Journal of Cancer Research and Immuno-Oncology
Open Access
ISSN: 2684-1266
ISSN: 2684-1266
Research Article - (2025)Volume 11, Issue 1
Background: In today’s world the use of medicine, especially of plant origin, is acquiring a lot of significance in restorative applications because of the relatively low harmfulness when contrasted with designed pharmaceuticals.
Methods: Methanolic extracts of Spondias pinnata, and Syzygium cumini were evaluated for antioxidant properties using DPPH and ABTS radical scavenging assays. MTT assay for the extract was carried out against the HCT116 cell line. HCT1116 cells treated with 40 and 80 μg/mL of S. cumini were studied using Annexin V FITC flow cytometry. PCR analysis of the caspase3 gene in HCT116 cells treated with 40 and 80 μg/mL of S. cumini extract was done to confirm apoptosis. A hemolysis assay was performed to determine the toxicity effect of the plant extracts on human RBCs.
Results: IC50 of S. cumini and S. pinnata were found to be 23.72 and 56.51 μg/mL; 47.64 ug/mL and 19.7 μg/mL for DPPH and ABTS radical scavenging activity. MTT assay showed an IC50 value of 59.43 and 137 μg/mL for S. cumini and S. pinnata respectively. Flow cytometry study indicated that 17.2 and 4%, and, 20.49% at a concentration of 40, 80 μg/mL have attained early apoptosis while the rest have attended late apoptosis and necrosis. PCR analysis of caspase3 indicates that S. cumini has demonstrated superior anti-cancer activity against HCT116 cells.
Conclusion: Downstream studies with flow cytometry on apoptosis and PCR analysis of the caspase3 gene suggest that S. cumini has comparably shown better anti-cancer activity against HCT116 cells proving the potential as a therapeutic agent.
Antioxidant; Syzygium cumini; Spondias pinnata; Annexin V FITC; Caspase3
In today’s world the use of traditional medicine and body care products, especially of plant origin, are gaining considerable significance in restorative applications as they are less harmful and better accepted by a wider public. Malignant growth is the most powerful driver of death among diseases worldwide, claiming the lives of more than 10 million people in 2020. Colorectal cancer was responsible for 10% of all deaths in the year 2020. Changes in habitual bowel movements, bowel motions, involuntary weight loss, biliousness, vomit, uneasiness, anorexia, and abdominal distension are all commonly diagnosed indicators of colon cancer. Rectal bleeding is more prevalent and obvious in distal tumors than in proximal [1]. Spondias pinnata is extremely well known for its injury recuperating properties and is suggested in the treatment of stomach pains and diarrhoea. S. pinnata bark concentrate can be utilized to treat conditions like Anorexia and Nausea. Juice of the leaves of this plant was used to treat ear infections. The bark concentrate is also regularly taken as a Sri Lankan standard prescription to treat diabetes mellitus and has effective antihyperglycemic activity in Wistar rodents. The plant is known to be a rich source of carbs, flavonoids, glucosides, tannins, phytosterols, phenolic compounds, sterols, terpenoids, and saponins. The methanolic extracts of S. pinnata leaves exhibited good antimicrobial and antioxidant properties. The analgesic effect of S. pinnata bark ethanolic extract proved to be dosedependent as observed in the acetic acid and formalin tests [2]. These effects were comparable with that of acetylsalicylic acid.
The results of this study lead credit to the traditional uses of S. pinnata, especially as an analgesic. The potential for the development of anticancer therapeutic from S. pinnata is evidenced by the studies on its bark extracts against human lung and breast cancer.
Syzygium cumini fruits and seeds are utilized to treat diabetes mellitus for a few centuries as medication in south Asia. The seeds are utilized as astringent and diuretic. They have anti-inflammatory, hypoglycaemic, lipid reducing fever-reducing, psychopharmacological, and cell reinforcement properties. The plant is also known to poses antibacterial properties. The leaves contain alkaloids, flavonoids, saponins, tannins, glycosides, phenols, proteins, triterpenoids, steroids and oils, and fats. As per the scientific documentary available, most of the work done is on bark extracts of S. pinnata and fruit, seeds extract of S. cumini [3]. The leaves being abundantly available throughout the year, they can be a major source of phytochemicals that may combat colorectal carcinoma. Leaf extract of both species was tested for their anti-cancerous activity in the MTT assay. So far, no studies have been reported on the effect of leaf extract on gene expression and apoptosis in cellular model systems. In the present study, we report that both S. pinnata and S. cumini leaf extracts exhibited cytotoxicity to HCT116 cells and caspase 3 gene expression was elevated in the presence of S. cumini.
Reagents and cell culture
Dulbecco’s modified eagle medium, fetal bovine serum, doxorubicin, propidium iodide, ABTS, DPPH, and quercetin were obtained from Sigma Aldrich. Methanol (HPLC grade), EDTA, and agarose were purchased from Thomas baker [4].
HCT116 cell lines were procured from American Type Culture Collection (ATCC). Stock cells were cultured in DMEM (Dulbecco’s Modified Eagle Medium) supplemented with 10% inactivated Fetal Bovine Serum (FBS), penicillin (100 IU/mL), streptomycin (100 μg/mL) in a humidified atmosphere of 5% CO2 at 37°C until confluent. The monolayer cell culture was trypsinized and the cell count was acclimated to 5.0 × 105 cells/mL utilizing DMEM medium containing 10% FBS.
Methanolic extraction
The leaves of S. pinnata and S. cumini were washed with clean water and shade dried and powdered. The extracts of S. pinnata and S. cumini were prepared by adding 30 g of dried powder to150 mL of methanol. The mixture was heated at 56°C for 4 hours with occasional stirring and the contents were filtered using Whatman filter paper. The filtrate was kept in a hot water bath at 60°C to vaporize the solvent [5].
Assessment of antioxidant activity
DPPH radical scavenging assay: DPPH was prepared in HPLC grade methanol (0.166 mg/mL). The test sample preparation was done by dissolving 1 mg of plant extract in 1 mL of CH3OH and serial two-fold dilutions were made in the range of 6.25 μg/mL to 400 μg/mL in methanol. Quercetin was used as a reference standard and positive control in the range of 0.312 μg/mL to 10 μg/ml. The reaction mixture contained 80 μL of DPPH solution and 160 μL of the test sample and reference standard in pre-designated tubes [6]. The reaction mixture was read at 510 nm (spectramax i3X, Molecular devices). The anti-oxidant property of the samples was expressed in terms of % inhibition and IC50 values were calculated by using graph pad prism software. Triplicates were maintained for each concentration.
ABTS antioxidant assay: The test was proceeded according to Auddy et al. 2,2'-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) and ammonium persulphate mixture were prepared by reacting ABTS solution (7 mM) with 2.45 mM ammonium persulfate and the mixture was allowed to stand in dark at room temperature for 16 hours before use. The OD734 of the mixture was adjusted to 1.0 with PBS. To 225 μL of ABTS solution 25 μL of the sample was added and the absorbance was recorded at 734 nm. The percentage inhibition was calculated at various concentrations (as mentioned in the DPPH assay) and the IC50 value was calculated. Triplicates were maintained for each concentration [7].
In vitro cytotoxic assay
To each well of the 96-well microtiter plate, 100 μL of the HCT 116 cell suspension (50,000 cells/well) was added. After 24 h, the supernatant was removed, and the cells were treated with the plant extracts concentration ranging from 320 μg/mL to 10 μg/ml. Doxorubicin was used as a positive control (100 μM to 3.125 μM). The plate was incubated at 37°C and 5% CO2 for 24 hours. The culture medium was removed and 100 μL of MTT (5 mg/10 mL in PBS) was added to each well. The plate was incubated for 4 h at 37°C and 5% CO2. The supernatant was removed and 100 μL of DMSO was added and the plates were gently shaken to solubilize the formazan crystals. OD590 was read (Spectramax i3X) and % inhibition was calculated using the below-given formula. IC50 was derived from a dose-response curve in graph pad prism [8,9].
Cell treatment
HCT116 cells were seeded into a 6-well plate (1 × 106 cells/well) in DMEM containing 10% FBS and were incubated for 18 hours [10]. The cell culture medium along with the dead floating cells was removed and Plain DMEM medium containing the test samples (40 μg/mL and 80 μg/mL) was added. Doxorubicin was standard (25 μM/mL) and control was DMEM containing 0.8% DMSO. After 24 hours the cells were taken for staining or RNA isolation.
PI ANNEXIN V-FITC staining
The cells were washed twice with cold PBS and then resuspended in 1 mL binding buffer and staining was performed eBioscience™ Annexin V Apoptosis Detection Kit following the manufacturer’s instructions. To 500 μL of cell suspension 10 μL of PI and 5 μL, Annexin V is added, incubated for 15 minutes at RT in dark and the cells were analyzed by flow cytometer [11].
RNA extraction and cDNA synthesis
HCT116 cells treated with S. cumini were transferred into 15 mL tubes and centrifuged at 5000 rpm for 5 min at 4°C. To the pellet, 650 μL of TRIzol was added, the contents were mixed well, and incubated on ice for 20 minutes, and 300 μL of chloroform was added and gently mixed by inversion. The mixture was incubated on ice for 10 min and the contents were centrifuged at 12000 rpm for 15 min at 4°C. The upper aqueous layer was carefully transferred to a new sterile 1.5 mL centrifuge tube and an equal amount of ice-cold isopropanol was added and incubated at -20°C for 1 hr. The mixture was centrifuged at 12000 rpm for 15 min at 4°C. The pellet was washed in 70% ethanol, air-dried at RT, and resuspended in 30 μL of DEPC treated water. The cDNA was synthesized from 2 μL of RNA using the Verso cDNA synthesis kit (Thermo Fisher Scientific) with oligo dT primer according to the manufacturer’s instructions. The reaction volume was set to 20 μl and the synthesis was performed at 42°C for 60 min, followed by inactivation at 85°C for 5 min [12].
Polymerase chain reaction
The Polymerase Chain Reaction (PCR) mix (final volume of 20 μL) contained 1 μL of cDNA, 10 μL of Red Taq Master Mix 2x (Amplicon), and 1 μM of each complementary primer specific for CASPASE 3 (initial denaturing at 94°C for 5 min, 35 cycles of amplification: 94°C for 30 seconds, 47°C for 30 seconds, and 72°C for 1 min). β-Actin was the internal control (annealing 55°C, final elongation at 72°C for 10 min). Ten microliters of the final amplification product were loaded onto 2% ethidium-stained agarose gel and visualized (ChemiDoc XRS, BIO RAD) and gel pictures were analyzed by Image J. The values were normalized to β-Actin intensity levels [13].
Statistical analysis
The results were expressed as mean ± standard deviation. Descriptive statistics were used to analyze the mean, standard deviation, variation, and statistical significance (p<0.01).
| Gene | Primer pair | Sequence | Tm | Product size (bp) |
|---|---|---|---|---|
| β–Actin | FP | TCCTCCTGAGCGCAAGTACTCT | 62.1 | 153 |
| RP | GCTCAGTAACAGTCCGCCTAGAA | 62.4 | ||
| CASPASE 3 | FP | ACATGGCGTGTCATAA AATACC | 51.88 | 127 |
| RP | CACAAAGCGACTGGATGAAC | 51.83 |
Table 1: Details of primer sequence used in PCR to amplify β-Actin and caspase3 genes.
Cell lines and culture
HCT116 Cell lines which are derived from a colonic carcinoma patient are tumorigenic in nude mice. These cells are adherent with epithelial like morphology. We cultured them in DMEM with 10% FBS [14].
Methanolic extraction
The total yield of crude extract obtained from leaves of S. pinnata and S. cumini using methanol as solvent was 7.7 and 9.7 grams, respectively, per 100 grams of dried leaf powder.
Assessment of antioxidant activity
The antioxidant property is measured in terms of the IC50 value. In the case of the DPPH radical scavenging assay, the standard used was Quercetin which gave an IC50 value of 0.9047 μg/mL. S. cumini and S. pinnata had an IC50 of 23.72 μg/mL and 56.51 μg/mL, respectively. Whereas, IC50 values of ABTS radical scavenging activity of quercetin, S. pinnata, and S. cumini were found to be 1.775 μg/mL, 47.64 μg/mL, and 19.72 μg/mL, respectively (Figures 1 and 2) [15].
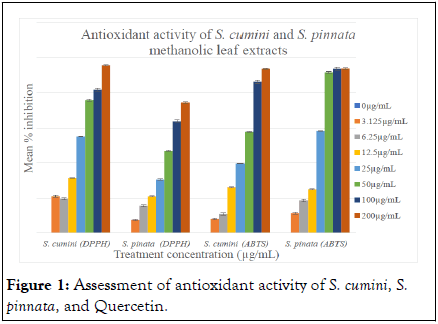
Figure 1: Assessment of antioxidant activity of S. cumini, S. pinnata, and Quercetin.
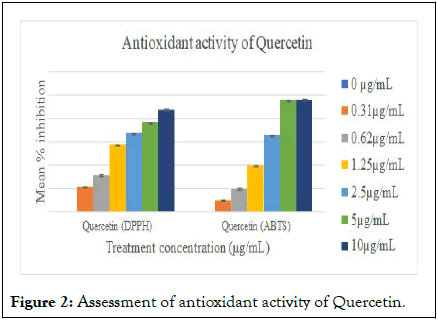
Figure 2: Assessment of antioxidant activity of Quercetin.
In vitro cytotoxic activity
MTT assay was conducted on HCT116 cell lines in presence of various concentrations of methanolic leaf extracts of both S. pinnata and S. cumini. Triplicates are maintained for each concentration and doxirubicin was used as standard. The IC50 values obtained for doxorubicin, S. pinnata, and S. cumini were 17.04 μM/mL, 137 μg/mL, and 59.43 μg/mL, respectively (Figures 3 and 4). It is evident that among the two plant samples S. cumini exhibited better cytotoxicity [16].
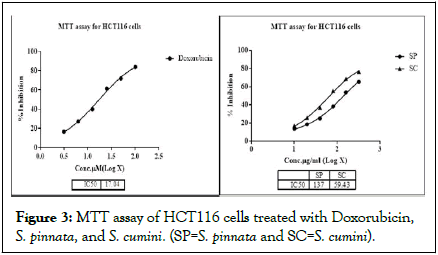
Figure 3: MTT assay of HCT116 cells treated with Doxorubicin, S. pinnata, and S. cumini. (SP=S. pinnata and SC=S. cumini).

Figure 4: Cytotoxicity observed in HCT116 cells after treatment: A-control, B and C=Doxorubicin 3.125 and 100 μM/mL, D and E=S. cumini 10 and 320 μg/mL, F and G=S. pinnata 10 and 320 μg/mL. Cell necrosis is more in presence of S. cumini leaf extract.
PI-ANNEXIN V-FITC staining
The test shows that HCT116 cells treated with S. cumini at 40 μg/mL and 80 μg/mL have induced early and late apoptosis in a dose-dependent manner. About 17.24%, 5.96% of cells have attained early and late apoptosis in cells treated at 40 μg/mL, whereas 20.49%, 11.81% of cells have attained early and late apoptosis at 80 μg/mL (Figure 5). HCT-116 cells treated with doxorubicin (25 μM/mL) attained 15.67% early and 23.54% late apoptosis [17].
| Sample | Viable cells % (PI -ve, FITC -ve) | Early apoptotic % (FITC +ve, PI -ve) | Late apoptotic % (PI +ve, FITC +ve) | Necrotic cells % (PI +ve, FITC -ve) |
|---|---|---|---|---|
| Control | 98.48 | 1.17 | 0.06 | 0.29 |
| S. cumini 40 µg/mL | 74.5 | 17.24 | 5.96 | 2.3 |
| S. cumini 80 µg/mL | 63.63 | 20.49 | 11.81 | 4.07 |
| Doxorubicin 25 µM/mL | 46.22 | 15.67 | 23.54 | 14.57 |
Table 2: FACS analysis of apoptosis detection in HCT116 cells.
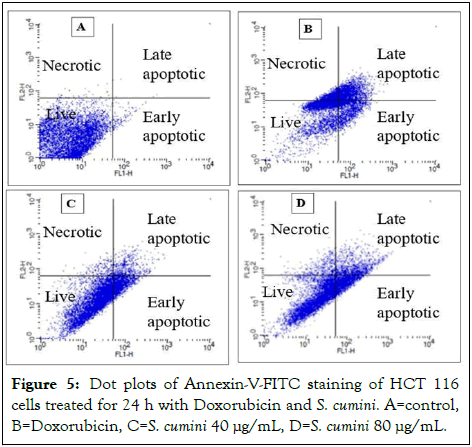
Figure 5: Dot plots of Annexin-V-FITC staining of HCT 116 cells treated for 24 h with Doxorubicin and S. cumini. A=control, B=Doxorubicin, C=S. cumini 40 μg/mL, D=S. cumini 80 μg/mL.
Gene expression analysis
The effect of S. cumini on CASPASE 3 gene expression was studied in HCT-116 cells by semi-quantitative-PCR (Figure 6). cDNA was synthesized using the mRNA extracted from the pre-treated HCT116 cells. The internal control β-Actin was used to normalize the gene expression. On treatment with S. cumini leaf extract expression of CASPASE 3 was up-regulated 1.48 and 1.89 folds at 40 μg/mL and 80 μg/mL, respectively.
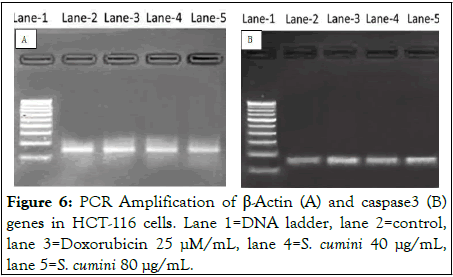
Figure 6: PCR Amplification of β-Actin (A) and caspase3 (B) genes in HCT-116 cells. Lane 1=DNA ladder, lane 2=control, lane 3=Doxorubicin 25 μM/mL, lane 4=S. cumini 40 μg/mL, lane 5=S. cumini 80 μg/mL.
Phytochemical compounds from the plants are utilized to treat various kinds of diseases. About 25-50% of the current drugs are obtained from plant-based compounds. It has been evidenced that oxidative stress is a contributing factor in the development of many illnesses. Oxidativetive stress is caused in cells when free radicals bind to organic macromolecules including proteins, lipids, and chromosomes, resulting in nucleic acid and protein damage as well as lipid peroxidation. In addition, malignancy, atherosclerosis, cardiovascular disease, autoimmune diseases, and aging are all caused by these alterations. Antioxidant enzymes (SOD) and catalase, as well as substances like vitamin c, tocopherol, and glutathione, equip most human cells to shield against free radical damage. Plant-based supplements are helpful to combat oxidative damage [18].
In the present study, we have examined the methanolic extracts of S. cumini and S. pinnata leaves for antioxidant and anticancer activity against human colorectal carcinoma. The presence of alkaloids, glycosides, tannins, terpenoids, flavonoids, and phenols, common in both the samples was revealed in the previous studies.
A study on phytochemical analysis by various researchers resulted in the isolation of 24-methylene cycloartanone, lignoceric acid, sitosterol, and D-glucoside from S. pinnata. The content in S. pinnata includes sterols, flavonoids, polysaccharides, gums, β-amyrin, oleanolic acid, amino acids including glycine, cysteine, serine, alanine, leucine, daucosterol, cycloartanone-24 methylene, lignoceric acid, ellagitosinoic acid, lellagitosinsin gallon, lellocitgerinsin gallon, lellocitosinsin gallon, lellocitosino β carotene18DPPH and ABTS radical scavenging test depend on the estimation of the scavenging limit of anti-oxidants towards DPPH and ABTS. The antioxidant assessment using DPPH and ABTS radical scavenging assays indicated S. cumini (23.72 ± 0.17 μg/mL) to possess better antioxidant capability than S. pinnata.
We have conducted an in vitro cytotoxicity study to estimate the effects of both the samples against HCT116 cells using MTT assay. The decrease of tetrazolium salts is generally acknowledged as a dependable method to analyse cell viability. The yellow tetrazolium MTT is reduced by metabolically active cells, partially by the activity of the dehydrogenase enzyme. The subsequent intracellular purple formazan can be solubilized using DMSO and evaluated spectrophotometrically. The present study reveals the IC50 value of S. pinnata and S. cumini to be, 137 ± 0.38 μg/mL, and 59.43 ± 0.27 μg/mL, respectively. The results show that IC50 values are comparable with other parts of the plant. As reported, 70% methanol extract of S. pinnata stem bark has cytotoxic activity against cancer cells of human lung adenocarcinoma with IC50 147.84 μg/mL whereas for human breast adenocarcinoma of 149.34 μg/mL. S. cumini methanolic leaves extract exhibited an IC50 of 378.35 ± 2.84 and 35.21 μg/mL against cervical and lung carcinoma respectively [19]. As observed in the MTT assay S. cumini was indicated to have better cytotoxic activity in comparison to S. pinnata, hence the further study was continued only with S. cumini.
Apoptosis is a cell death process characterized by morphological and biochemical features occurring at different stages. Once triggered, apoptosis proceeds with different kinetics depending on cell types and culminates with cell disruption and formation of apoptotic bodies. By attaching Fluorescein Isothiocyanate (FITC) to Annexin V, it is feasible to recognize and quantitate apoptotic cells on a solitary cell premise by flow cytometry. Staining cells at the same time with FITC-Annexin V (green fluorescence) and the propidium iodide (red fluorescence) permits the separation of intact cells, early apoptotic and late apoptotic or necrotic cells. Our work has shown that the treatment of HCT116 cells with S. cumini cells has undergone early, late apoptosis and necrosis (Table 2). S. cumini fruit skin is known to induce apoptosis in human cervical cancer cells and human oral squamous carcinoma cells. S. pinnata induces apoptosis in human lung and breast carcinoma.
There are two kinds of apoptotic pathways in cells: intrinsic and extrinsic. Caspase 8 is a significant piece of the extrinsic pathway. The activation of caspase 8, is driven by external factors. However, the intrinsic pathway includes the release of cytochrome from mitochondria, which causes necrobiosis by activating caspase 9 and other apoptosis inducers. Both apoptotic pathways conclude by caspase 9 and caspase 8 activating caspase 3. At now, other apoptosis mediators break procaspase 3, causing caspase 3 activation, which is a significant stage inside the beginning of apoptosis [20]. PCR analysis of the pro-apoptotic gene, caspase3, is observed to upregulated in the treatment of HCT116 cells with S. cumini in a dose-dependent manner.
S. cumini has comparably shown better anti-cancer activity against HCT116 cells proving its potential as a therapeutic agent. Our work also reveals that the solvent and protocol adopted for the extraction of samples have a profound effect on biological activity against human colorectal cancer cells. However, more data needs to be established to further prove the efficacy of the sample by performing animal model-based studies and toxicity studies. It is also essential to find out the exact phytochemical compound responsible for the anti-cancer activity shown by the compound.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Suneetha YK, Neha HL, Anand S (2025) Non-proliferating Effect of Syzygium cumini and Spondias pinnata Against Colorectal Carcinoma. J Cancer Res Immunooncol. 11:237.
Received: 26-Feb-2023, Manuscript No. JCRIO-23-21947; Editor assigned: 01-Mar-2023, Pre QC No. JCRIO-23-21947 (PQ); Reviewed: 15-Mar-2023, QC No. JCRIO-23-21947; Revised: 02-Jan-2025, Manuscript No. JCRIO-23-21947 (R); Published: 09-Jan-2025 , DOI: 10.35248/2684-1266.25.11.237
Copyright: © 2025 Suneetha YK, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.