Gynecology & Obstetrics
Open Access
ISSN: 2161-0932
ISSN: 2161-0932
Research - (2019)Volume 9, Issue 11
Objective: Performance of feminine cleansers can be improved by increasing antimicrobial potency and favouring the prolonged contact on vulvo-vaginal mucosa of active ingredients, that permits the increase of the persistence of their activity, and consequently of their efficacy.
The presence of specific polymers in topical formulations allows the cleansing solution to adhere to mucosal substrates.
The associations of xanthan gum with lambda carrageenan from Chondrus crispus has a high mucoadhesive potential. The aim of our study was to evaluate the mucoadhesive potential and the safety of an innovative cleanser for feminine hygiene (SA3) compared with a reference cleanser (SA) in a double-blind design.
Material and Methods: The cleansers were provided at dilutions: the reference product containing thymol from Thymus vulgaris extract at pH 3.5 (SA), and the test one with Zinc Coco Sulfate for its antimicrobial action, and Xanthan gum and Chondrus crispus extract as mucoadhesive agents (SA3) added to SA.
The pro-sensitizing test was performed on cultures of human monocytes THP-1. The expression of two membrane markers, CD86 and CD54, was evaluated and compared with the sensitizing 2,4-dinit rochlorobenzene as a positive control, and with untreated cells as a negative control. Light scattering images were used to analyze the morphology of THP-1 cells.
The mucoadhesivity was assessed as percentage of inhibition of the lectin-binding glycoprotein.
Results: No changes in CD86 and CD54 expression were evidenced in the cells exposed to the test product, compared to the untreated cells. Furthermore, no significant alteration of the cell morphology was observed for SA3. Mucoadhesion% values showed a statistically significant difference in favor of SA3 even at 1:2 and 1:5 dilutions. At the higher dilution, SA3 lost only 23.2% of mucoadhesion versus the lower one, compared to the 45% lost between the two SA dilutions.
Conclusion: No pro-sensitizing potential and no significant alteration of the cell morphology were observed for SA3 compared to the untreated cells. SA3 is safe, with high mucoadhesion to the vulvo-vaginal mucosa, significant even at higher dilution.
Feminine hygiene; Mucoadhesion; Xanthan gum; Zinc coceth sulfate, Plant extracts; Cleanser
The vulvo-vaginal cavity can be the site of various fungal and bacterial infections that can be successfully treated by a local administration of anti-infective and anti-inflammatory drugs.
A correct lifestyle schedules routine daily intimate hygiene by cosmetic products aimed to protect the physiological vaginal microbiome through formulations containing microbiologically active ingredients.
Different feminine detergents are present on the market, but they need scientific documentation to support the claim of antimicrobial and anti-inflammatory activity [1]. Research and Development in cosmetology have been working for improving the performance of these cleansers, following a double way strategy: increasing antimicrobial potency and persistence of activity. This rationale has been applied to a standard cleanser (SA) containing the microbiologically active Thymus vulgaris extract at acid pH [2,3]. The innovative formulation (SA3) has been designed adding Zinc Coceth Sulfate to potentiate the antimicrobial action and a mucoadhesive system to extend the persistence in situ of the active ingredients of SA. The increased antimicrobial effect was ascertained in a specific in vitro study versus SA [4].
Prolonged contact of a formulation on vaginal mucosa permits to increase the efficacy not only of drugs but even of cosmetic products or medical devices containing active ingredients intended for vaginal application can benefit of extended contact with the mucosa.
The capability of mucoadhesive formulations to adhere to mucosal substrates is generally due to the presence of specific polymers [5].
The association of xanthan gum with lambda carrageenan from Chondrus crispus was selected considering their highest mucoadhesive potential reported in previously published studies [6].
A new surfactant system more respectful of the vaginal environment, with emollient and moisturizing activity, completed this innovative formulation.
The objectives of our study were to preliminarily check the safety of this new cleanser indicated for feminine hygiene, with active ingredients from plant extracts in a mucoadhesive system at acid pH with mild surfactants (SA3=Saugella Attiva new, Mylan) performing in vitro analysis of its pro-sensitizing potential, then to evaluate its mucoadhesive property compared with the reference cleanser (SA) based on traditional surfactants in a double-blind design.
The pro-sensitizing test was performed on cultures of human monocytes THP-1, used as dendritic cell model, treated with a non-cytotoxic concentration of the tested product by Bio Basic Europe, Pavia, Italy.
As positive control 2,4-dinit rochlorobenzene (DNCB 4 g/mL), well known sensitizing substance, was used, while the negative control was represented by untreated cells maintained in culture medium.
Then tested sample was used at 20 g/mL concentration. THP-1 cells were cultured in RPMi-1640 medium supplemented with fetal bovine serum (10%), 2-mercaptoethanol (0.05 mM) and antibiotics (1%). Cells were incubated at standard conditions (37°C, 5% CO2).
Good cell culture practices were used. The variation in the expression of two membrane markers, CD86 and CD54, was evaluated: a significant increase in the expression of these molecules on cell surface is indicative of the activation of an immune response and therefore the potential sensitizing action of the test substance. In detail, a <10% change of CD86 and CD54 co-expression corresponds to insignificant variation, a 10 change is a significant variation and proves pro-sensitising potential.
Light scattering images were used to analyze the morphology of THP-1 cells. The parameters determined in cytofluorimeter analysis are the intensity of Forward Scatter (FSC) and the intensity of Side Scatter (SCC).
The combination of two types of signal (FSC and SSC) allows obtaining a diagram of dispersion called cytogram (a dot plots), in which different cell populations can be evidenced, based on the physical characteristics only. FSC is proportional to dimension and form of the cells and is very sensitive to the properties of cell surface, distinguishing e.g. from alive and dead cells.
The study on mucoadhesivity was planned and carried out according to a controlled, double-blind design, comparing the test product SA3 (Saugella Attiva new, Mylan) versus the reference SA (Saugella Attiva, Mylan) as traditional cleanser.
The main ingredients of SA3 are Zinc coco sulfate, disodium capryloyl glutamate, and coco-glucoside as surfactants, propylene glycol, glycerin as a humectant, glyceryl oleate as emollient, lactic acid and caprylyl glycol as skin conditioning, xanthan gum and Chondrus crispus as gel-forming mucoadhesive agents.
SA reference traditional formulation does not contain zinc, muco-adhesive polymers, moisturizing agents, and the innovative non-aggressive surfactants included in SA3.
Both cleansers were buffered at pH 3.5 and contained Thymus vulgaris extract as antimicrobial and anti-inflammatory [7].
To maintain double-blind condition of the investigator up to the end of the study, the two solutions were supplied in identical bottles, labeled only with a progressive number randomly assigned to each product [8].
The key code was delivered in a sealed envelope to the investigator that disclosed it only after the completion of the determinations and the statistical analysis.
The muco-adhesivity model comes from the optimization of previous experimental protocols and scientific work on the subject. This model can be applied to buccal or vaginal mucosal cells. Initially, the cells are treated with biotinylated lectin, a protein contained in some Leguminosae (Canavalia ensiformis), which shows a high affinity for mannoside and glucoside residues present in the membrane glycoproteins.
Thus, the sites of the glycoproteins of the mucosal membranes are engaged with biotinylated lectin. The presence of biotin (vitamin H) in the lectin structure is indispensable for the next step.
The cells, previously treated with biotinylated lectin, are added to streptavidin-peroxidase allowing, thanks to the high affinity between biotin and streptavidin, to form the complex proteinglucose- lectin-biotin-streptavidin-peroxidase.
The cells are then washed, and the protein-glucose-lectin-biotinstreptavidin- peroxidase complex is quantified, thanks to the presence of peroxidase, by the reaction of the vegetablephenylenediamine oxidation.
The reaction of oxidation reported below, will, in fact, be catalyzed by the complex protein-glucose-lectin-biotinstreptavidin- peroxidase:
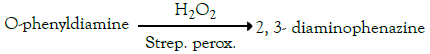
The intensity of the yellow-orange coloring of the solution (measured with a spectrophotometer at λ=450 nm) is proportional to the amount of glycoprotein-lectin bonds and therefore to the number of available sites (glycoproteins) for the mucoadhesion. The absorbance value determined is "control."
In the determination of the mucoadhesiveness of a product, the cells are treated beforehand with this (incubation at 30°C for 15 minutes before treatment with lectin).
If the product in question contains mucoadhesive substances, these will bind to glucoside and mannosidic sites present in the membrane glycoproteins.
In the next step, adding the sequence of the biotinylated lectin, streptavidin peroxidase and ortho-phenylenediamine will be obtained, compared to the control, a coloration of lower intensity and this is because a part of the glucosidic sites available for binding to the Con-A have already been engaged by the mucoadhesive substances present in the test product.
In fact, the initial link between the mucoadhesive substances, contained in the product to be tested, and the glucoside sites, without prejudice to the next part of the conjugation Con-A with the streptavidin-peroxidase complex and the subsequent color development after the addition of hydrogen peroxide.
The decrease in absorbance is proportional to the ability of the test substances of mucoadhesion to the mucosal cells.
The mucoadhesive capacity is expressed as a percentage of inhibition of the glycoprotein-lectin binding, which reflects the extent of mucosal sites occupied by the product (which can be also expressed as a percentage of mucoadhesion of the product) according to the equation:
% mucoadhesion of the product = (1 ˗ abs product/abs control) × 100. The mucous cells used for the test were EpiVaginal kit VEC-100, MatTek Corporation.
The statistical analysis was performed applying the Student’s ttest. The level of significance was taken to be α=0.05 (type 1 error) and power β=0.90 (type 2 error).
The pro-sensitizing test did not show a significant change in the expression of CD86 and CD54 in the cells treated with the test product compared to the not treated cells.
The not treated control gave a nil% change of CD86 and CD54 expression, the positive control respectively +73.4% and +55.4% as mean value and the tested sample 4.6% and 3.1%.
No significant alteration of the THP1-1 cell morphology was observed. In fact, the light scattering images underline that the cells treated with the tested sample, do not undergo a substantial change of morphology, being the image superimposable to the one of untreated control cells (Figure 1) [9-21].
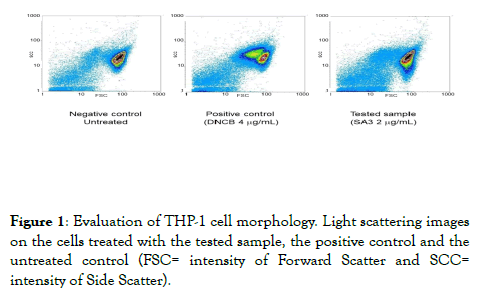
Figure 1. Evaluation of THP-1 cell morphology. Light scattering images on the cells treated with the tested sample, the positive control and the untreated control (FSC= intensity of Forward Scatter and SCC= intensity of Side Scatter).
The number of cells that undergo an alteration of the morphology is negligible and comparable to the one of the untreated cells (Figure 2).
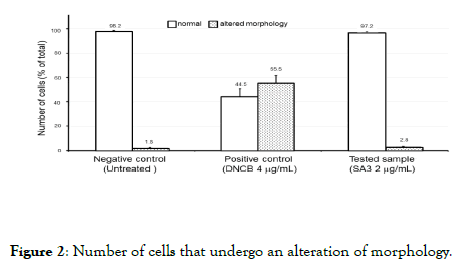
Figure 2. Number of cells that undergo an alteration of morphology.
Mucoadhesion% values showed a statistically significant difference in favor of SA3 at the two dilutions tested (1:2 and 1:5). At the higher dilution, SA3 lost only 23.2% of mucoadhesion versus the lower one, compared to the 45% lost between the two SA dilutions (Figure 3).
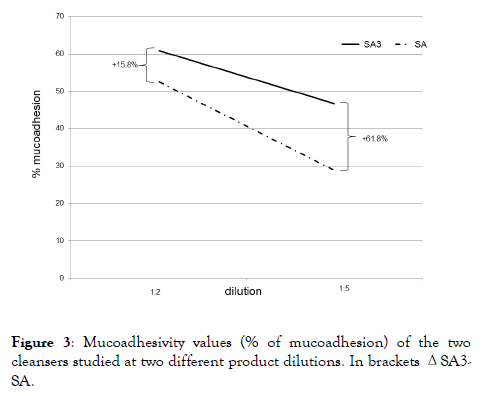
Figure 3. Mucoadhesivity values (% of mucoadhesion) of the two cleansers studied at two different product dilutions. In brackets ΔSA3- SA.
Mucoadhesion is used in the pharmaceutical field to enhance localized active product delivery allowing two surfaces, represented by a mucous membrane and a product layer, to adhere to each other. In the first step, the mucoadhesive macromolecules establish the adhesive interactions with the contact or wetting phase followed by the consolidation phase.
The application of this technology to the gynecological field requires careful consideration of the consolidation as the formulations are exposed to significant removing stresses.
The outcomes of the study evidenced the safety of the product for the lack of co-expression of the membrane markers, meaning that SA3 has no pro-sensitising activity. The immune response for the tested sample, in terms of CD86/CD54 expression, proved to be lower than the one obtained with the DNCB “positive” control and comparable to the one obtained with the untreated “negative” control.
Safety and activity are the pivotal issues that should be considered for products used in personal care, and even more for intimate hygiene purposes. The innovative non-aggressive surfactants combined with the other ingredients contained in SA3 are safe, non-toxic and well-tolerated.
Moreover, zinc contained in the formulation has anti-irritant properties and is useful for keeping the skin in healthy conditions, supporting collagen synthesis, fibroblast proliferation, and repair processes and favoring epidermis maturation.
The activity of thymol, that is the main active component of SA3, is improved by prolonged contact with the vulvovaginal mucosa, thanks to the mucoadhesiveness remaining high even when the formulation was progressively diluted and can give reliable protection to the genital area.
In fact, thymol has antibacterial and antimycotic action, without interfering with Lactobacilli growth. The possibility of longer permanence in situ allows thymol to express better the pharmacological and microbiological properties of thymol.
Thymol affects the envelope of Candida inducing morphostructural damages of the Candida envelope, reducing the ability of the fungi to adhere to mucosal cells, thus decreasing their virulence and infectiousness. It inhibits C. albicans filamentation affecting the invasive capability of the fungus, disturbing the cell membrane and Candida metabolism.
Thymol can antagonize native and mature biofilms of Candida and important factors conditioning the virulence and pathogenicity of these two pathogens, having a high tendency to develop biofilms that allow microbes to tolerate higher concentrations of antibiotics. Biofilm, presence increases the possibility of recurrence of vulvovaginal infections. Moreover, the persistence of thymol on the vulvovaginal site is useful for its antioxidant and anti-inflammatory effects.
The experimental results on the degree of mucoadhesion recognize the successful scouting in research and development around the degree of cross-linking, chain length and the presence of various functional groupings of the various polymer examined.
In conclusion, SA3 is a safe cleanser with high mucoadhesion to the vaginal mucous membrane, remaining significant even when the formulation is progressively diluted: these properties make it suitable for successful use in the daily intimate feminine hygiene.
Benvenuti C and Gasparri F are consultants and Zanardi A is employed for Mylan. No competing financial interests exist.
Citation: Benvenuti C, Gasparri F, Zanardi A (2019) Mucoadhesive Properties of a New Intimate Hygiene Cleanser against Vulvovaginal Candidiasis. Gynecol Obstet (Sunnyvale ) 9:513. doi: 10.35248/2161-10932.19.9.513
Received: 22-Oct-2019 Accepted: 30-Oct-2019 Published: 06-Nov-2019
Copyright: © 2019 Benvenuti C, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.