Journal of Leukemia
Open Access
ISSN: 2329-6917
ISSN: 2329-6917
Research Article - (2013) Volume 1, Issue 3
Keywords: Oral mucositis; Saliva biomarkers; Acute lymphoblastic leukemia; Children
Under normal conditions the saliva is saturated with calcium, magnesium and phosphorus ions that promote maintaining the integrity of the teeth and oral mucosa. The relationship between micronutrient ions in the saliva is particularly important to maintain proper functioning of the oral mucosa. The abnormalities in the secretion of saliva and changes in its composition in patients undergoing chemotherapy are often observed. This leads to pathological changes in the oral mucosa - mucositis. Oral mucositis is a common and difficult to treat complication during high-dose chemotherapy and radiotherapy followed by hematopoietic stem-cell support (HSCT) [1-4]. At present the condition is often referred to as mucosal barrier injury – MBI.
The aim of this study was to assess the impact of oral hygiene and the concentration of calcium, phosphate and magnesium ions in the saliva on oral mucositis in children with acute lymphoblastic leukaemia during anticancer therapy.
The study included 78 children aged from 2 to 18 yrs suffering from acute lymphoblastic leukaemia (ALL). In the study group, 5 children had leukaemia recurrence confirmed by the cerebrospinal fluid tests, 2 children had recurrence of leukaemia in the bone marrow, in 7 children the CNS was affected and 3 children had Down’s syndrome. The children with acute lymphoblastic leukaemia were examined in three stages: examination 1 – prior to chemotherapy; examination 2 – after the initiation of chemotherapy up to five months of treatment; examination 3 – from 6mths to 1.5 year of anti-cancer treatment. The children were treated according to the ALLIC BFM 2002 program.
Clinical dental examination was conducted by a dentist using basic diagnostic tools in artificial lightning.
Oral hygiene status was assessed using the Oral Hygiene Simplified Index (OHI-S Index), according to Greene and Vermillion, with the use of the Plaque Test.
In the group of patients with ALL, oral mucosa conditions were assessed clinically according to five-grade WHO classification of oral mucositis. Changes in the oral mucosa were monitored every day.
In the group of children examined unstimulated saliva was collected in the morning two hours after a meal to determine the concentration of calcium, phosphorus and magnesium ions. The samples of collected saliva were centrifuged for 15 min at 5,000 rot/min. The centrifuged saliva samples were frozen at -80°C, until the time of biochemical scores.
The concentration of calcium ions was determined by the colorimetric method of Arsenazo III at neutral pH, the complex was read at 660nm wavelength with the use of reagent kit Konelab™ CALCIUM, Thermo Electron Corporation.
The concentration of phosphorus ions was determined calorimetrically by the direct method without deproteinization by measuring UV absorption of the resulting phosphorus-molybdenum complex formed in the acidic environment/absorption at 340 nm wavelength is proportional to the concentration of phosphate ions in the material tested. The measurement was done using reagent kit Phosphorus UV BioMerieux.
The concentrations of magnesium ions were determined by colorimetric method without deproteinization, using calmagite as chromogen and the reagent kit Mg-Kit, BioMérieux.
Microelements were determined on a biochemical analyzer Konelab Kone Pro.
The results were analyzed statistically. The measurable parameters were presented as mean, Median, minimum and maximum values and standard deviation. Mann-Whitney U test was used to compare 2 independent groups, Spearman’s Rank Order Correlation test was used to analyze the relationship between two quantitative parameters; p<0.05 was assumed as statistically significant. The database and the statistical analyses were performed using STATISTICA 8.0 software (StatSoft, Poland).
Table 1 shows oral health status of children with acute lymphoblastic leukemia and generally healthy children (expressed as OHI-S index).
| Children | index | N | X | Me | SD | Mann-Whitney U Test (Z) | Significance level (p) |
|---|---|---|---|---|---|---|---|
| ALL examination 1 | OHI-S | 78 | 0.64 | 0.4 | 0.85 | 3.42736 | 0.00061* |
| ALL Examination 2 | 54 | 0.68 | 0.5 | 0.74 | 2.65201 | 0.00800* | |
| ALL examination 3 | 63 | 0.49 | 0.0 | 0.69 | 4.36259 | 0.00001* | |
| healthy | 78 | 0.98 | 1.0 | 0.70 | Test results compared to generally healthy children | ||
Table 1: Oral hygiene index (OHI-S) in children with acute lymphoblastic leukemia and in healthy children
In the group of children with acute lymphoblastic leukemia, the value of OHI-S index determined in examination 1 was 0.64 ± 0.85, in examination 2 it was 0.68 ± 0.74 and in examination 3 it was the lowest 0.49 ± 0.69. However, in healthy children the value of OHI-S index was the highest 0.98 ± 0.70. The comparison of OHI-S values found statistically significantly lower values in the children with acute lymphoblastic leukaemia compared to healthy controls, indicating better oral health in the group of children with acute lymphoblastic leukaemia. In the group of patients, oral hygiene status was worse in examination 2, however it was still better than in the group of healthy controls (Table 1).
Our results found lesions of the mucositis type in the children with acute lymphoblastic leukaemia which were detected after 48 hours to 6 months of chemotherapy; those were of varied intensity with periods without any pathological changes, which was related to the intensity of chemotherapy. Mucosal opacity followed by redness usually occurred within 2-4 days from the Methotrexate infusion. Most intensive oral mucosal lesions developed over the first month of chemotherapy. Changes in the oral mucosa were of different severity. The following pathologies were observed: localized erythema of the mucosa (Grade 1) in 35% children, pseudomembraneous mucosa (Grade 2) in 18% children, ulcers with extensive erythema (Grade 3) in 40% children, massive mucosal ulcers and tissue necrosis (Grade 4) in 4% patients. Wounds and difficult to heal ulcers were related to poor blood parameters. It was observed that healing was faster, especially with regard to oral mucosa ulceration, when blood morphological parameters improved. The lesions of mucositis type were neutropeniadependent. Each child with neutropenia had fungal complications in the oral mucosa. Generally, no lesions were observed in the periods between subsequent protocols. After 6 months of chemotherapy, oral mucosa lesions were less intense and were observed in 3.17% of the children examined. Redness and erosions were the most frequent. No ulcers in the oral cavity were observed.
Tables 2-4 present the concentrations of calcium, phosphorus and magnesium ions in the saliva of children with acute lymphoblastic leukaemia and the control group.
| Children | Ca2+ 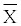 |
Me | Min. | Max | SD | Mann-Whitney U Test (Z) | Significance level (p) |
|---|---|---|---|---|---|---|---|
| ALL examination 1 | 1.7 | 1.2 | 0.59 | 9.94 | 1.99 | -0.0144 | 0.9885 |
| ALL Examination 2 | 1.4 | 1.2 | 0.62 | 5.77 | 0.78 | -0.0792 | 0.9369 |
| ALL examination 3 | 1.3 | 1.0 | 0.56 | 4.06 | 0.75 | 1.8279 | 0.0676 |
| Healthy | 1.4 | 1.2 | 0.33 | 4.25 | 0.75 | Test results compared to generally healthy children | |
Table 2: The concentration of calcium ions in the saliva of children with acute lymphoblastic leukemia and in healthy children (mmol/l).
| Children | P3+ 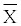 |
Me | Min. | Max | SD | Mann-Whitney U Test (Z) | Significance level (p) |
|---|---|---|---|---|---|---|---|
| ALL examination 1 | 7.6 | 3.8 | 1.66 | 31.28 | 7.78 | 3.3617 | 0.0008* |
| ALL Examination 2 | 11.7 | 4.4 | 2.00 | 38.40 | 11.07 | 1.6391 | 0.1012 |
| ALL examination 3 | 10.4 | 3.8 | 0.58 | 38.88 | 11.19 | 3.0167 | 0.0025* |
| Healthy | 14.4 | 18.6 | 2.01 | 35.32 | 9.61 | Test results compared to generally healthy children | |
Table 3: The concentration of phosphorus ions in the saliva of children with acute lymphoblastic leukemia and in healthy children (mmol/l).
| Children | Mg2+ 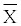 |
Me | Min. | Max | SD | Mann-Whitney U Test (Z) | Significance level (p) |
|---|---|---|---|---|---|---|---|
| ALL examination 1 | 0.2 | 0.2 | 0.02 | 1.00 | 0.17 | -1.2662 | 0.2054 |
| ALL Examination 2 | 0.3 | 0.2 | 0.01 | 1.87 | 0.34 | -2.3409 | 0.0192* |
| ALL examination 3 | 0.2 | 0.2 | 0.02 | 1.07 | 0.23 | -1.5888 | 0.1121 |
| Healthy | 0.2 | 0.1 | 0.03 | 0.97 | 0.16 | Test results compared to generally healthy children | |
Table 4: The concentration of magnesium ions in the saliva of children with acute lymphoblastic leukemia and in healthy children (in mmol/l).
Biochemical studies found that the concentration of calcium ions in the saliva of patients with acute lymphoblastic leukemia decreased during anticancer therapy. The mean value of the calcium ion concentration in the saliva of children with acute lymphoblastic leukaemia in examination 1 was 1.7 ± 1.99 mmol/l, in examination 2 it was reduced to the value of 1.4 ± 0.78 mmol/l, and the lowest value was observed in examination 3 - 1.3 ± 0.75 mmol/l The average concentration of calcium in the saliva of children with acute lymphoblastic leukaemia in examination 3 was lower compared with healthy children, and the difference was close to statistical significance (p=0.0676) (Table 2).
The results of biochemical tests showed that the concentration of phosphorus ions in the saliva of patients with acute lymphoblastic leukemia before chemotherapy was significantly lower compared to the controls (p < 0.0001, Table 3). In examinations 2 the concentration of phosphorus ions in the saliva of children with acute lymphoblastic leukaemia was increased, but compared to otherwise healthy children the values were still significantly lower (p < 0.005) (Table 3).
The analysis of examination 1 results revealed that worsened oral hygiene was related to decreased concentration of phosphorus ions in the saliva of children with acute lymphoblastic leukaemia (Spearman rank correlations R=-0.303; t=-1.93, p=0.06; Figure 1).
Biochemical the research showed that the concentration of magnesium ions in the saliva of patients with acute lymphoblastic leukemia in the period from 1 to 5 months of chemotherapy was significantly higher compared to the control group (p = 0.0192, Table 4).
In recent years, the saliva has been more and more often used to evaluate biomarkers in different and sometimes serious diseases. Due to non-invasive character of the method to obtain material for study, saliva tests have become a good method to diagnose various oral 5 pathologies as they enable the evaluation of the effects of treatment, and monitoring of patients after treatment with minimal discomfort for both patient and doctor [5,6].
Abnormal secretion of saliva is often observed in the patients on chemo-and radiotherapy. In case of salivary gland dysfunction, the majority of protective properties of the saliva disappear. However, the researchers agree that the saliva saturated with calcium and phosphorus ions promotes remineralization of the tooth enamel [7]. Ca2+ ions are involved in changes of cell membrane potential which affects the enzymatic pathways of many metabolic processes that lead to the production of fibrin [8,9]. Moreover, Ca2+ ions play a very important role in tissue repair and regulation mechanisms by being an essential part of the intracellular signal transduction system that brings about cell growth and metabolism [10].
The most common complaints of patients who suffer from damage to the mucosal barrier include burning and dryness of the mouth, difficulty speaking and eating food, and altered taste. Using an oral solution containing supersaturated calcium phosphate, e.g. Caphosol, is an option to treat or alleviate such symptoms, as it can reduce the frequency, severity and duration of oral mucositis [11-13]. Supersaturated calcium phosphate reduces the frequency, intensity and duration of oral mucositis in patients undergoing HSCT [12]. It might be related to leveling serum Ca2+ ions and phosphate ions in the saliva of patients undergoing chemotherapy, which needs further examination though.
Moreover, the scientists who studied various microelement concentrations in the saliva in other diseases, observed oral pathologies too. Bloniarz et al. [14] found higher concentrations of calcium, magnesium and sodium in the saliva of patients with oral carcinoma planoepitheliale spinocellulare [14]. Brik et al. [15] demonstrated that patients with oligoarticular inflammations had mean concentration of calcium and phosphate in the saliva by 25% lower compared to the patients with systemic inflammation of polyarthritis and control patients. The concentrations of Mg ions, total protein and α-amylase in the saliva in the patients with oligoarticular inflammations were lowered in comparison to the patients in the control group [15].
Inadequate oral hygiene may lead to oral mucosal inflammations. They pose significant problems in patients undergoing chemotherapy as oral lesions are painful, cause ulcerations and eventually result in poor nutrition, underhydration, and may create life threat eventually. Complications that develop within the oral cavity are likely to prolong treatment time and increase its costs too [3,16-20].
Patients on chemotherapy are recommended to change their oral hygiene habits, which are often improper, to maintain good oral hygiene. Doctors agree that regular tooth brushing, two times daily at the least, the application of oral rinses and effective motivating patient to thoroughly brush all tooth surfaces and clean the soft tissues surrounding them are of utmost importance to lower the risk of developing complications within the oral cavity. Adequate oral hygiene helps avoid many adverse side effects, particularly in patients with lowered immunity and patients undergoing anticancer treatment certainly belong to the group of high risk [4,21-25].
Oral hygiene status and lowered concentrations of calcium, phosphate and magnesium ions in the saliva in children with ALL during chemotherapy may affect the intensity the pathological changes in the oral mucosa in that group of patients.