International Journal of Physical Medicine & Rehabilitation
Open Access
ISSN: 2329-9096
ISSN: 2329-9096
Mini Review - (2020)Volume 8, Issue 3
Chronic thromboembolic PH (CTEPH) and pulmonary arterial hypertension (PAH) are two subtypes of PH. CTEPH is characterized by organic thrombotic obstructions of pulmonary arteries, which reduce pulmonary vascular reserve. As new therapies are developed for CTEPH and PAH, prompt screening for the presence of PH, diagnosis and distinction between CTEPH and PAH have become increasingly important. Although their pathophysiology differs, the clinical presentation at rest is highly similar between CTEPH and PAH, as both disorders have only non-specific symptoms. Therefore, differentiating between CTEPH and PAH using non-invasive techniques remains challenging.
This mini-review focuses on the role of ventilatory gas analysis in the management of PH patients, presents recent studies dealing with ventilatory gas analysis, and discusses the latest use of ventilatory gas analysis for diagnosing, assessing (in terms of severity), and distinguishing between CTEPH and PAH.
Pulmonary hypertension; Pulmonary gas exchange
Pulmonary hypertension (PH) is defined as a mean pulmonary arterial pressure (PAP) ≥ 25 mmHg at rest on right heart catheterization (RHC) [1]. Chronic thromboembolic PH (CTEPH) and pulmonary arterial hypertension (PAH) are two subtypes of PH. Characterized by organic thrombotic obstructions of pulmonary arteries with reduced pulmonary vascular reserve, CTEPH is the only form of PH known to be potentially curable. However, without appropriate treatment, the prognosis of CTEPH is very poor, with a 5-year survival rate of 10% in those with a mean PAP>50 mmHg [2]. With the development of new therapies for CTEPH and PAH, it has become increasingly important to promptly screen for the presence of PH, diagnose CTEPH and PAH, and distinguish them from each other. Having only non-specific symptoms, CTEPH and PAH share fairly similar clinical presentations despite resulting from different pathophysiological processes. Therefore, differentiating between CTEPH and PAH using noninvasive techniques remains challenging.
According to the latest PH guidelines, RHC not only is mandatory in the diagnosis of PAH and CTEPH but also may prove useful in assessing the severity of PH and determining reversibility. However, RHC is invasive and imposes a heavy burden on patients if repeated for follow-up purposes. Current guidelines recommend transthoracic echocardiography as the first noninvasive diagnostic tool in patients with suspected PH [3]. Yet, echocardiography may underestimate PAP in mild PH and is unable to detect PH in the early stages [4,5]. Moreover, an increase in resting PAP is a late marker of pulmonary vascular disease because approximately 50% of the pulmonary circulation is already obstructed before an increase in resting PAP is detected. Therefore, several current noninvasive screening modalities depend on the detection of PAP elevation, and thus fail to identify a mild increase in PAP at an early stage of PH.
Cardiopulmonary exercise test (CPX) with ventilatory gas analysis is useful in that it helps to (1) detect disorders such as exercise-induced dyspnea, (2) support the diagnosis of PH in patients with echocardiographic findings suggestive of PH, and (3) evaluate the severity of PH [3,6]. Moreover, CPX has proven to be both a suitable tool for establishing and guiding therapeutic management of PH and a complementary diagnostic tool for the detection of CTEPH in patients with suspected PH but normal echocardiography [7,8]. Held, et al. reported that a score combining the minute ventilation-carbon dioxide production (VE/VCO2) slope, alveolar-arterial oxygen tension difference [P(A‐a)O2], capillary to end-tidal carbon dioxide gradient [P(c ‐ ET)CO2], and end-tidal CO2 pressure (PETCO2) could detect CTEPH in electrocardiographically normal patients with a sensitivity of 83.3% and a specificity of 92.2% [7].
However, in exercise tests, clinicians may sometimes impose more stress than expected on critically ill patients with PH, which might not be suitable for more severe PH patients with right heart failure at risk for syncope and arrhythmias. Furthermore, CPX requires expertise and specialist facilities which limits its use at present and therefore is not currently recommended as a screening tool for PH [1,3,9].
Postural change from sitting to supine position usually improves ventilation/perfusion mismatch in healthy individuals through the pulmonary vascular reserve and increased functional pulmonary blood flow [10-12], leading to an increase in PETCO2 and a decrease in VE/VCO2 (Figure 1) [13]. Meanwhile, reduced pulmonary vascular reserve, which develops in conditions such as PH, results in the attenuation of the redistribution of pulmonary perfusion in response to postural change [14].
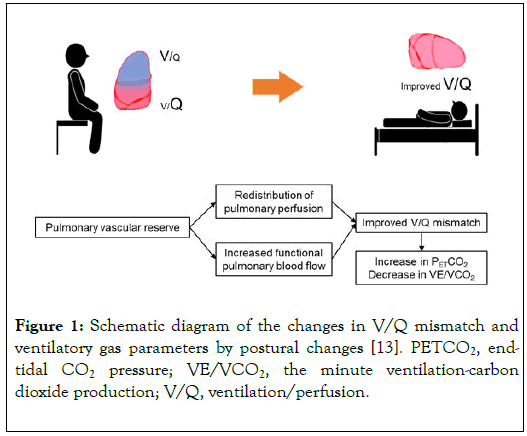
Figure 1. Schematic diagram of the changes in V/Q mismatch and ventilatory gas parameters by postural changes [13]. PETCO2, endtidal CO2 pressure; VE/VCO2, the minute ventilation-carbon dioxide production; V/Q, ventilation/perfusion.
A previous study showed that patients with PH had notably reduced perfusion redistribution after postural change compared to controls (p<0.0001). In the same study, the functional vascular reserve of the pulmonary circulation was assessed by quantifying the degree of perfusion redistribution following postural change, which was found to correlate with markers of PH severity, such as mean PAP (mPAP), 6-min walk distance, and the World Health Organization functional class. In addition, the posture change method has proven superior in discriminating between normal and PH values to other methods relying on parameters measured in only one posture [15].
Recently, a new evaluation method using the mechanism has been reported [13,16].
We reported the usefulness of ventilatory gas analysis with postural changes for the assessment of PH severity [16]. Various ventilatory gas analysis parameters were examined in the supine and sitting postures, and their respective postural differences were subsequently calculated (Δ supine-sitting). In addition, we examined hemodynamic and ventilatory gas analysis parameters before and after treatment with balloon pulmonary angioplasty (BPA), which is an interventional therapy for CTEPH patients.
In terms of ventilatory gas analysis parameters, supine PETCO2 and ΔPETCO2 were significantly lower in the improved CTEPH group (mPA<25 mmHg after receiving optimal therapies including vasodilator therapy and surgical therapies, BPA or both) than in the control group (patients with suspected PH but normal mPAP values), whereas hemodynamic and echocardiographic parameters were comparable between these groups. Moreover, supine PETCO2 and Δ PETCO2 were found to correlate significantly with mPAP (R2=0.507, p<0.001and R2=0.470, p<0.001 respectively). These findings suggest that mild abnormalities in the pulmonary circulation can possibly be detected using ventilatory gas analysis in different postures and that low supine PETCO2 and Δ PETCO2 may be useful, sensitive, and noninvasive markers for the evaluation of mPAP. Furthermore, supine PETCO2 significantly increased after BPA (p<0.001), and the improvement in mPAP by BPA paralleled the improvement in supine PETCO2 (R2=0.478, p<0.001) [16].
This study is the first to show that ventilatory gas analysis in different positions can serve as a useful method for evaluating not only mPAP but also CTEPH patients’ response to therapy.
We also conducted ventilatory gas analysis in different postures to distinguish between CTEPH and PAH [13]. Δ PETCO2 was significantly lower in patients with CTEPH and PAH than in those without PH (both p<0.001). Δ PETCO2<0 mmHg could effectively differentiate PH from non-PH (area under the curve [AUC]=0.969, sensitivity=89%, specificity=100%). In response to a postural change from sitting to supine, VE/VCO2 increased significantly in the CTEPH group (p<0.001), whereas the PAH group experienced a meaningful decrease in the same ratio (p=0.001). Besides, ΔVE/VCO2 appeared to be higher in CTEPH than PAH, but no differences in hemodynamic and echocardiographic parameters were observed between the two groups. Furthermore, ΔVE/VCO2>0.8 could effectively differentiate CTEPH from PAH (AUC=0.849, sensitivity=78%, specificity=88%) (Figure 2).
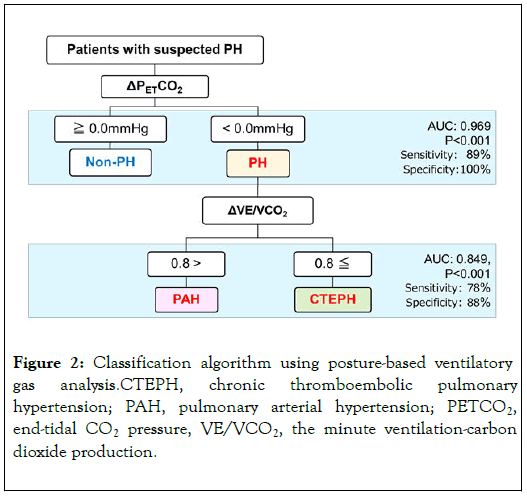
Figure 2. Classification algorithm using posture-based ventilatory gas analysis.CTEPH, chronic thromboembolic pulmonary hypertension; PAH, pulmonary arterial hypertension; PETCO2, end-tidal CO2 pressure, VE/VCO2, the minute ventilation-carbon dioxide production.
It was demonstrated in the same study that intrapulmonary shunt increased in line with mPAP, was greater in CTEPH than in PAH, and correlated with mPAP. Intrapulmonary shunt could also result in lower PETCO2 and higher VE/VCO2 values [17]. Moreover, changes in body posture were used to shed light on the effects of intrapulmonary shunts on the pulmonary circulation and gas exchange [18]. These changes were found to be more prominent in CTEPH, which has greater intrapulmonary shunt, than in PAH [19,20].
To recapitulate, patients with Δ PETCO2<0 mmHg would be likely to have PH, and those with Δ VE/VCO2>0.8 might presumably be regarded as patients with suspected CTEPH.
The main findings about ventilatory gas analysis in different positions are as follows: i) supine PETCO2 and Δ PETCO2 are useful for the evaluation of mPAP, which is one of the parameters of CTEPH severity; ii) supine PETCO2 may help assess response to therapies such as BPA; iii) Δ VE/VCO2 could effectively differentiate CTEPH from PAH.
The strength of the study lies in the use of a feasible and practical approach to screening for PH by dint of simple postural changes. This methodology may seem appealing to patients who are either unable to exercise due to cardiovascular or orthopedic limitations or simply reluctant to perform exercise. In addition, this procedure can be performed on an outpatient basis with minimal effort. The present postural change method may have important clinical applications such as diagnosis of PH, evaluation of serial changes related to the clinical course or therapeutic interventions in CTEPH patients, and discrimination between CTEPH and PAH.
The role of ventilatory gas analysis with postural changes in the assessment of PH merits further research. RHC remains an essential tool for making diagnosis, assessing disease severity, and evaluating response to therapy in patients with PH. Then again, ventilatory gas analysis with postural changes may represent a simple, safe, and feasible approach to use in clinical practice. This noninvasive method can be both repeatedly performed in the course of treatment and clinically implemented as an extremely useful screening tool. In addition, it might reduce the frequency of RHC attempts made to manage patients with PH.
The authors have no potential conflicts of interest to disclose.
Citation: Akizuki M, Kohzuki M (2020) Non-Invasive Screening for Pulmonary Hypertension Using Ventilatory Gas Analysis. Int J Phys Med Rehabil 8:547. DOI: 10.35248/2329-9096.20.08.547
Received: 26-Apr-2020 Accepted: 08-May-2020 Published: 15-May-2020 , DOI: 10.35248/2329-9096.20.08.547
Copyright: ©2020 Akizuki M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.