of the outer to inner body shifts in the virus-host relationship for the three main animal host domains of the world today; wildlife, humans, and livestock.
Figures 1 and 2 are best viewed in conjunction. Presented in Figure 1 is an outer to inner-body alignment of viruses and organ systems based on the organ system tropism of 36 of the world’s main livestock viruses. A distinction is made between epithelial viruses interfacing the external environment and viruses colonizing the inner-body, in part or in full. Also indicated is the resulting outer to inner-body line-up of virus families.
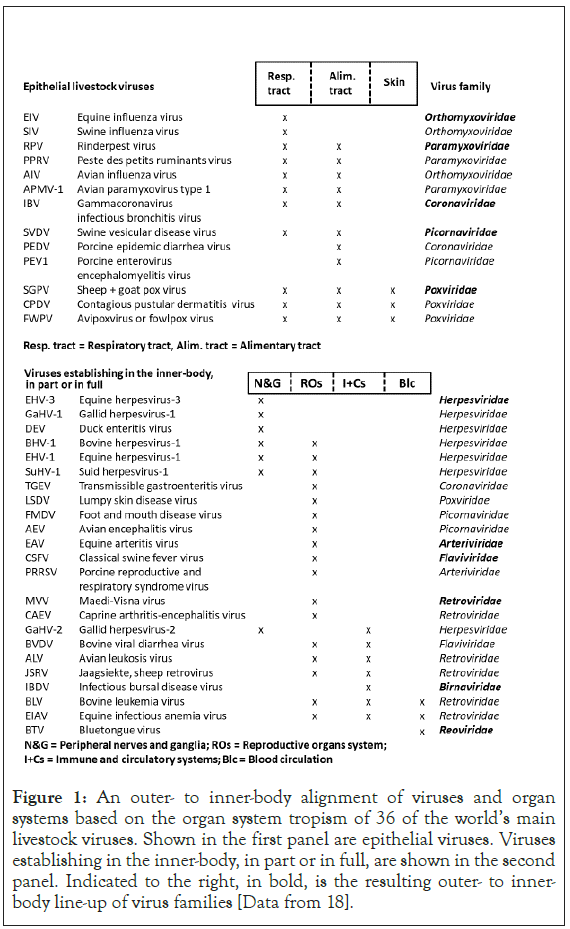
Figure 1: An outer- to inner-body alignment of viruses and organ systems based on the organ system tropism of 36 of the world’s main livestock viruses. Shown in the first panel are epithelial viruses. Viruses establishing in the inner-body, in part or in full, are shown in the second panel. Indicated to the right, in bold, is the resulting outer- to innerbody line-up of virus families [Data from 18].
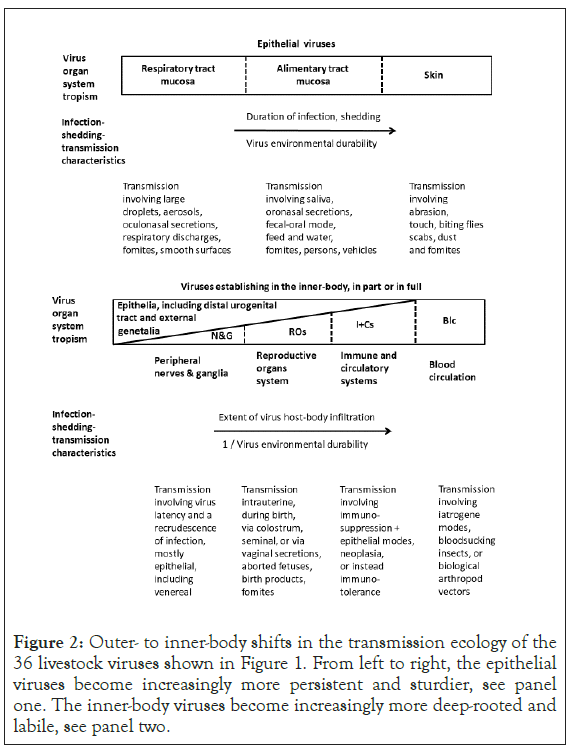
Figure 2: Outer- to inner-body shifts in the transmission ecology of the 36 livestock viruses shown in Figure 1. From left to right, the epithelial viruses become increasingly more persistent and sturdier, see panel one. The inner-body viruses become increasingly more deep-rooted and labile, see panel two.
The matching virus ecological details are presented in Figure 2. From respiratory tract to alimentary tract to skin, the virus becomes increasingly more persistent, well established in the outer-body environment. As the virus becomes more exposed also to the conditions prevailing in the environment outside the hostbody, the virus environmental durability increases. As shown in the second panel of Figure 2, in the inner-body, the virus becomes increasingly more deep-rooted and labile, from peripheral nerves and ganglia to the reproductive organs system to the immune and circulatory systems to just the blood circulation.
Shown in Figure 3 is an outer to inner-body line-up of 19 common human viruses belonging to the same families as shown in bold in Figure 1, as per the organ system tropism of the individual human viruses. Indicated to the right, in bold, is the ensuing outer to inner-body, human virus family line-up. The line-up is broadly identical to the one shown in Figure 1 except for the apparent greater prominence of respiratory human viruses.
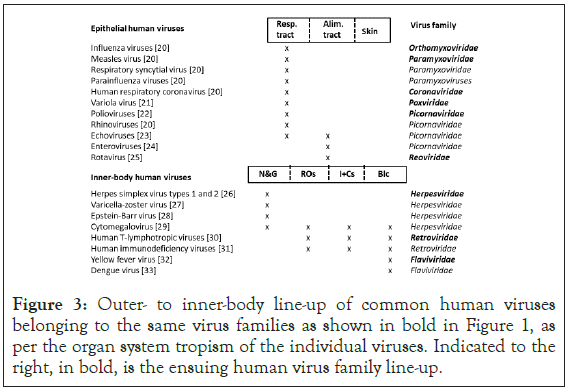
Figure 3: Outer- to inner-body line-up of common human viruses belonging to the same virus families as shown in bold in Figure 1, as per the organ system tropism of the individual viruses. Indicated to the right, in bold, is the ensuing human virus family line-up.
Presented in Figure 4 is evidence for evolution towards virus-host mutualism, defined in terms of minimal virus replication costs coupled to minimal host damage, organ system by organ system. The acute respiratory virus may colonize just parts of the upper respiratory tract. The infection may abate in a matter of days. Also, the virus may be shed already during the incubation period. The virus quickly spreads through a herd or flock. The host immune system may be hardly provoked. Recurrent infection waves may take the form of an annual cycle, a winter epidemic. From respiratory to alimentary tract to skin the infection changes from acute to persistent. Enteric viruses are generally more environmentally robust than respiratory viruses. Enteric viruses may continue to be shed in feces also after the cessation of clinical signs. Skin viruses are utmost persistent, both on the host-body and in the environment.
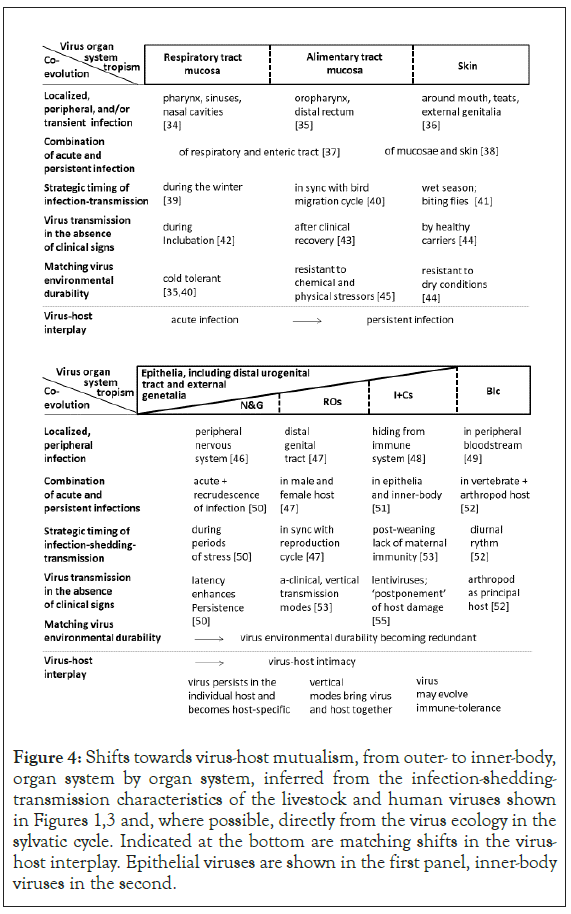
Figure 4: Shifts towards virus-host mutualism, from outer- to inner-body, organ system by organ system, inferred from the infection-shedding- transmission characteristics of the livestock and human viruses shown in Figures 1,3 and, where possible, directly from the virus ecology in the sylvatic cycle. Indicated at the bottom are matching shifts in the virus- host interplay. Epithelial viruses are shown in the first panel, inner-body viruses in the second.
Inner-body viruses colonizing nerves and ganglia tend to persist in the individual host and evolve as host-specific. Viruses establishing in the reproductive organs system may transmit vertically. Viruses colonizing the immune and circulatory system may evolve immune-tolerance. The latter may permit sustained virus circulation in the bloodstream. For the fully internalized viruses, the virus environmental durability is redundant. For the arboviruses, virus- host co-evolution extends to the arthropod host ecology and behavior.
In brief, virus-host mutualism gains in prominence from outer to inner-body.
Presented in Figure 5 are examples, 20 in total, of viruses that have newly emerged in livestock over the past 150 years, shown in order of emergence. Also indicated are the virus organ system tropism, the livestock host category, and a chronological virus family line-up.
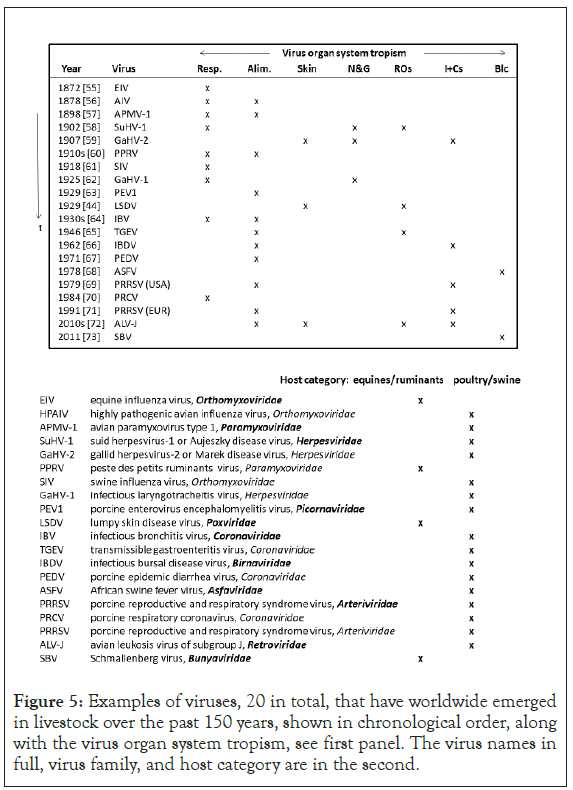
Figure 5: Examples of viruses, 20 in total, that have worldwide emerged in livestock over the past 150 years, shown in chronological order, along with the virus organ system tropism, see first panel. The virus names in full, virus family, and host category are in the second.
Respiratory and enteric virus transmission modes prevailed in all but four emergence events. The exceptions formed gallid herpesvirus-2 (GaHV-2) or Marek disease virus, shed from feather follicles, Lumpy Skin Disease Virus (LSDV), transmitting via biting flies, plus two arboviruses, African Swine Fever Virus (ASFV) and Schmallenberg virus (SBV). Poultry and pigs appeared as host in all but four disease emergence events. The exceptions formed Equine Influenza Virus (EIV), Peste des Petits Ruminants Virus (PPRV), and again LSDV and SBV. The virus family line-up is broadly identical to the Figure 1 line-up, with viruses of the Orthomyxoviridae and Paramyxoviridae families on top of the list.
Explored in Figure 6 is evidence for virus-host antagonism, again from outer to inner-body, organ system by organ system. As indicated in the first panel of Figure 6, an acute, generalized respiratory or enteric tract infection may result in profuse virus shedding, supporting swift onward transmission and translating in boom-and-bust disease dynamics. Instead, a generalized, persisting skin condition may support more continual virus shedding and transmission. Thus, from respiratory tract to alimentary tract to skin the disease dynamics change from epizootic to enzootic forms. Indicated in the second panel of Figure 6 are two examples of inner body-based pathology contributing to the overall virus transmission success.
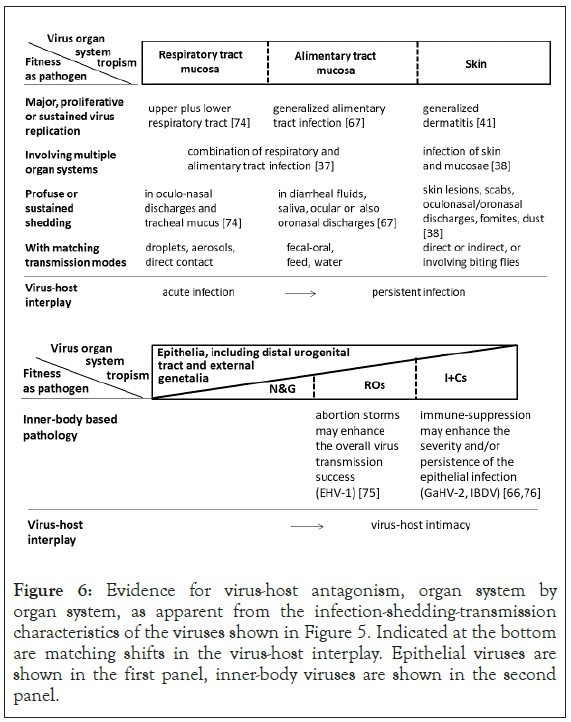
Figure 6: Evidence for virus-host antagonism, organ system by organ system, as apparent from the infection-shedding-transmission characteristics of the viruses shown in Figure 5. Indicated at the bottom are matching shifts in the virus-host interplay. Epithelial viruses are shown in the first panel, inner-body viruses are shown in the second panel.
In brief, virus-host antagonism increases from inner to outer-body. Presented in Figure 7 is the role of the host domain in mutualism-antagonism evolution. For this, the virus evolution trajectory is examined starting from a host shift from wildlife to humans or livestock. Epithelial viruses are contrasted to non-epithelial or fully internalized viruses. A-clinical, enteric viruses of wild birds or bats in humans and livestock evolve as a respiratory or also enteric pathogen. The new host may not be genetically related as the virus may jump from an avian to a mammal host. A-clinical, internalized wildlife viruses, in humans and livestock tend to evolve to return to the original, sylvatic virus trait profile. The new host tends to be genetically related. The virus evolution trajectory may be extensive [20-100].

Figure 7: The role of the host domain in mutualism-antagonism evolution. Explored are virus host shifts from wildlife to humans or livestock. Epithelial viruses are contrasted to non-epithelial, fully internalized viruses. As emerges from panel one and two, a-clinical enteric viruses of wildlife, in humans and livestock evolve as respiratory or also enteric pathogen. As emerges from panels one and three, a-clinical internalized viruses evolve to return to the sylvatic virus trait profile, very gradually so.
Presented in Figure 8 is a synthesis of the collective findings of this study. Revealed are typical outer to inner-body shifts in the virus-host relationship for the three main animal host domains of the world today; wildlife, humans, and livestock. Consistent with Figures 1 and 2, the evidence for evolution towards virus-host mutualism increases from outer to inner-body. Consistent with Figures 1 and 3, the evidence for virus-host antagonism increases from inner to outer-body. Respiratory and enteric viruses tend to evolve viral virulence in both the human and livestock host. Consistent with Figures 1 to 4, from the human to the livestock host domain, viral virulence gains in significance. Relatively mild respiratory viruses are common in the human host. In poultry and pigs, hyper-virulent respiratory and enteric viruses are common.
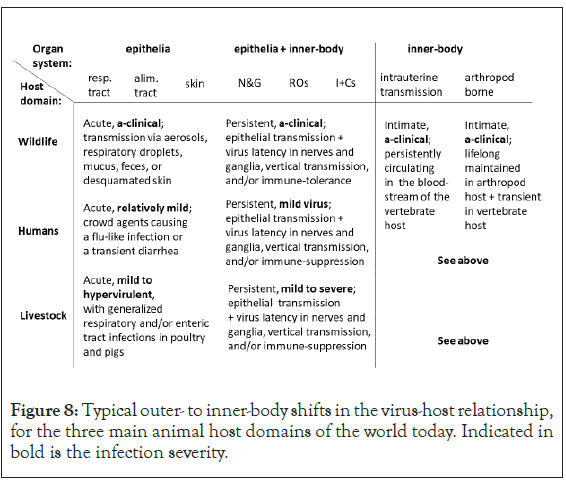
Figure 8: Typical outer- to inner-body shifts in the virus-host relationship, for the three main animal host domains of the world today. Indicated in bold is the infection severity.
The start of the evolution of human crowd agents arguably dates back to the Neolithic agricultural revolution [101], when humans settled on a more permanent basis in the fertile places around the world. The human populations gradually yet progressively increased in size. Humans and livestock frequently shared the same environment, facilitating an exchange of viruses between the two host domains. For example, the measles virus is believed to have evolved from the rinderpest virus between the 11th and 12th centuries [102], shifting from the enteric tract of bovines to the respiratory tract of humans. Both human and livestock populations continued to grow, eventually supporting pandemic and panzootic forms of disease. During the fourteenth century, Black Death, caused by the Yersinia pestis bacterium, ravaged virtually every civilization in the Old World, killing 75-200 million people [103]. In 1709, the Rinderpest Virus (RPV) started to cause devastating a panzootic [104]. Some 200 million cattle died of rinderpest in eighteenth century Europe. Between 1888 and 1897, rinderpest virus spread to sub-Saharan Africa, presumably for the first time, killing over 90 percent of the cattle and countless wildlife [105]. During the 1910s in Africa, the rinderpest virus evolved in small ruminants [106] (PPRV), transmitting primarily v via the respiratory route.
Over the past 150 years, coincident with the gradual emergence of global scale, intertwined, human, poultry and pig host contact networks [107] a suit of novel, initially highly damaging viruses emerged starting with Influenza type A viruses. Over time, the human, swine and equine Influenza type A viruses have all evolved as relatively mild. In general, human respiratory viruses tend to evolve as relatively mild. Apart from Influenza type C, B and A, also human respiratory coronaviruses, rhinoviruses, adenoviruses, and parainfluenza viruses cause mild infections [20]. The rationale is that a respiratory virus may gain in fitness by evolving subtle, swift transmission modes not provoking the host defenses, opening the door to continual, widespread virus circulation and build-up of an ever more diverse virus gene pool. Also, host radiation may become integral to virus fitness. The cold tolerance of the respiratory influenza viruses arguably stems from the fecal-oral cycle in the ancestral wild water-bird host breeding at higher latitudes in the Holarctic Region [40].
Distinctly different from the above described crowd agents are the hyper-virulent respiratory and enteric viruses evolving in poultry and pigs. Given the infinite supplies of susceptible hosts, modern poultry and pig husbandry evidently select for hyper-virulence, supporting boom-and-bust disease dynamics, including panzootics and, in case of a zoonotic virus, pandemics. In poultry and pigs, the combination of hyper-virulent and also less virulent viruses secures sustained global virus circulation, with new, aggressive viruses showing up haphazardly.
The chronological line-up of virus families broadly agrees with the line-up derived in Figure 1, indicating that the number of relatively deep-rooted poultry and pig viruses that are capable of performing a virulence jump may be finite. Modern poultry and pig mass rearing tends to select against evolution of deep-rooted viruses.
Finally, regarding the fully internalized viruses, which includes the arboviruses, the transmission ecology is again different. Whereas myxoviruses and also coronaviruses may continually adjust virulence levels to host availability, shift from enteric to respiratory tract, or also to a new host, the internalized viruses evolve an intimate, stable virus-host relationship. Upon being transferred to a new, genetically related, host, say as a result of ecological perturbation, the virus tends to eventually evolve to return to the trait profile observed in the sylvatic cycle. Projected on a long evolutionary timescale, there appears no incentive for an internalized virus to evolve pathogenic fitness.
Still, in the short to medium-term, anthropogenic, curious disease ecologies may result. For example, the African Swine Fever Virus (ASFV) originates from grassy woodland areas in sub-Saharan Africa where the virus circulates in Ornithodorus ticks and warthogs as host [52]. The ticks occasionally transmit the virus to domestic pigs. In the domestic cycle, swill feeding involving food scraps containing virus contaminated pig meat products explains how the virus is exported to new countries and continents. Today, in central parts of Europe at higher latitudes, the virus may overwinter in carcasses of wild boars that have succumbed of ASFV [108]. During spring, there may be an upsurge of the disease [108].
The collective results of this study corroborate the notion that in natural ecosystems friendly virus-host relationships tend to be selected for. Evolution of viral virulence and other forms of pathogenic fitness, including immune-suppression, primarily occur in animal mass rearing and so are anthropogenic in nature. The crowd agents circulating in humans as host take an intermediary position.
No external funding was used for this manuscript
The author JS declares NO competing interest.
The author is grateful to Marjan Leneman, Epke Le Rutte, Lenny Hogerwerf, Marius Gilbert, Jelle Bruinsma, Paul Van Bronswijk, Dorothea van Ooyen, and Marleen Slingenbergh for discussion.
Citation: Slingenbergh J (2021) Outer to Inner-body Shifts in the Virus-Host Relationship for the Three Main Animal Host Domains of the World Today: Wildlife, Humans, and Livestock. Immunome Res. 17:186.
Received: 11-Dec-2020 Accepted: 25-Dec-2020 Published: 01-Jan-2021
Copyright: © 2021 Slingenbergh J. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.