Journal of Clinical & Experimental Dermatology Research
Open Access
ISSN: 2155-9554
ISSN: 2155-9554
Case Report - (2019)Volume 10, Issue 5
Apocrine hidrocystomas of the eyelids are cystic tumors that have their origin in the glandular portion of the apocrine gland; in most cases they cause only mild or absent symptoms, but they may annoy patients when in huge number, usually interfering with the field of view and disfiguring of upper part of the face. Consequently, those affected usually ask these lesions to be removed. Although a solitary lesion can be treated easily with surgical excision, the elimination of multiple lesions may be problematic because of their number and locations. Many approaches were proposed for solving this particular clinical presentation. We present a difficult case of multiple apocrine hidrocystomas successfully treated using carbon dioxide laser with a good cosmetic outcome taking the opportunity for a literature review.
Multiple apocrine hidrocystomas; Eyelid's cystic tumors; Carbon dioxide laser; Ocular cysts, Apocrine cystadenoma
A 74 years old man presented to the Skin Clinic of Iris Polispecialistic Center for the occurrence of multiple slow growing cysts of eyelid and zygomatic region in last 5 years (Figure 1).
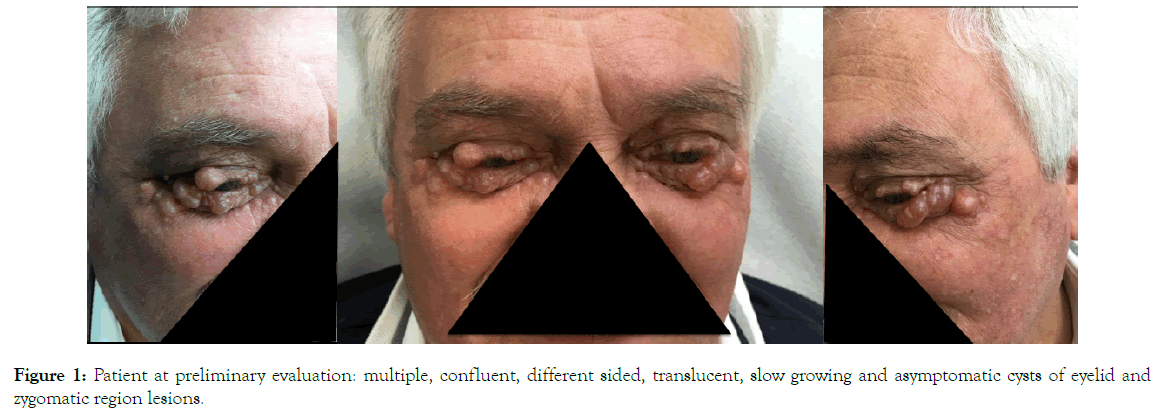
Figure 1. Patient at preliminary evaluation: multiple, confluent, different sided, translucent, slow growing and asymptomatic cysts of eyelid and zygomatic region lesions.
At clinical examination the cysts presented multiple, confluent, different sized, translucent, filled with a watery fluid and asymptomatic. On local examination, the lesions were observed on both the upper and lower eyelids reaching the tarsal margins and on both the zygomatic areas.
A clinical diagnosis of hidrocystoma was offered. Patient’s medical history included arthrodesis for lumbar discali hernias, carotid and coronary stenting and hypercholesterolaemia; he took baby aspirin, atorvastatin and lansoprazole. The patient had no known medical allergies.
The patient underwent, under local anesthesia, an excisional biopsy of one lesion and the specimen was subjected to a histopathological examination that confirmed the clinical hypothesis of multiple apocrine cystadenomas.
Many approaches were proposed for solving this particular clinical presentation. We decided to perform a carbon dioxide laser ablation. In mild sedation and with topical anesthesia with Oxybrupocaine Hydrochloride (Novesin) 4 mg/ml eyedrops we placed laser resistant eyeball shields. Then we completed anesthesia with local injection of lidocaine 2% on the eyelids and the zygomatic areas to treat (Figure 2).
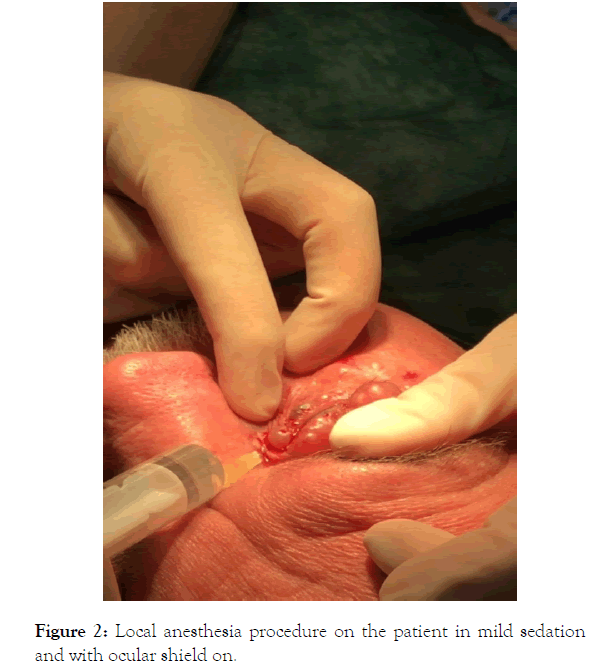
Figure 2. Local anesthesia procedure on the patient in mild sedation and with ocular shield on.
Then we performed the ablation of each cyst with a Deka SmartXide 2 Touch in HighPulse modality using 0.4-0.8 W at 50 Hz down to its back wall in the papillary dermis (Figure 3). During the procedure we took care to clear with a gauze moistened with sodium chloride solution the treated areas from the necrotic tissue in order to reduce the risk of carbonization e consequently of retracting scars. At the end of the operation a cold pack was left on for 30 minutes and then we applied an antibiotic tobramycin cream with a q-tip. We send home the patient with simple, cheap and transparent plastic glasses to leave on during the day to protect the eyes form dirty and dust and with a prescription of continuing antibiotic treatment topical and adding a systemic one for few days, anti-oedema tablets, paracetamol when needed, cold packs, and with the recommendation to hold the head up while sleeping. The patient came back for a clinical evaluation after 7 days, 30 days and 60 days.
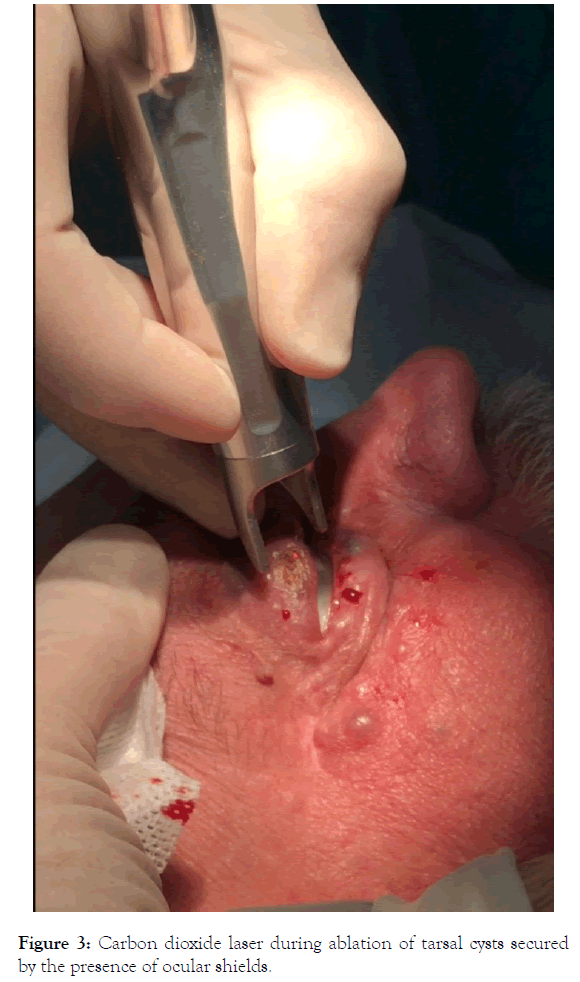
Figure 3. Carbon dioxide laser during ablation of tarsal cysts secured by the presence of ocular shields.
The cosmetic result was really satisfying with an almost complete disappearance of the lesions (Figure 4). The patient referred a good tolerability of the treatment with only a bit of discomfort in the first days after the treatment (Figure 5).
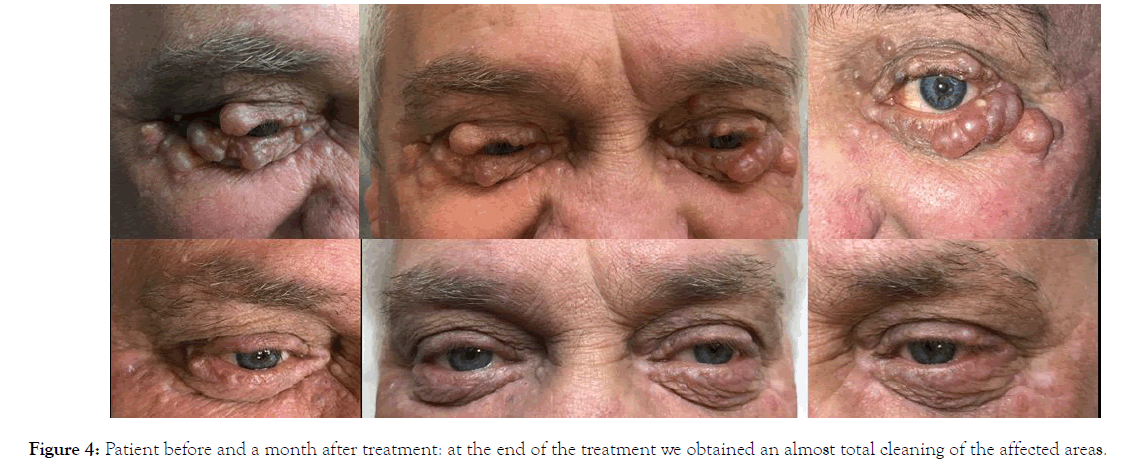
Figure 4. Patient before and a month after treatment: at the end of the treatment we obtained an almost total cleaning of the affected areas.
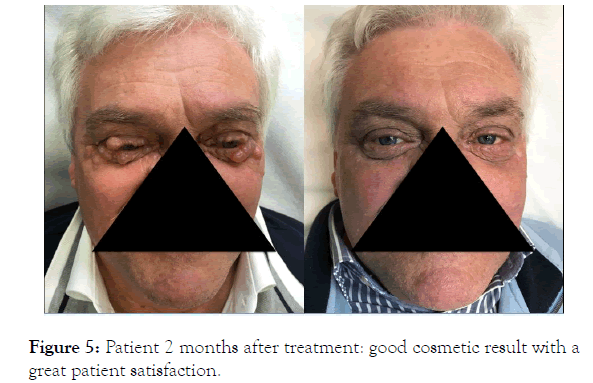
Figure 5. Patient 2 months after treatment: good cosmetic result with a great patient satisfaction.
We specified to the patient the typical tendency of this pathology to recur over the years and the likely need to repeat the treatment in the future.
Apocrine hidrocystomas, which are also known as apocrine cystadenomas and apocrine retention cysts, most commonly appear as asymptomatic and solitary or occasionally multiple dome-shaped, cystic translucent nodules presenting a smooth surface with color which varies from skin color to grayish or blue-black because of the Tyndall phenomenon or the presence of lipofuscin pigments in the fluid [1-7].
They were first described by Mehregan in 1964 that reported 17 cases of a benign neoplasm located on the face that he named “apocrine cystadenoma” underlining that the tumor had to be considered an adenomatous cystic proliferation of the apocrine gland [1,8-10].
In 1968, Grinspan et al. first reported a case of a patient with multiple lesions located on the face [5]. Apocrine cystoadenomas usually involve the periocular area, particularly lateral to the outer canthus but they may occur on ears, scalp, neck, trunk, shoulders, axilla, feet, anus [2,11-13] and genitalia where they have to be differentiated from the median raphe cyst of the penis or from the hidroadenoma papilliferum of the vulva [14-17].
In rare cases, they occur in greater numbers and usually confined to head and neck region most of all in periocular area in so called Robinson type [18-21].
The lesions increase slowly in size and may become 10 mm or more in diameter.
Unlike eccrine hidrocystomas, apocrine hidrocystomas have not been related to differences in temperature.
The usually appears in adult, without any relation with laboratory abnormalities, and without any predilection for sex or race [1,21-24].
Bilateral multiple apocrine hidrocystomas are uncommon and are usually associated with two rare inherited syndromes, namely the Schöpf-Schulz-Passarge Syndrome and the Goltz-Gorlin Syndrome also known as Jessner-Cole Syndrome or focal dermal hypoplasia Syndrome [25-28].
The main differential diagnosis is represented by eccrine hidrocystomas, described by Robinson since 1893 [29] that are ductal retention cysts of eccrine sweat glands, more common in the eyelid region. They usually appear as cystic translucent lesions covered by smooth and shiny skin, clearer and smaller than apocrine hidrocystomas, with a diameter ranging from 1 mm to 6 mm. They generally occur on the eyelids, especially near, but non directly involving, the tarsal margin, and on the cheeks affecting most of all adult women (sex ratio 8:1).
Heat, humidity, and perspiration can cause them to become larger, more numerous, and more symptomatic, than they tend increasing in number and size in summer and decreasing in winter [30-34]. Multiple eccrine hidrocystoma has been reported to be associated with Grave’s disease [35], Parkinson’s disease [36], idiopathic craniofacial hyperhidrosis and prolactinoma [37].
Other differential diagnosis of solitary apocrine hidrocystoma includes epidermoid or pilar cysts, cystic basal cell epithelioma and melanoma [1,7,38].
The other main differential diagnosis of the rare cases of multiples apocrine hidrocystomas are syringomas, milia, trichoepitheliomas, and angiofibromas [21-24].
At dermoscopic evaluation the lesions show a homogeneous pale gray or bluish pattern, whitish cotton wool-like structures, linear vessels, and nonconstant focal brownish orange areas. The grayish color is probably owed to the diffraction effect caused by the presence of sialomucin, the whitish structures are due to a reflection of the connective tissue and the brownish orange structures result from the presence of clear cells containing important amounts of glycogen. The dermoscopic feature of the peripheral linear vessels corresponds to the dilated vessels of the papillary dermis.
In contrast eccrine hidrocystomas present as a large uniloculated cyst lined by two layers of cuboidal to flattened epithelial cells, sometimes with squamous metaplasia, with or without connection to an eccrine duct.
In general, eccrine hidrocystomas are considered to represent dilatations of eccrine ducts due to retention of eccrine secretions. In contrast, apocrine cystadenomas are mostly thought to be true adenomas of the apocrine sweat gland coils because the secretory cells do not appear flattened as seen with a true retention cyst [1,2,7,20,31].
Furthermore, when the lesions are located on the margins of the eyelids, where Moll's glands are present, they must be differentiated from the retention cysts of these apocrine glands. The distinction is not difficult due to the presence of flattened epithelium and absence of columnar cells and papillary projections in this latter condition [40]. Alessi et al. theorized the lesions in these areas are not due to an adenomatous cystic proliferation of the normal Moll's glands, but they take their origin from ectopic residues of fetal apocrine glands [20]. Their hypothesis was in accordance with Kruse and collegues [41,42] who considered that the lesions only occur in areas where previous apocrine glands have usually been resorbed. This postulate was corroborated by the fact that there were no reports of multiple apocrine hidrocystomas in apocrine gland-bearing areas such as the axilla, groin and perineum.
Coming back down to nomenclature, despite clinical practice, and because of the probably different histogenesis of these tumors, the use of the name apocrine cystadenoma has been advocated to distinguish it clearly from the presumably nonneoplastic eccrine hidrocystoma, and use of the terms "apocrine hidrocystoma" or "eccrine cystadenoma" has been discouraged [43-45].
But, up to now, the precise histogenetic derivation and the cellular differentiation of these tumors and consequently their classification and nomenclature has been debatable. That’s because Immunohistochemical studies of their expression of the carcinoembryonic antigen (CEA) and of S1OO have not been unequivocal.
De Viragh et al. in 1997 for example suggested to use the term apocrine cystadenoma for the secretory type of cysts, because this cystic tumor has its origin in or differentiates towards the glandular portion of the apocrine gland, and that it is probably a neoplasm. In contrast, he suggested the term hidrocystoma, without specification of a sweat gland type, should replace the diagnosis of so-called eccrine hidrocystoma. Hidrocystoma in his classification indeed stood for a cystic tumor of a sweat gland duct. Staining for SMA (Smooth Muscle Actin) should in his study allow a distinction between a hidrocystoma and a cystadenoma, since SMA had proven to be a helpful marker to differentiate a secretory from an excretory type of sweat gland cyst. Furthermore, he divided hidrocystomas into eccrine or apocrine. This further distinction was possible only after staining for human milk fat globulin 1 (HMFG), that isn’t expressed in eccrine glands, but that it is found in the mammary and in the sebaceous gland, and in the apocrine secretory coil and ducts [43].
Lots of therapy is reported for handling of apocrine hidrocystomas. Solitary lesion could easily treated surgically by enucleation [44], or marsupialization [45].
On the other hand elimination of multiple lesions is problematic because of their number and location and surgical removal of multiple lesions often results in disfiguring scarring. Lots of methods for their management are described in literature such as electrodessication [46], sclerotherapy [47], blepharoplasty [48], trichloroacetic acid (TCA) [49,50], diode laser [51] and carbon dioxide laser [52-55].
Although solitary apocrine hidrocystomas can be treated easily with surgical excision, the management of multiple lesions could be a clinical challenge. In this last decades a lot of solutions have been proposed and among them laser therapy. Since these lesions are benign cystic neoplasms, a carbon dioxide laser approach could be taken into account, but this last option was fully described only in two articles. In these two publications, respectively of Bickey et al. in 1989 and Del Pozo et al. in 2001 a total of 11 lesions in three adult patients were treated with carbon dioxide laser vaporization using a continuous and defocused mode, with a power density of 5 J/cm2 with referred good cleaning and cosmetic outcome [54,55].
The technological progress of last decades led to development of new modality of energy emission in CO2 lasers.
The first CO2 laser systems, which used continuous wave delivery systems, were efficient at ablating and also cutting the tissues, but the high incidence of possible scars, and pigmentary modification limited their usage in the vaporization of thin, superficial layers.
Then, in the last 20 years, technological development permitted creation of high-energy, “SuperPulsed” and “UltraPulsed” systems which were able to emit shorter pulses with high peaks of power. This, in turn, allowed laser surgeons to ablate epidermal and dermal tissue with minimal risk of scarring, resulting in a precise and adequate vaporization of superficial skin layers, and limiting thermal damage to surrounding tissues. In fact, considering the pulse mode of the CO2 lasers, the earlier continuous wave lasers used low power and long pulse, that, for this duration, led heat in the surrounding tissue resulting in wide conical-shaped zone of thermal damage and consequently scars and dyschromia. On the other side the newer SuperPulse lasers produced a higher power peak in a shorter pulse width, enabling ablation threshold to be reached remaining under tissue thermal relaxation time of 0.8 ms, resulting in very quick ablation of target tissue with minimal thermal damage and then minimizing the injury of the underlying tissue. The newly introduced UltraPulse lasers, then, create a sustainable high pulse power, delivering about 6 times more power, then CW laser, but the pulse width is even shorter of SuperPulse laser and reaches ablation threshold well before thermal relaxation time.
Therefore, UltraPulsed CO2 lasers are more powerful, penetrate deeper and have less collateral thermal effect.
That’s why we decide to use ultrapulsed carbon dioxide in the therapy attempt to treat this difficult case of multiple apocrine hidrocystomas, even though well aware of the limitations of a relapsing tendency of this condition.
At the end of the treatment we obtained an almost total cleaning of the affected areas from the cystic lesions with a good cosmetic result and a great patient satisfaction.
With the limitation of a single case we feel that carbon dioxide ablation wherever performed with the newest technology could be a strategy to consider in the management of single and multiple apocrine hidrocystomas.
Citation: Russo A, Cannarozzo G, Sannino M, De Luca G (2019) Multiple Apocrine Hidrocystomas of the Eyelids: A Case Successfully Treated Using UltraPulsed Carbon Dioxide Laser and Literature Review. J Clin Exp Dermatol Res. 10:5. Doi: 10.35248/2155-9554.19.10.507
Received: 22-Sep-2019 Accepted: 07-Oct-2019 Published: 14-Oct-2019 , DOI: 10.35248/2155-9554.19.10.507
Copyright: © 2019 Russo A. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.