Medicinal & Aromatic Plants
Open Access
ISSN: 2167-0412
ISSN: 2167-0412
Research Article - (2020)Volume 9, Issue 2
Centella asiatica is an herbaceous plant commonly known as Gotu Kola and belongs to Apiaceae family. It is found in most tropical and subtropical countries growing in swampy areas. It is a tasteless, odourless plant and it is traditionally used for the treatment of a wide variety of disorders. Its leaves and roots are used as vegetables and for medicinal purposes. Knowledge of their contributions to human nutrition and contents of bioactive components is lacking and has limited their use. Therefore this study evaluated the Nutrients content and phytochemical composition of Centella asiatica leaves using standard methods. The result of proximate composition revealed moisture (13.10 ± 1.07%), ash (16.5 ± 0.45%), protein (8.35 ± 1.28%), lipid (1.20 ± 0.10%), fiber (17.00 ± 1.87%) and carbohydrate (43.81 ± 0.70%) contents. Physicochemical result revealed Saponification value of 238.43 mg/KOH. Fatty acid composition revealed a high concentration of palmitic acid (55.70%) as saturated and Linoleic acid (17.50%) as unsaturated fatty acids; while amino acid composition showed high level of glutamate (13.389 g/100 g) as nonessential and Histidine (11.64 g/100 g) as essential amino acids respectively. The phytochemical composition revealed the presence of bioactive compounds such as; Proanthocyanin (11.964 μg/g), Rutin (11.8883 μg/g), Nanngenin (3.0122 μg/g),Quinine (10.4490 μg/g), Flav-3-ol(2.5900 μg/g), Spartein (3.0122 μg/g), Phenol (18.8713 μg/g), Flavonones (2.1836 μg/g), Steroids (18.8974 μg/g), Kaempferol (0.7273 μg/g), Phytate (1.6851 μg/g), Naringenin (2.7523 μg/g), Resveratol (10.8596 μg/g), Tannin (4.4377 μg/g) and Ribalinidine (3.0500 μg/g). The presence of these nutrients and bioactive phytochemicals in Centella asiatica leaves makes them useful in pharmaceutical and food industries.
Nutrients; Phytochemical composition; Centella asiatica; Leaves
In recent years, there has been an increased effort to find food and beverages with high nutrients and health-promoting properties. Herbs, traditionally used in folk medicine, have attracted consumer interest because of their long historical consumption and ready acceptability [1]. Plants are known to provide food, clothing, shelter and medicine [2]. Centella asiatica commonly called Gotu Kola or pennywort is a herbaceous, frost tender perennial plant in the flowering plants family called Apiaceae. It is native to India and other parts of Asia such as China, Sri Lanka, Nepal and Madagascar and has been utilized in folk medicine in different countries [3]. Centella asiatica has been reported as being used for the treatment of varicose veins, chronic venous insufficiency, psoriasis, minor wounds, analgesic and anti-inflammatory agent [4]. Centella asiatica is widely available in Madagascar and is commonly used as tea, soft drink and syrup [5].
It is important to note that natural food and food ingredients from plants have been proved to be safer and healthier than synthetic ones [6]. Living things generally, require a number of organic compounds for growth and development. Plant materials contain most of the needed micro and macro nutrients in addition to water [7]. Most regions in Africa depend on starchbased foods; thus protein deficiency is generally found among the population. To alleviate the situation, efforts are being made to explore the lesser known wild edible plants as sources of nutrient supplements. Increasing the utilization of wild edible herbs in our diet may increase the nutrients as well as dietary fiber and can be a food-based approach to ensuring the intake of these nutrients [6]. Gupta et al. [8] have emphasized that analyzing such plants for various nutrients would enable identification of unconventional food resources.
The medicinal properties of plants like antidiabetic, antimicrobial, antidiuretic, antioxidants, etc. are mainly dependent on the type, nature and concentration of secondary metabolites present. Reports have shown that some of the most important bioactive compounds are phytochemicals such as alkaloids, saponins, flavonoids, tannins, sterols and phenolic compounds [9,10]. These phytochemicals are plant derived metabolites naturally occurring in medicinal plant leaves, stem and roots where they are used as defence mechanism to protect the plant from various diseases. The phytochemicals are secondary metabolites that exhibit various important pharmacological activities [10]. Centella asiatica is utilized traditionally as medicine in the treatment of diverse ailments. However, the scientific knowledge on its nutrient content and chemical constituents is grossly inadequate. Hence, this research investigated the nutrient and phytochemical composition of the leaves of Centella asiatica.
Collection of plant material and identification
Centella asiatica (Gotu kola) leaves were collected from the surroundings of Bonny Island in Rivers State, Nigeria. The plant material was identified in the Department of Plant science and Biotechnology in the University of Port-Harcourt by Dr. Chimezie Ekeke.
Preparation of plant sample
The leaves were sorted, cleaned and dried at room temperature for 14 days. The dried leaves were pulverized with electric blender, transferred into an airtight container and labelled for future use.
Determination of proximate composition
The proximate composition (moisture, ash, protein, fat, fiber and carbohydrate) of the Centella asiatica leaves was performed using Association of Official Analytical Chemists methods [11]. Moisture content of the sample was determined by air drying at room temperature (25-28°C) for 14 days. The crude protein was determined using micro-Kjeldahl method. Crude lipid was determined by Soxhlet extraction method. The ash content was determined using muffle furnace set at 550°C for 4 hours until constant weight of ash was obtained. Crude fiber was determined according to the method of Saura-Calixto [12]. The carbohydrate content was obtained by difference.
Determination of physicochemical properties
Physicochemical parameters of Centella asiatica leaves such as refractive index, density, viscosity, acid value, peroxide value, iodine value, saponification value, free fatty acid and thiobarbituric acid were estimated by AOAC method [13].
Determination of fatty acid composition
The fatty acid composition was determined by GC/MS. Powdered sample (10g) was subjected to soxhlet extraction with 300 mL of n-hexane for 24 hours, and then evaporated to dryness using a rotary evaporator at 40°C. Sample was prepared by dissolving 1 mL of filtered residue in 50 mL chloroform and evaporated at room temperature; followed by the addition of 1 mL of reagent (benzene and methanol 20: 55 Vol %) and sample heated for 30 minutes at 40°C. Organic sample was extracted with hexane and taken for GC analysis.
Determination of amino acid composition
Sample weighing 0.1 g was hydrolysed with 6N HCl at 110°C for 24 hours. After hydrolysis, the contents were vacuum filtered (using Whatman #541, Maidstone, England). Sample derivatization procedure was done by a modification of the method of Elkin et al. [14] by adding 30 μL of methanol-waterphenyl isocyanate (2: 2: 1 v/v) agitating and then removing the solvents under nitrogen stream. Chromatographic procedure was evaluated using gradient elution: Eluent A was aqueous buffer of 0.5 mL/L triethylamine and 0.14 M sodium acetate titrated with glacial acetic acid to pH 6.20 while Eluent B was acetonitrile-water (60: 40 v/v).
GC-FID Identification and quantification of phytochemical constituents
For the GC-FID analysis, 1g of the powdered Centella asiatica sample was weighed and transferred into a test tube. Exactly 15 mL of ethanol and 10 mL of 50% w/v potassium hydroxide were added to the sample in the test tube. The test tube was allowed to stand in a water bath at 60°C for 60 minutes. Then the content of the test tube was carefully transferred into a separatory funnel and the tube rinsed into the same funnel with 10 mL of cold water, 10 mL of hot water, 20 mL of ethanol and 3 mL of hexane. The extract in the test tube was washed three times with 10 mL of 10% v/v ethanol solution. The extract solution was then dried with anhydrous sodium sulphate and the solvent was evaporated. A sample of the extract was then made soluble in 100 μL of pyridine of which 20 μL was transferred into a vial on the Gas Chromatography machine for phytochemical analysis. The GC-FID phytochemical analysis was performed on a BUCK M910 Gas Chromatograph (GC) (BUCK Scientific, USA), equipped with a flame ionization detector (FID) [15].
Statistical Package for Social Sciences (SPSS) version 22 was used to process and analyze the data obtained. Values were expressed as means ± standard error mean (SEM).
Results of the proximate composition of Centella asiatica leaves are contained in Table 1. Carbohydrate (43.81 ± 0.70%) was highest followed by crude fiber (17.00 ± 1.87%) and ash (16.55 ± 0.45%) contents.
| Proximate Compositions | Concentration (%) |
|---|---|
| Moisture | 13.10 ± 1.07 |
| Ash | 16.55 ± 0.45 |
| Protein | 8.35 ± 1.28 |
| Lipid | 1.20 ± 0.10 |
| Fiber | 17.00 ± 1.87 |
| Carbohydrate | 43.81 ± 0.70 |
Table 1: Proximate composition of Centella asiatica leaves.
Results of the physicochemical properties of the Centella asiatica leaves are presented in Table 2. The saponification value was highest (238.43 mg/kOH) while the free fatty acid content had the least value (0.56%).
| Parameters | Concentration |
|---|---|
| Saponification value (mg/KOH) | 236.43 ± 2.13 |
| Peroxide value (mEq/kg) | 26.80 ± 0.13 |
| Acid value (%) | 1.12 ± 0.10 |
| Free fatty acid (%) | 0.56 ± 0.01 |
| Iodine value (g I2/100 g) | 62.60 ± 1.07 |
| Refractive index(at 40°C) | 1.42 ± 0.11 |
| Viscosity (Pa.S) | 0.99 ± 0.00 |
| Density (g/mL) | 0.97 ± 0.04 |
| Thiobarbituric acid (mg/kg) | 1.92 ± 0.05 |
Table 2: Physicochemical composition of Centella asiatica leaves.
The fatty acid composition of Centella asiatica leaves is shown in Table 3. The leaves contained a considerable amount of fatty acids with 78.48% saturated and 21.53% unsaturated. The predominant fatty acids are palmitic acid (55.70%), linoleic acid (17.50%) and lauric acid (13.73%). Whereas the highest saturated fatty acid was palmitic acid (55.70%), linolenic acid (17.50%) was the highest unsaturated fatty acid. Myristic acid was found in trace amount of 0.50%.
| Fatty acid | Concentration (%) |
|---|---|
| Saturated fatty acids | |
| Myristic acid (C14) | 0.50 |
| Palmitic acid (C16) | 55.70 |
| Stearic acid (C18) | 8.55 |
| Lauric acid (C12) | 13.73 |
| Total | 78.48 |
| Unsaturated fatty acids | |
| Oleic acid (C18: 1) | ND |
| Arachidonic acid (C20: 4) | ND |
| Arachidic acid (C20) | ND |
| Linolenic acid (C18: 3) | 4.03 |
| Linoleic acid (C18: 2) | 17.50 |
| Total | 21.53 |
Table 3: Fatty acid composition of Centella asiatica leaves.
The amino acid composition of Centella asiatica leaves is contained in Table 4. The results indicated high levels of the glutamate (13.389 g/100 g), Histidine (11.64 g/100 g), Lysine (9.72 g/100 g), Isoleucine (9.56 g/100 g), Aspartate (9.37 g/100 g) and phenylalanine (8.49 g/100 g). The results also showed higher amount of essential amino acids compared to the nonessential amino acids.
| Amino acid | Concentration (g/100 g) |
|---|---|
| Valine | 4.48 |
| Threonine | 3.68 |
| Isoleucine | 9.56 |
| Leucine | 6.48 |
| Lysine | 9.72 |
| Methionine | 1.50 |
| Tryptophan | 1.12 |
| Phenylalanine | 8.49 |
| Histidine | 11.64 |
| Glycine | 3.79 |
| Alanine | 4.68 |
| Serine | 5.88 |
| Proline | 3.07 |
| Aspartate | 9.37 |
| Tyrosine | 7.79 |
| Cysteine | 1.42 |
| Glutamate | 13.39 |
| Arginine | 6.55 |
Table 4: Amino acid composition of Centella asiatica leaves.
Gas chromatogram of the ethanol extract of Centella asiatica leaves is presented in Figure 1. Results of the quantitative analysis revealed the presence of steroids (18.90 μg), phenols (18.87 μg), proanthocyanin (11.96 μg) and rutin (11.89 μg), while kampferol (0.73 μg) had the lowest value (Figure 2).
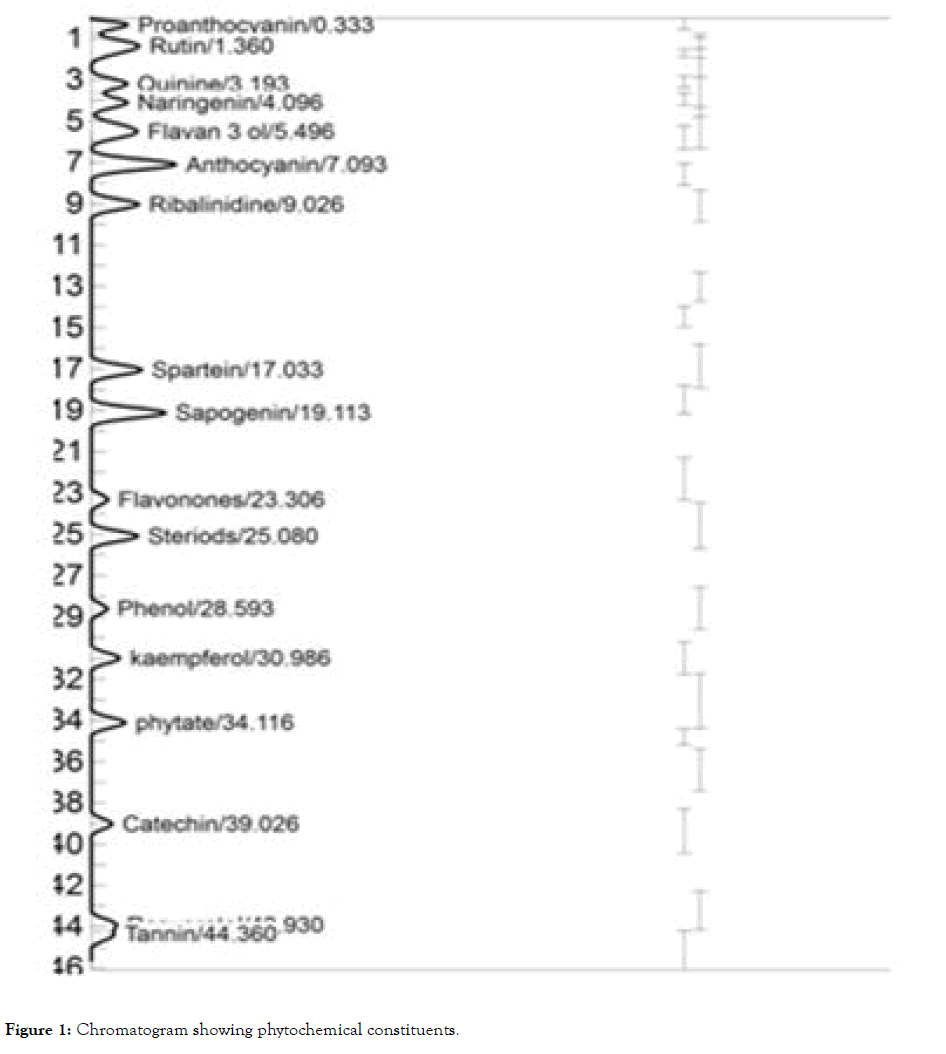
Figure 1: Chromatogram showing phytochemical constituents.
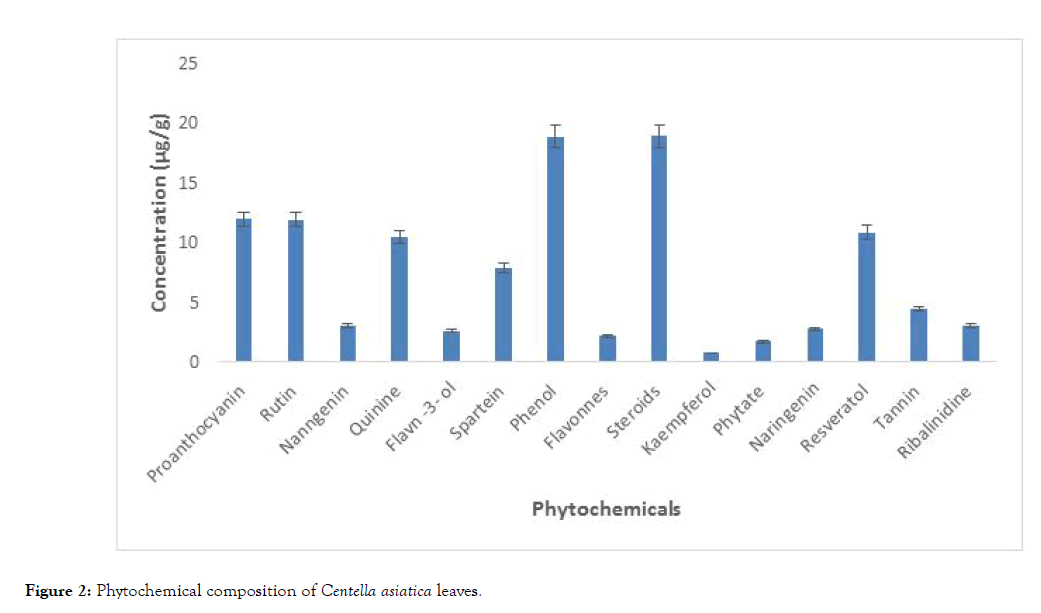
Figure 2: Phytochemical composition of Centella asiatica leaves.
The proximate analysis data indicated that C. asiatica leaves contained an appreciable amount of carbohydrate, crude fiber and ash. The result suggests that carbohydrate is the major nutrient contained in the leaves. The high content of carbohydrate and ash recorded in this study agree with the report by Mertz et al. [16] on different species of Centella asiatica grown in Madagascar with carbohydrate content ranging from 42.9-52.0% and ash content ranging from 13.5-16.4%. Ogunka- Nnoka and Nwabueze, [17] and Gopalan et al. [18] reported carbohydrate content of 38.48% in Jatropha tanjorensis leaf-stalk and 20.0 to 66.8% carbohydrate in some conventional Indian leafy vegetables respectively. Carbohydrates provide the body with the necessary energy required to drive cellular metabolism as well as a raw material for many industries. The high ash content is an indication of good sources of minerals for human nutrition. The substantial amount of fiber shows that the leaves of Centella asiatica can help in keeping the digestive system healthy by removing potential toxicants from the body and prevents the absorption of excess cholesterol. It also adds bulk to the diet and prevents the intake of excess starchy foods [19]. There were variations in the amount of fiber, ash, protein and moisture contents of the present study when compared to the reports of Mertz et al. [16].
The physicochemical properties of the Centella asiatica leaves revealed high saponification, iodine and peroxide values. The saponification value in this study was higher than the value for Ipomoea involucrata leaf (196.50 mg KOH/g) and Conarium indicum nut oil (175.47 mg KOH/g) but comparable to coconut oil (246.28 mg KOH/g) as reported by Opene et al. [19], Rehman et al. [20] and Rahman et al. [21] respectively. Oils with this level of saponification value will serve useful purpose in the soap making industries [22]. The iodine value of oil is a measure of its unsaturation and is a useful criterion for purity and identification. The oil from Centella asiatica is regarded as nondrying because the iodine value is below 100 I2/100 g [21]. The low peroxide value of Centella asiatica oil (Table 2) indicates that the oil is stable. The low free fatty acid content is indicative of the low enzymatic hydrolysis; this could be advantageous as oil high free fatty acid develops off flavour during storage. Free fatty acid and peroxide values are a valuable measure of the oil quality [23]. The refractive index of oils is an important optical parameter that analyzes the light rays traversing through materials medium and can be used as a tool to determine adulteration of oils [24].
The major saturated fatty acids in Centella asiatica oil were palmitic and lauric acids and the predominant unsaturated fatty acid was linoleic acid followed by linolenic acid. Palmitic acid was the most abundant saturated fatty acid found in the leaves of Centella asiatica, this corresponds to what was reported for leaf- stalk of Jatropha tanjorensis [17]; although the value reported for Jatropha tanjorensis was lower than that of the Present study.
Palmitic acid has been reported to increase cholesterol level [25]. On the contrary, Davis, [26] demonstrated that palmitic acid has no hypercholesterolemic effect if the intake of linolenic acid is greater than 4.5% of energy. Lauric acid as the next predominant saturated fatty acid is effective in preventing tooth decay [27]. Linoleic fatty acid (omega-6) is used for reducing the risk of heart disease, lowering total cholesterol levels, low density lipoprotein level, raising High density lipoprotein levels and reducing cancer risk in humans [28].
Amino acids are building blocks of proteins in muscle fibers and other structures in the body. They help to transport nutrients, prevent illness and perform other functions. Its deficiency can result in decreased immunity, digestive problems, depression, slowed growth in children and many other health issues [29].
The amino acid profile of the leaves of Centella asiatica showed ten essential amino acids including histidine and arginine and eight non-essential amino acids. The highest essential amino acid found was histidine followed by lysine, Isoleucine and phenylalanine; while glutamate and aspartate were recorded as highest in the nonessential amino acids category (Table 4). The results obtained in the present study were lower compared to the values obtained for these amino acids as reported by Mertz et al. [16] for the Centella asiatica from Madagascar; but higher than the values (except for glutamate) obtained by Ogunka-Nnoka and Nwabueze, [17] for leaf-stalk of Jatropha tanjorensis. Histidine facilitates growth, the creation of blood cells and tissue repair. It also helps to maintain the special covering over nerve cells, which is called the myelin sheath [29]. Lysine plays a vital role in building muscle, maintaining bone strength, aiding recovery from injury and regulating hormones, antibodies and enzymes. It may also have antiviral effects [30]. Isoleucine helps in wound healing, immunity, blood sugar regulation and hormone production [31]. Phenylalanine helps the body to use other amino acids as well as proteins and enzymes. It is needed in treating brain disorder and the normal functioning of the central nervous system. Its deficiency can lead to poor weight gain [32]. Glutamate as a nonessential amino acid plays critical roles in nutrition metabolism and signalling. Post-translational carboxylation of glutamyl residues increases their affinity for calcium and plays a major role in haemostasis [33]. Aspartate was found to be very important in the functioning of RNA and DNA, as well as in the production of immunoglobulin and antibody synthesis. It also helps the body to promote a robust metabolism and in the treatment of depression and fatigue [34]. The leaves of Centella asiatica may serve as nutritional supplement in addressing nutritional and health related issues.
Phytochemical analysis is very useful in the evaluation of active biological components of plants with medicinal value. Steroids, phenols, proanthocyanin and rutin were high in the Centella asiatica leaves compared to the other phytochemical components (Figure 2). Steroids are important in wound recovery; improve athlete’s performance and other health promoting effects. Prolonged intake of steroids can lead to risk of liver and kidney diseases as well as prostate cancer, high blood pressure and elevated levels of cholesterol leading to heart disease [34]. Phenolic compounds can be a major determinant of antioxidant potentials of food plants and therefore, a natural source of antioxidants [35]. Due to its antioxidant property, phenols have the ability to scavenge free radicals and neutralise its effect thereby preventing cardiovascular diseases and cancer [36]. Rutin a flavonoid has been shown to have anti-ulcer, antibacterial, antiviral, and anti-thrombosis activities [34]. Kaempferol as an alkaloid has been shown to have anti-ulcer, anti-inflammatory, antiviral and anti-cancer activities. Alkaloids generally play some important metabolic role in living organisms, causing some physiological changes and are involved in protective function in animals [37]. The presence of these phytochemicals in Centella asiatica leaves shows that it could serve important physiological function with good nutritional and therapeutic value.
The result of the present investigation revealed that Centella asiatica leaves are rich sources of nutrients such as carbohydrate, crude fiber, ash and proteins. Most of the amino acids found were essential amino acids while the physiochemical properties revealed high saponification value and stability to rancidity. The leaves are rich in bioactive components that possess wide range of biological activity and therapeutic value.
Authors have no conflict of interest.
Citation: Ogunka-Nnoka CU, Igwe FU, Agwu J, Peter OJ, Wolugbom PH (2020) Nutrient and Phytochemical Composition of Centella asiatica Leaves. Med Aromat Plants (Los Angeles) 9: 346. doi: 10.35248/2167-0412.20.9.346
Received: 17-Apr-2020 Accepted: 25-Apr-2020 Published: 30-Apr-2020 , DOI: 10.35248/2167-0412.20.9.346
Copyright: This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.