Journal of Pharmaceutical Care & Health Systems
Open Access
ISSN: 2376-0419
ISSN: 2376-0419
Research Article - (2022)Volume 9, Issue 1
We report a successful formulation of (CEF) ion paired Solid Lipid Nanoparticle (SLN) with a demonstrated improvement of the intestinal membrane permeability up to 41 folds. Cefepime is a parentally administered fourthgeneration cephalosporin zwitterion. The class 3 BCS drug has high water solubility and low permeability, which result in low oral bioavailability. Our goal is to overcome the low oral bioavailability challenge using double techniques, a molecular alteration ion pairing, and a Nano carrier formulation approaches.
The CEF ion pair was prepared by freeze-drying dispersions of an anionic lipid, Sodium Stearate (NaSA), with CEF. Then a combination of Precirol ATO ® 5 and Compritol 888 ATO ® lipids was used in preparing the SLNs using the microemulsion-ultrasonication technique. The enhanced drug permeability was confirmed using an ex vivo rat intestine in a side-by-side chamber.
The IR and Raman spectra confirmed the formation of the ion pair. The ex vivo studies showed a significant increase in the permeability and the percentage of drug transported of the encapsulated ion paired CEF compared to the unencapsulated control CEF by approximately 13 and 41 folds respectively. We thus concluded that SLN is a propitious formulation for improving the CEF oral bioavailability and that the CEF-stearate ion pair can further enhance the CEF SLN encapsulation and intestinal permeability.
Cefepime; Oral delivery; Solid lipid nanoparticles; Ion pair
With the antibiotics' cross-resistance and the emerging allergies among different classes, there are increasingly restricted antibiotics treatment options, and physicians are left with limited choices of antibiotics. If an effective antibiotic is only available as injectable, an inevitable increase in patient risk and healthcare costs is expected, which invokes another challenge.
Oral administration of therapeutic agents has many advantages such as relative safety, patients' compliance, and ease of selfadministration. Cefepime is a fourth-generation cephalosporin. It presents a challenge in oral absorption due to its physicochemical properties and thus only available as a parenteral dosage form [1].
The goal of the study was to design a CEF effective oral delivery system. Cefepime is a zwitterion ion with high water solubility and poor lipid permeability, all of which result in very poor oral bioavailability (Figure 1).
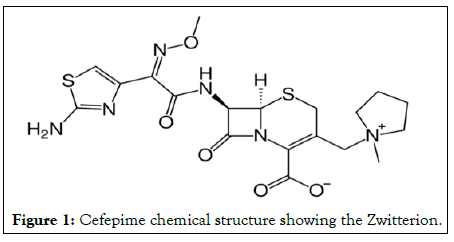
Figure 1: Cefepime chemical structure showing the Zwitterion.
Based on the Biopharmaceutical Classification System (BCS), it is classified as a class 3 drug. Our formulation strategy was first to neutralize the CEF positive charge, using a negatively charged lipid molecule to create a counter ion pair, to facilitate the intestinal membrane permeability and improve the drug SLN incorporated and second to encapsulate the charged drug molecules into lipid carrier to enhance its membrane permeability (Figure 2) [2].
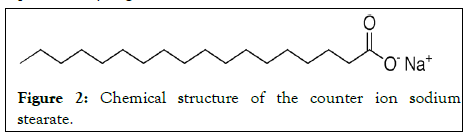
Figure 2: Chemical structure of the counter ion sodium stearate.
The use of lipid-based systems for oral delivery formulation design is advancing. This, for the most part, is due to its ability to enhance oral bioavailability and reduce plasma profile variability.
In addition, formulation versatility and lipid excipients in lipidbased systems are better characterized, resulting in a more likely than before successful scale-up [3,4]. The Intestinal Lymphatic System (ILS), the major route of lipid absorption, has been studied and used for site-specific oral absorption of peptides, proteins, drugs, vaccines, and poorly soluble drugs. Many mechanisms of oral delivery of therapeutic agents to or through lymphatic circulation have been studied and evaluated. These include par cellular mechanism, exploitation of M cells for vaccine delivery, and Tran’s cellular mechanism of the drug and Nano carrier absorption [5,6]. Various lipid formulations have also been studied, especially SLNs, and exploited as potential carriers for drugs through intestinal lymphatic delivery. Another important SLN feature is their stability at gastric physiologic pH [7].
Cefepime HCl was purchased from Tecoland Corporation. Compritol 888 ATO ® and Precirol ® ATO 5 were generously supplied by Gattefosse USA. Sodium stearate was purchased from Alfa Aesar. Simulated gastric fluid, simulated intestinal fluid was purchased from Ricca Chemicals; mammalian ringer solution from electron microscopy sciences; Cremophor from Sigma-Aldrich. Intestinal tissues used in this study were excised from animals purchased from Charles River Laboratory. All other chemicals and reagents were of analytical grade unless otherwise specified.
Preparation of CEF-ion paired loaded SLNs
NaSA-CEF ion pairing: The NaSA-CEF ion pair was prepared by hydrophobic ion-pairing technique performed by dissolving 8.2, 16.3, or 24.5 mg of NaSA in 5 ml water at 80°C. Enough CEF was then added to create a 2.5 mg/ml final mixture concentration. The prepared CEF-sodium stearate ion pair dispersions’ molar ratios were 1:1, 1:2, and 1:3 of CEF:NaSA. The dispersions were frozen at −75°C for 12 hr. then dried for 96 hr. cycle using free zone triad cascade benchtop freeze dry system from labconco. The freeze-dried NaSA-CEF ion pair was further characterized and used in the preparation of SLN.
Preparation of SLNs: Two CEF-loaded SLNs formulations were prepared in a total volume of 10 ml. The formulations had either 5% or 7% total lipid content. The 5% CEF-SLNs were prepared by melting 0.5 g of PA5 lipid at 70°C. As of the 7% CEF-SLNs, 0.5 g of PA5 is melted with 0.2g of C8A lipids at 80°C in a scintillation vial. Then 25 mg of CEF were dispersed in the above-melted lipids under magnetic stirring at 600 rpm for each formulation.
In a separate vial, 0.2 ml of a cremaphore was mixed with 9.3 ml or 9.1 ml of water to the 5% CEF-SLNs and 7% CEF-SLNs respectively. The surfactant mixture was preheated to 70°C then added drop-wise to the lipid-drug dispersions. Each mixture was solicited using Qsonica ultrasonic micro tip processor for 5 minutes. The nanoemulsions were left to cool under gentle shaking for 3 minutes. The method was adopted from few adjusted parameters [8].
The NaSA-CEF-SLNs were prepared using W/O/W emulsions by ultra-sonication. In Table 1 summarize the composition of the three studied optimized formulations.
Spectroscopic analysis of ion-pairing NaSA-CEFSLNs
The Raman and IR spectroscopy were used to confirm the NaSA-CEF ion pairing interaction.
Raman spectroscopy analysis: The Raman spectra of CEF, sodium stearate and lyophilized NaSA-CEF SLNS were collected. This analysis aimed to identify the drug-lipid ionic interaction or complex formation at a molecular level. The analysis focus was on the carboxylate peak region of 1420-1460 cm-1 using RAMANRXN1™ microprobe from Kaiser optical systems and HoloGRAMS™/HoloMap™ mapping software. The Raman spectral collection was done at 10 accumulations in 10 seconds setup.
IR spectroscopy analysis: The shift in the IR peak positions of the bonds indicates environmental change around CEF and sodium stearate molecules. IR peak analysis was performed on CEF, sodium stearate, and lyophilized NaSA-CEF SLNS spectra to detect molecular interactions. Nicolet FT-IR spectrometer IS series, Thermo Scientific Waltham, MA was used in the study.
Characterization of CEF-loaded SLNs
Entrapment efficiency: The entrapment efficiency was determined by ultra-filtration. After the nanoemulsion was cooled, 20 μl was diluted in 10 ml distilled water. Five hundred μl were transferred to a microcone (Molecular weight cut-off 20,000 Da).
Particle size, polydispersity, and zeta potential
The mean particle size and polydispersity index of the SLNs formulations were determined by Dynamic Light Scattering at the 90°C scattering angle using Brookhaven 90 plus size analyzer.
Differential scanning calorimetry of CEF and lipids
Differential Scanning Calorimetry (DSC) analysis was performed using a Seiko SSC/5200, Instruments. An approximately 5 mg sample of CEF, CA8, or PO5 individually or pre-melted mixtures were used in the DSC studies. The heating rate was 10°C per minute up until 20°C above the melting point of each sample. The melting points are 15°C, 7°C, and 60°C for CEF, C8A, and PA5, respectively.
Differential scanning calorimetry
The DSC analysis of CEF showed a single sharp peak indicating the crystalline nature of the CEF (Figure 3). The lipids thermo grams showed a broad melting point, due to the mixed chains length fatty nature of the fats. The DSC thermo grams help in understanding the physicochemical properties of the pure compounds and mixtures. Complex lipids of monoglyceride, diglyceride, and triglyceride mixture form less perfect crystals with many imperfections that allow for more drug encapsulation (9, 10). In Table 1 summarizes the melting point of the used lipids. The detailed thermograms are in S4 supporting materials.
| Compound | Melting point (°C) |
|---|---|
| Compritol 888 | 72 |
| Precirol ATO ® 5 | 55 |
| Pre-melted mixture of both lipids at 90°C | 57 |
Table 1: The melting points of the lipids used in the study using DSC.
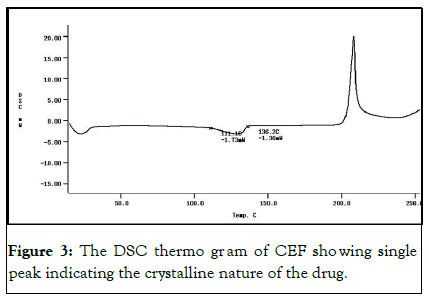
Figure 3: The DSC thermo gr am of CEF sho wing single peak indicating the crystalline nature of the drug.
Spectroscopic analysis of NaSA-CEF-SLNs
Spectroscopic analysis was conducted using Raman and IR spectroscopy in Figures 4 and 5 to study the CEF-ion pair formation and confirm the conjugation of CEF with the counter stearate ion lipid molecule.
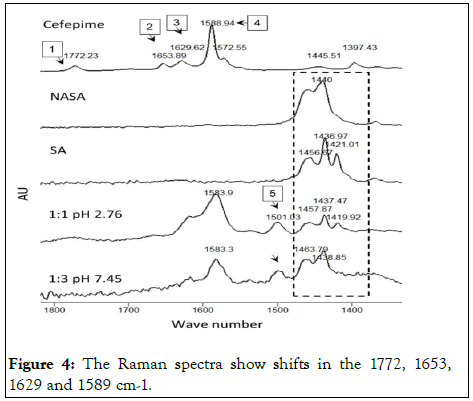
Figure 4: The Raman spectra show shifts in the 1772, 1653, 1629 and 1589 cm-1.
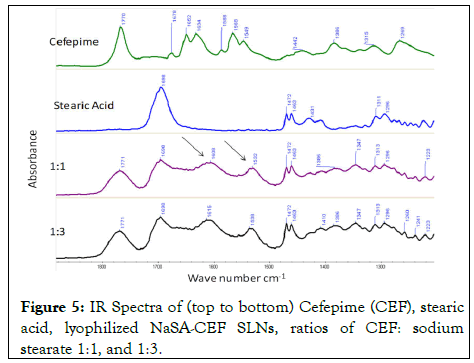
Figure 5: IR Spectra of (top to bottom) Cefepime (CEF), stearic acid, lyophilized NaSA-CEF SLNs, ratios of CEF: sodium stearate 1:1, and 1:3.
Raman spectroscopy
The appearance of a new peak at 1500 cm-1, the shift of 1589 cm-1 (the CEF asymmetric COOH) and 1630 cm-1 (the CEF C=O), and the disappearance of the 1654 cm-1 (CEF C=C) confirm the interaction between CEF polar groups and the NaSA.
The observed changes in the (1420-1460 cm-1) region of the stearate were suspected to be due to molecular ionization rather than interaction. To confirm that, we compared the collected spectra of the NaSA-CEF 1:1 and 1:3 mixtures with sodium stearate (ionized) and stearic acid (unionized) reference materials. These findings are highlighted in Figure 4.
IR spectroscopy
The new peaks at 1532 and 1608 cm-1 suggest an environmental change around the bonds and suggest an interaction between the NaSA and CEF. The IR peaks’ shifts, in a similar region change of the Raman, confirm the suggested interactions observed in the Raman spectra in Figures 4 and 5.
Solid lipid nanoparticle formulation and optimization
The composition of the optimized CEF-SLN is indicated in Table 2. Other surfactants e.g. Tween 80 and different concentration ranges were tested but did not yield SLN within the sought specifications. The entrapment efficiency, particle size, polydispersity index, and zeta potential were used to optimize the CEF-SLNs formulations (Table 3).
| SLNs composition (%) | 5% CEF-SLNs | 7% CEF-SLNs | NaSA-CEF-SLNs (1:3) |
|---|---|---|---|
| CEF | 0.25 | 0.25 | 0.25 |
| C8A | -- | 2 | -- |
| PA5 | 5 | 5 | 5 |
| NaSA | -- | -- | 0.326 |
| SAA | 2 | 2 | 2 |
| H2O | 92.75 | 90.75 | 92.42 |
Table 2: Composition of the three studied SLN formulations.
| Formulation | Entrapment efficiency % | Particles size (nm) |
Polydispersity index |
Zeta potential |
|---|---|---|---|---|
| 5% CEF-SLNs | 21 ± 5 | 111 ± 30* | 0.189 ± 0.026 | -12.1 ± 4.86 |
| 7% CEF-SLNs | 24 ± 6 | 283 ± 80 | 0.292 ± 0.021 | -20.7 ± 2.29 |
| NaSA-CEF-SLNs | 37 ± 8* | 110 ± 11* | 0.226 ± 0.019 | -38.7 ± 0.53 |
Note: Data expressed as Mean ± SD (n=3); *significant difference (p<0.05).
Table 3: Characterization of CEF-SLNs formulations.
Care was taken in choosing a suitable lipid to fulfill the aim of this formulation by balancing between the physicochemical properties of the lipids and the desired formulation characteristics. Different lipids such as stearic acid and glyceryl monostearate and polymers such as PEG1450 were screened for possible SLN formulations but were excluded due to failure in one or more of the following reasons; large particle size, low entrapment efficiency, complete In - vitro release , and appearance.
If an oral fourth-generation CEF was successfully formulated, that would be a great success as many of the current oral cephalosporins are susceptible to bacterial resistance, a public health concern.
Cefepime, a BCS class 3, a charged molecule, is currently only administered intravenously due to its poor lipid permeability. Solubility and membrane permeability are the two factors that contribute to the drug's oral bioavailability. Improved drug permeability through formulation, ion-pairing, or both would enable us to formulate an oral dosage form successfully.
In this study, we have utilized two techniques to increase the permeability of the quaternary ammonium CEF molecule; incorporation of the drug in solid lipid nanoparticle in addition to blocking the drug charge through conjugation with an anionic lipid, stearate. Each of the two techniques contributed to improving the permeability of CEF compared to the unencapsulated drug.
In another study, Cefepime was used as a model drug to investigate the encapsulation efficiency and release profile from a hydrophilic drug delivery platform using chitosan microspheres. The study showed possible entrapment, but no in vivo relevance, permeability, and potential scale up were discussed [1].
Although prodrugs have been utilized to improve the lipophilicity of molecules, the covalent linking might result in a toxic or less effective form of the drug. Our alternative approach improves the drug lipophilicity without possible interference with the therapeutic efficiency [2].
In this study, SLNs were investigated as a potential carrier to enhance the intestinal uptake for CEF. An initial screening of the cefepime’s and the lipids’ physicochemical properties was performed as a first step in determining potential lipids, surfactants, drug concentration, and other excipients to achieve a successful formulation. Cefepime is a crystalline hydrophilic molecule with poor lipid permeability. In the screening process, lipids with a mixture of short, medium, and long-chain fatty acids were favored in order to maximize CEF encapsulation through dispersion between the imperfect crystals of the lipids.
In determining the lipid formulation to be used, we have investigated different percentages of lipids and SLNs preparation techniques. The tested lipids concentration ranged from 2-10% total lipid mixtures. In addition, other surfactants at multiple ratios were studied. Using our optimization criteria of particle size, encapsulation efficiency and zeta potential we have narrowed down our optimized formulations to two formulas with unpaired CEF in Table 1.
Solid lipid nanoparticles have been widely considered to advance the biopharmaceutical properties and pharmacokinetics of drugs. It has been used to create a long acting injectable drug delivery platform for intramuscular and subcutaneous administration in addition to the successful delivery of mRNA [3,4].
When it comes to safety, lipids used in SLNs are thought to be well accepted in vivo since they are generally made of physiological compounds and, thus, digestion should cut the risk of severe and prolonged toxicity. However, formulators must take care attention of the possible toxicity from surfactants.
The toxicity profile of the developed SLNs was not assessed. However, the lipids used in all formulations are categorized as GRAS, and the surfactant used in all formulations did not exceed 2% of the total prepared volume.
Exploring the possible chemical complexion by ion-pairing of CEF to further block the surface charges and better encapsulate CEF in SLN was our second step of enhancing encapsulation and improving permeability NaSA was the anion of choice.
Sodium stearate dissociates in water to a negatively charged stearate, which can be used to block the positively charged quaternary nitrogen of CEF. Coupling of the NaSA and the CEF was achievable at both molar ratios of 1:3 and 1:1 CEFNaSA.
Maximizing the yield was not in the scope of this study. Therefore, it was decided to use the 1:3 molar ratios in the subsequent steps of loading the SLN formulation. The reason being that the 1:3 ion pair product was easier to handle than the 1:1 ion pair, which was sticky and carried a static charge.
The vibrational modes of the unpaired and ion paired CEF bonds were studied using IR and Raman. The molecule vibration is a function of many factors, among which are ionic and hydrogen bonding. The shift in the 1629 cm-1 Raman peak of the CEF (C=C peak), the appearance of a new peak at 1501 cm-1, and the change in the ionic functional carbonyl group of the β-lactam at 1775 cm-1 confirm CEF sodium stearate molecular interactions. In addition, the fingerprinting region of the IR spectra suggests changes around the vibration of the carbonyl groups of sodium stearate.
Both IR and Raman complement each other and confirm the changes in the CEF bonds’ vibrations due to changes in the environment around the CEF molecule before and after ion pairing. These changes could be attributed to ion-ion pairing or dipole-dipole molecular interaction.
The apparent permeability of the CEF formulations was performed across excised rat jejunum tissues using ex-vivo side by-side diffusion technique. The apparent permeability parameter is commonly used in such studies to indicate the passive transport of a molecule. The rat jejunal tissue has high similarity to human jejunal tissue structurally in tight junctions and mechanically where most drug absorption processes occur [4,5].
Ion pairing of the CEF with the NaSA improved both the permeability of the un-encapsulated CEF-NaSA and the encapsulation of CEF-NaSA into the SLN and thus improved the CEF permeability. The net effect of the two combined mechanisms was more than 40 folds improvement of the CEF permeability compared to the untreated CEF solution.
The formation of different drug complexes and ion pairs have been utilized to improve drug bioavailability to facilitate drug penetration into specific tissues, for example, but not limited to the retina and inner ear [7-9]. A study showed an improved ocular bioavailability of Timolol by sorbic acid ion pairing [10].
An increase in the negative zeta potential of the NaSA-CEF nanoparticles compared to the CEF nanoparticles was also observed. This could be attributed to the anionic fats (stearate) incorporation in the SLN, resulting in an increased surface negative charge.
The CEF-NaSA ion pair SLN negative zeta potential was significantly higher than the CEF-SLN formulas. The increased negative zeta potential of the SLN can provide physical stability of SLN through high repulsion. The particles' surface charge on the cellular uptake and permeability was studied and found to be significant yet small in the range of -2 to -16 mv. Yet, a considerable decrease was confirmed for higher charge levels [1]. One reasoning is that the intestinal mucus exhibits a net negative charge due to the silica and sulfonic acid structure; a slightly negative particle can allow particle movement within the mucus layer as long as the mucus is not dense and its structure is sufficiently broken. On the other hand, positively charged particles could be immobilized due to charge interaction with the negatively charged mucus [2].
In terms of the effect of SLN size, the average particle size of 110 nm favors the penetration of the particles through the intestinal membrane. This explains the difference in the net improved permeability of CEF from the 5% CEF-SLNs with an average size of 111(nm) compared to the 7% CEF-SLNs with an average size of 283 (nm), although the encapsulation of the drug in the latter was higher. Thus we concluded that the net effect depends on both the type and the magnitude of the charge on the particles and the mucus structure, as confirmed by another study [3,4].
Currently the CEF is only available as an injectable form. Our efforts to prepare it in a oral formulation will maximize the therapeutic use of this antibiotic. The CEF ion pair formulation was successfully prepared and evaluated. The loaded SLN showed significant improvement of the CEF permeability and the ion pairing of the CEF with sodium stearate further enhanced the CEF encapsulation in SLN and its penetrability as well. The NaSA-CEF ion paired SLN is thus a promising oral formula for CEF and may present a strategy not only to formulate the CEF as oral dosage form but also to solve the oral impermeability of ionized drugs.
Citation: AlBenayan W, Karzoun B, Atef E, Rizkalla C (2022) Novel Solid Lipid Nanoparticles Formulation of Ion paired Cefepime for Enhanced Oral Absorption. J Pharma Care Health Sys.9:237.
Received: 04-Jan-2022, Manuscript No. JPCHS-22-45923; Editor assigned: 10-Jan-2022, Pre QC No. JPCHS-22-45923 (PQ); Reviewed: 24-Jan-2022, QC No. JPCHS-22-45923; Revised: 28-Jan-2022, Manuscript No. JPCHS-22-45923 (R); Published: 04-Feb-2022 , DOI: 10/35248/2376-0419.2022.237
Copyright: © 2022 AlBenayan W, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.