Research Article - (2018) Volume 4, Issue 2
Molecular Phylogenetic Taxonomy and Descriptive Analysis on Hexangium sigani Goto & Ozaki, 1929 (Digenea: Microscaphidiidae) from Three Different Siganus spp. Fishes from Red Sea, Egypt.
*Corresponding Author: HS Mohamadain, Department of Zoology, Faculty of Sciences, South Valley University, 83523, Qena, Egypt Email:
Abstract
Three different common species of Rabbitfishes in the Red Sea region were found to be naturally infected by Hexangium sigani Goto and Ozaki, 1929. The encountered parasites were described morphologically and morphometrically by means of light and scanning electron microscopy. Present specimens presented and exhibited a wide range of variability inside the same host and at same locality and accordingly all previous synonyms of Hexangium sigani were presented, shown here and discussed with the previously described forms. These variations included testes position relative to each other and relative to ovary, body spination and uterus extension but these differences were considered to be of minor importance. The SEM disclosed well differentiated three forms of sensory papillae; oral papillae, genital papillae and body papillae which may reflect a variation in the functions they performed. Furthermore, the true nature of male genital system in all Hexangium spp. were reviewed and elucidated the absence of cirrus sac in all known species and probably some fibrous tissues may be around the seminal vesicle. Also, Key to species of Hexangium Goto and Ozaki, 1929 was added. Molecular data characterized Hexangium sigani within Microscaphidiidae and referred to an interrelationship between Microscaphidiidae & Mesometridae which in need to more future analyses to give a deeper understanding. It is worth mentioning that SEM study of this parasite was done for the first time from Egypt with an addition of many ultrastructural details; most of which are of taxonomical importance. For the first time, Siganus luridus represented a new host record of H. sigani.
Keywords: Egypt; Microscaphidiidae; Hexangium; Hexangium sigani Goto and Ozaki, 1929; Siganus; Rabbitfishes; Red Sea
Introduction
Goto and Ozaki erected Hexangium with H. sigani from Siganus fuscescens in Misaki and Takamatsu, Japan as its type species [1]. Yamaguti partly re-described this species from the same host in the Inland Sea of Japan and recorded it late from the intestine of a Siganus sp. of the Celebes [2,3]. Tubangui and Masilungan added H. affinum as the second species from Siganus javus of Manila, the Philippines [4]. From the same geographical region, Annereaux described H. secundum from a single specimen obtained from Siganus guttatus of Mercedes, Samar, the Philippines [5].
Nagaty established Arthurloossia as the fourth genus in the family Mesometridae Poche, 1926 and described its type species Arthurloossia loossi which had been collected from two fish species, Hipposcarus harid and Siganus canaliculatus in the Red Sea of Hurgada, Egypt [6]. Investigations clarified that Arthurloossia Nagaty, 1954 was congeneric with H. sigani with very few and insignificant variations [7]. So, Arthurloossia was reassigned as a synonym of Hexangium Goto and Ozaki, 1929. In addition, Arthurloossia loossi Nagaty, 1954 synonymized a junior synonym of Hexangium sigani due to the great identity [7]. Also, Yamaguti erected a new subfamily Hexangiinae in the family Angiodictyidae Looss, 1902 for Hexangium [7].
Razarihelisoa recorded H. sigani from non siganid fish Neoniphon sammara (Syn. Holocentrus samara) and observed the great similarities among H. affinum, H. secundum, its specimens and Yamaguti’s description of H. sigani [8]. Accordingly, he cast doubt about the validity of H. affinum, H. secundum and H. loossi, and considered them as synonyms of H. sigani. The subsequent studies by Velasquez and Fischthal & Kuntz revealed them agreement with the previous author in considering H. affinum, H. secundum and H. loossi as synonyms of H. sigani [9,10]. Gupta and Miglani expressed a different point of view and refused these synonymies [11]. Nevertheless, subsequent reports of H. sigani have continued to accept these synonymies [12-17].
Manter described H. elongatum from Naso sp., of Fiji [18]. Since ventral body-surface of H. elongatum concaved anteriorly and modified to form accessory attachment organ, Jones and Blair eliminated H. elongatum from Microscaphidiidae Looss, 1900 and transferred it inside Mesometridae Poche, 1926 as a new genus Parawardula [19]. Jones and Blair due to the very close similarities with to Wardula Poche, 1926 and Parawardula elongate [18] n. comb. as the type species [19].
Machida and Uchida described another species H. leptosomum from Naso unieornis off Okinawa [21]. This species characterized by concaved ventral body-surface anteriorly and modified to form accessory attachment organ, tandem testes, the caeca almost reach the posterior extremity and lack an oesophageal bulb so, Blair transferred this species into Mesometridae Poche, 1926 as a new genus Pseudohexangium with Pseudohexangium leptosomum n. comb. as the type species [20,21].
In 2005, Hassanine and Gibson described a new species H. brayi from Siganus luridus of Sharm El-Sheikh, South Sinai, Egypt [22]. Also, in 2013, two new species added; H. ecsomi from Siganus rivulatus of Red Sea, Saudi Arabia and H. saudii from the same fish species off Saudi coast of the Red Sea [16,23].
The most structuring taxonomy of Hexangium Goto and Ozaki, 1929 recognized only four accepted specie; H. brayi, H. sigani, H. ecsomi and H. saudi [24]. All these species are commonly known in the Red Sea region [17,23].
As part of an on-going study of the digenean tremadodes parasitizing some Red Sea fishes, the main purpose of this study was to increase our knowledge by the endohelminths of fish from the Red Sea through clarifying the morphological variations in internal organs’ shape and distribution and ultrastructural description of a questionable Microscaphidiid species Hexangium sigani collected from some Rabbitfishes, of Sharm El-Naga. Also, molecular characterization of Hexangium sigani within Microscaphidiidae. Lastly, providing a key to the species inside this genus.
Materials and Methods
Morphological data
A total of ninety four Rabbitfishes: seventy Siganus rivulatus Forsskal and Niebuhr, sixteen Siganus sutor Valenciennes and eight Siganus luridus Ruppell (Perciformes: Siganidae), were caught by small trawl in the Red Sea off Sharm El-Naga, Egypt, during the period from July 2011 to August 2012. Fish were transported as alive as possible with good aeration and cooling immediately to the laboratory, the alimentary tract from the esophagus to the anus removed and examined for endohelminths under a dissecting microscope and the surrounding peritoneal cavity examined by the aid of a magnifying hand lens. Digeneans were relaxed in 1 part unfiltered sea water to 3 parts tap water as per [25], observed alive, fixed in near boiling water for 2 min [26], then under very slight cover slip pressure in a 5% buffered formal saline solution [27], preserved in 70% ethanol, stained with alum carmine and mounted in a mixture of distyrene and a plasticizer dissolved in toluene-xylene (DPX). Drawings were prepared with the aid of a Zeiss Universal compound microscope using micro-projector or camera Lucida (PZO 01852 10x). Measurements for the species description are expressed in micrometers (µm) with ranges and means indicated; the number [n] of measurements is also noted where needed (Table 1). Comparative measurements were taken from the original species descriptions unless otherwise stated. If needed, some critical measurements that were not available from the original descriptions were calculated from original illustrations and are identified herein. The fish host was identified according to criteria established by [28-31]. The identification more confirmed through the fishbase website (http://www.fishbase.org). Digenea identification is based on Bray [32]. Ecological terms follow Bush et al. [33].
| Reference | Goto & Ozaki, (1929) | Tubangui & Masilufigain (1944) | Annereaux (1947) | Yamaguti, (1953) |
Nagaty (1954) | Velasquez (1961) | Fischthal & Kuntz (1964) | Gupta & Tandon (1983) | Present study | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Parasite Name | H. sigani | H. affine | H. secundum | H. sigani | A. loossi | H. sigani | H. sigani | H. sigani | H. sigani | ||
| Host(s) | Siganus fuscescens | Siganus javus | Siganus guttatus | Siganus sp. | Hipposcarus harid & Siganus canaliculatus | Siganus argenteus | Siganus spinus & Siganus canaliculatus & Stolephorus commersonnii | Johnius borneensis | Siganus rivulatus & Siganus luridus & Siganus sutor | ||
| Locality | Misaki and Takamatsu, Japan | Manila, Luzon, Philippines | Mercedes, Samar, Philippine Islands | Celebes Sea | Ghardaga, Egypt in Red Sea | Malabon, Rizal, Luzon Island. Philippines | Puerto Philippines. Princesa, Palawan Inland, | Bay of Bengal , at Puri, Orissa | Sharm El-Naga, safaga Egypt in Red Sea | ||
| Length [L] | 8,000−10, 000 | 2,700-3,000 | 4,170 | 3,600− 5,300 |
6,278−8,170 | 1,510− 4,500 |
2,005- 3,030 |
4,350− 6,540 |
3,431−3,958 (3,661) | ||
| Width [W] | 1,800− 2,200 |
800−1,000 | 1,020 | 700−1,150 | 1,360−2,150 | 310−1,100 | 425 –835 | 750−1,600 | 668−1,149 (917) | ||
| Width%* | 22−23b | 30−33b | 24b | 19−22b | 22−26b | 21−24b | 21−28b | 17−24b | 19−29 25 | ||
| Pharynx length × width | 380−450 × 380−450 | 200−240 × 200−240 | 210 × 210 | 120−130 × 130−150 | 264−290 × 264−290 | 110−250 × 110−250 | 138−160 × 159−222 |
200−280 ×150×300 | 141-217 (186) ×164-224 (194) | ||
| Ph. length%* | 4−5b | 7−8b | 5b | 2−3b | 3−4b | 5−7b | 5−7b | 4− 5b | 4−6 (5) | ||
| Oes. length | 1,450−1,650 | 400−460 | 560 | 500− 850 | 1/8 - 1/6 length of worm | 300−690 | 248−380 | 600−890 | 412−432 (420) | ||
| Oes. length %* | 17−18b | 14−15b | 13b | 14−16b | 13−17b | 15−20b | 12−13b | 13− 14b | 10−12 11 | ||
| Oes. bulb L × W | 250 × 250 | 120−150 × 120−150 | 130× 130 | Absent | [280−412 × 297−351]a | 60−120 × 110−250 | 108−158 × 90−169 | --- | 133-183 (114) × 80-152 (115) | ||
| Oes. bulb L%* | 2−3b | 4−5 | 3b | --- | 5a | 3−4b | 5b | --- | 3−4 (3) | ||
| Ph. length: Oes. bulb L | 1:0.56−0.66b | 1:0.60−0.63b | 1:0.62b | --- | 1:0.61− 1.08a | 1:0.48− 0.55b | 1:0.78− 0.99b | --- | 1:0.64− 0.94 1:0.79 | ||
| Ph. Width: Oes. bulb W | 1:0.56−0.66b | 1:0.60−0.63b | 1:0.62b | --- | 1:0.70− 0.99a | 1:0.48− 0.55b | 1:0.57− 0.76b | --- | 1:0.49− 0.68 1:0.59 | ||
| Cirrus sac length | 330−500 | 100−120 | Absent | Absent | Absent | Absent | 86−91 × 63−103 | Absent | Absent | ||
| Ovary length × width | about 1/2 testis diameter | 100−120 × 100−160 | 240 × 240 | 200−300 × 200−300 | 413−616 × | 57−291 ×55−322a | 114−222 × 145 −179 | 230−470 × 250−400 | 183−241 (204) ×163−264 (210) | ||
| Ovary length%* | 6b | 4b | 6b | 5−6b | 7−8b | 5− 6b | 6−7b | 5−7b | 5−6 (6) | ||
| Anterior Testes L × width | 900− 1,100 | 240−260 × 240−300 |
300 × 300 | 400−550 × 300−450 | 1,080− 1,554 × 790−925 | [100−619 × 84×525]a | 106–237 × 152–270 | 450−900 × 500−670 | 470−517 (415) × 404– 460 (344) |
||
| AT length%* | 11b | 8−9)b | 7b | 10−11b | 17−19b | --- | 5−8b | 10–14b | 12−14 (13) | ||
| Posterior Testes L × width | 900− 1,100 | 240−260 × 240−300 |
300 × 300 | 400−550 × 300−450 | 1,080− 1,554 × 790−925 | 103−671× 90−550a | 133–380 × 169 -280 |
570−850 × 510−750 | 433-520 (485) × 337-547 (437) | ||
| PT length%* | 11b | 8−9b | 7b | 10−11b | 17−19b | --- | 7−13b | 12–13b | 13−14 (13) | ||
| Mean testes L : Ovary L | 1:0.50b | 1:0.42− 0.46b | 1:0.80b | 1:0.50− 0.55b | 1: 0.38−0.40b | 1:0.45− 0.56a | 1:0.72− 0.95b | 1:0.40–0.54 b | 1: 0.40− 0.49 (1:0.47) | ||
| Mean testes W: Ovary W | 1:0.50b | 1:0.42− 0.53b | 1:0.80b | 1:0.67b | 1: 0.52−0.67b | 1:0.63− 0.80a | 1:0.65− 0.90b | 1:0.50− 0.56b |
1:0.44–0.52 (1:0.49) | ||
| OS to genital pore%* | 5a | --- | --- | --- | 5−7a | --- | --- | 9–10b | 7–8 (8) | ||
| Oes. bulb to ovary%* | 69a | --- | 63a | --- | 55−70a | --- | --- | --- | 63−70 (67) | ||
| Prebifurcal distance %* | 17a | − | 22a | --- | 16−18a | --- | --- | 21a | 21−23 (22) | ||
| Post-caecal distance%* | 25a | --- | 22a | --- | 18−26a | − | − | 26a | 26−28 (27) | ||
| Pre-genital pore distance%* | 11a | --- | --- | 10−11b | 10−18a | --- | --- | 12−13b | 13−14 (13) | ||
| Previtelline distance%* | 24a | --- | 34a | --- | 17−33a | --- | --- | 30a | 25−29 (28) | ||
| Post-vitelline distance%* | 32a | --- | 20a | --- | 21−33a | --- | --- | 35a | 28−34 (30) | ||
| Preovarian distance%* | 86a | --- | 85a | --- | 83−86a | --- | --- | 89a | 88−89 (89) | ||
| Post-ovarian distance %* | 9a | --- | 9a | --- | 6−11a | --- | --- | 6a | 8−11 (9) | ||
| Pre-testicular distance%* | 69a | --- | 79a | --- | 71−76a | --- | 75a | 70−72 (71) | |||
| posttesticular distance%* | 13a | --- | 15a | --- | 8−11a | --- | 4−18b | 12a | 7−13 (11) | ||
| Post-uterine distance%* | 8a | --- | 8a | --- | 4−8a | --- | --- | 4a | 6−9 (8) | ||
| egg length × width | 77–85 × 50–56 | 80–100 × 48–57 | 82–90 × 52–56 | 75-81× 54 | 86–90 × 054–62 | 70–80 × 50–60 | 77–88 × 43–56 | 80−90 × 50−60 | 75-82 (78) × 46-50 (48) | ||
All dimensions, measurements and percentages are calculated to [0] decimal places; all ratios are calculated to 2 decimal places.
*=Proportion of body length, IB=Intestinal bifurcation, OS=Oral sucker, VS=Ventral sucker, AT=Anterior testis, PT=Posterior testis, Oes.=Oesophagus.
aCalculated from figures of the original description: Annereaux (Figure 1) [5], Nagaty (Figure 6 & 7) [6], Velasquez (Figure 10-13) [9], Gupta & Tandon (Plate 3, Figure 1) [37].
bCalculated from measurements given in the original description.
---=Neither given in the original description nor available from the published illustrations.
Table 1: Comparison of the measurements, morphometric percentages and morphometric ratios of Hexangium sigani between the present specimens against the previously described questionable synonyms and forms.
Specimens were deposited in the Zoology Department Museum, Faculty of Sciences, South Valley University (SUV), Qena, Egypt.
Ultra-structural data
For scanning electron microscopy; the relaxed specimens were fixed for 6 h at 4°C in 2.5% glutaraldehyde in 0.1 M sodium cacodylate buffer then washed several times in the same buffer. The post fixation carried out in 1% osmium tctroxide for 2 h, specimens washed two times cacodylate buffer then dehydrated in ascending grades of ethanol series, transferred into pure acetone. Samples were then processed in a critical point drier "Bomer-900" with Freon 13 then sputter coated with gold in a Technics Hummer V and viewed with a JEOL JSM-5400LV SEM operated at 15 kV in electron microscopy unit, Assiut University [34].
Molecular data
Total genomic DNA was extracted using DNA Extraction Kit (QIAamp DNA Blood Mini Kit (Cat. No. 51140). A region from bp 92 to bp 112 off the 18S nuclear ribosomal DNA was chosen for the forward primer (5′-GGT TCC TTA GAT CGT ACA TGC-3′), bp 498 to 519 bp for the reverse primer (5′-GTA CTC ATT CGA ATT ACG GAG C-3′). PCR amplifications were carried out using Taq PCR Master Mix Kit (Qiagen, Cat.201443) in a total volume of 25 μl consisting of 12.5 μl of Taq PCR Master Mix, 0.5 μl of each primer, and 1 μl of DNA template, made up to 25 μl with Invitrogen™ ultraPURE™ distilled water.
The PCR Amplification was performed in a thermal cycler (Eppendorf) with the GastB programme:after an initial denaturation step to hot start the polymerase at 95°C for 15 min, 30 cycles of 1 min at 95°C, 2 min at 56C and 3 min at 72°C and an elongation step at 72°C [35]. Amplified DNA was purified using a QIAquick Gel Extraction Kit (Qiagen, Cat. No. 28704) according to the manufacturer's protocol. Amplified DNA fragments were sequenced directly using the ABI Prism Big Dye Terminator V.3.1 Cycle sequencing Kit on an ABI 310 DNA automated sequencer (Applied Biosystems) reactions were done in 20 μl mixture reaction, according to the instructions of the manufacturer, using the same primers used for PCR amplification. Sequencing had been carried out at Genetic Engineering Research Department (VACSERA), Cairo, Egypt.
Newly generated 18S sequences were aligned with sequences of species of Superfamily Paramphistomoidea taxa available on GenBank (Table 2). Alignments were performed using Clustal W tool in MEGA v7.0.26 software. The resultant alignments were refined by eye using MEGA v7.0.26 and the ends of each fragment were trimmed to match the shortest sequence in each alignment.
Minimum evolution (ME), Neighbour-joining (NJ), Maximum Parsimony (MP), maximum likelihood (ML) and Bayesian inference (BI) analyses were used to determine the relationships of the present isolate and to attempt molecular identification. Both Minimum evolution and Neighbour-joining analyses of Kimura-2-parameter distances were carried out using MEGA v7.0.26; nodal support was estimated using 1,000 bootstrap resamplings. Genetic distance matrices (p-distance model) were also calculated with MEGA v7.0.26. Maximum Parsimony was performed using MEGA v7.0.26; nodal support was estimated using 1,000 bootstrap resamplings and 50% consensus trees were calculated. Maximum Likelihood analysis was performed by PAUP v4.0a150 software; nodal support was estimated using 1,000 bootstrap resamplings and likelihood parameters set for ML analysis were based on the Akaike Information Criteria (AIC) test in MrModeltest 2.3. Bayesian inference utilizing Monte Carlo Markov Chain (MCMC) analysis in MrBayes 3.2.5. The likelihood parameters set for BI analysis were based on the Akaike Information Criteria (AIC) test in MrModeltest 2.3 and the number of generations used in this analysis was increasing until the standard deviation of split frequencies became <0.01 and the potential scale reduction factor (PSRF) approached one.
Results
Morphology
Family Microscaphidiidae Looss, 1900
(Syn. Angiodictydae Looss, 1902 )
Genus Hexangium Goto and Ozaki, 1929
Syn. Arthurloossia [6]
Hexangium sigani Goto and Ozaki, 1929 (Figures 1-4)
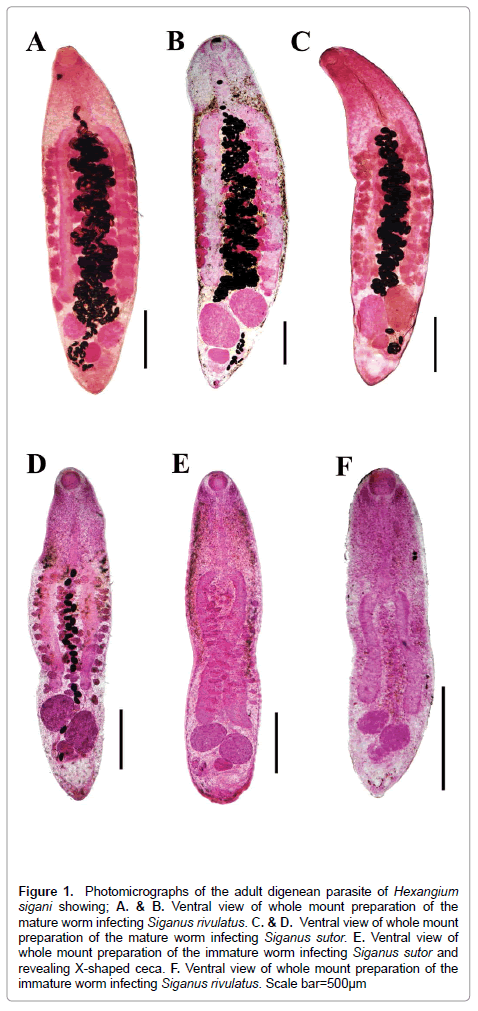
Figure 1: Photomicrographs of the adult digenean parasite of Hexangium sigani showing; A. & B. Ventral view of whole mount preparation of the mature worm infecting Siganus rivulatus. C. & D. Ventral view of whole mount preparation of the mature worm infecting Siganus sutor. E. Ventral view of whole mount preparation of the immature worm infecting Siganus sutor and revealing X-shaped ceca. F. Ventral view of whole mount preparation of the immature worm infecting Siganus rivulatus. Scale bar = 500μm
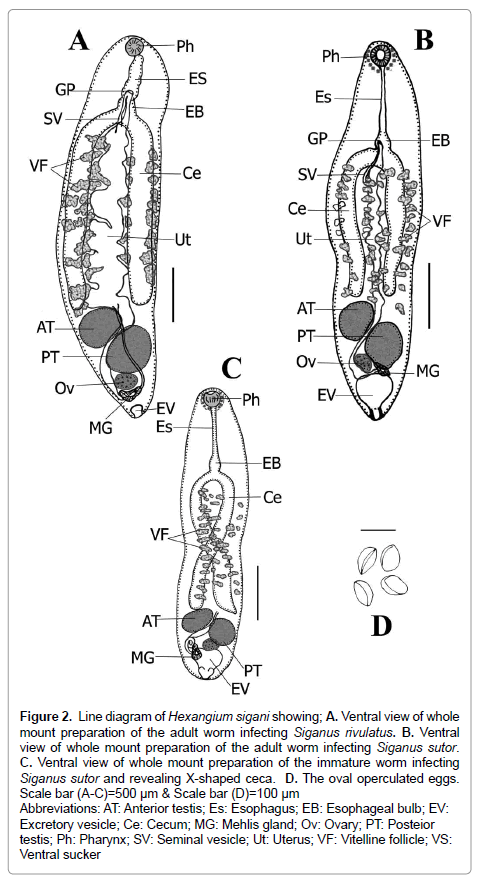
Figure 2: Line diagram of Hexangium sigani showing; A. Ventral view of whole mount preparation of the adult worm infecting Siganus rivulatus. B. Ventral view of whole mount preparation of the adult worm infecting Siganus sutor. C. Ventral view of whole mount preparation of the immature worm infecting Siganus sutor and revealing X-shaped ceca. D. The oval operculated eggs. Scale bar (A-C)=500 μm & Scale bar (D)=100 μm
Abbreviations: AT: Anterior testis; Es: Esophagus; EB: Esophageal bulb; EV: Excretory vesicle; Ce: Cecum; MG: Mehlis gland; Ov: Ovary; PT: Posteior testis; Ph: Pharynx; SV: Seminal vesicle; Ut: Uterus; VF: Vitelline follicle; VS: Ventral sucker
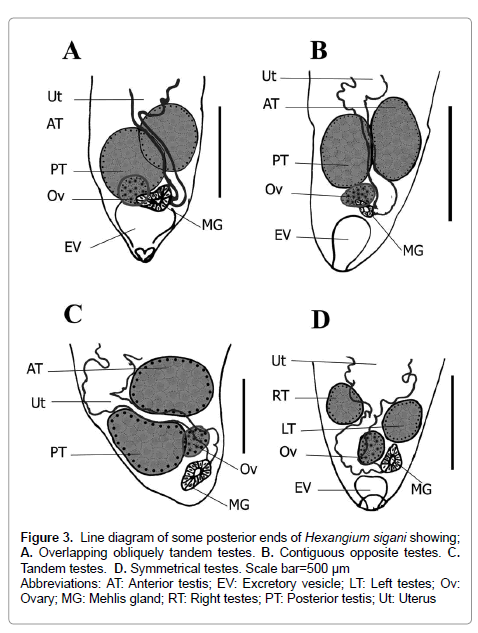
Figure 3: Line diagram of some posterior ends of Hexangium sigani showing; A. Overlapping obliquely tandem testes. B. Contiguous opposite testes. C. Tandem testes. D. Symmetrical testes. Scale bar=500 μm
Abbreviations; AT: Anterior testis; EV: Excretory vesicle; LT: Left testes; Ov: Ovary; MG: Mehlis gland; RT: Right testes; PT: Posterior testis; Ut: Uterus
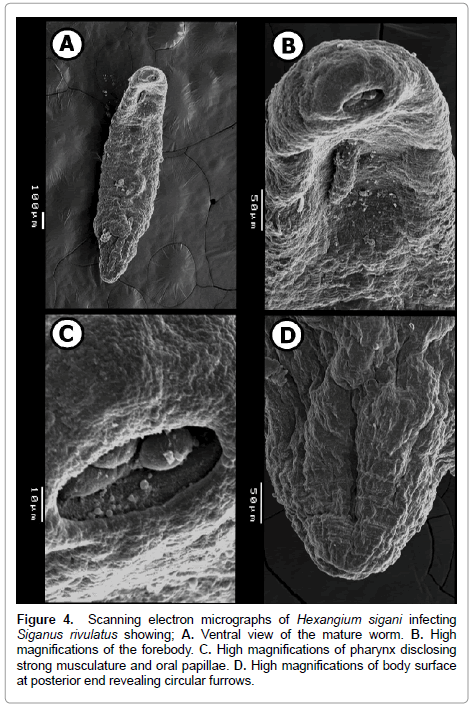
Figure 4: Scanning electron micrographs of Hexangium sigani infecting Siganus rivulatus showing; A. Ventral view of the mature worm. B. High magnifications of the forebody. C. High magnifications of pharynx disclosing strong musculature and oral papillae. D. High magnifications of body surface at posterior end revealing circular furrows.
(Syn. Arthurloossia loossi [6]; Hexangium affine [4]; Hexangium loossi [6,7]; Hexangium secundum [5].
Hosts: Siganus rivulatus Forsskal and Niebuhr; Siganus sutor Valenciennes; Siganus luridus Rüppell (Perciformes: Siganidae).
Locality: Northern Red Sea, off Sharm El-Naga, Makadi Bay, Southern Hurghada, Egypt (26°55.16’N, 33°56.05’E-26°53.59’N, 33°59.49’E, depth=0.5-2.5 m; July/2011-August/2012).
Site of infection: Intestine.
Deposited material: Deposited in Zoology Department, Faculty of Science, South Valley University (SVU), Qena, Egypt.
Prevalence: 44/70 S. rivulatus (62.9% infected); 2/16 S. sutor (12.5% infected); 2/8 S. luridus (25% infected).
Intensity: 1-8 worms/host specimen.
Mean intensity: 2.64 (116/44) in S. rivulatus; 2 (4/2) in S. sutor; 1 (2/2) in S. luridus.
Relative density/abundance: 1.66 (116/70) in S. rivulatus; 0.25 (4/16) in S. sutor; 0.25 (2/8) in S. luridus.
Records: 1. Goto and Ozaki [1], 2. Willey [36], 3. Yamaguti [2], 4. Tubangui and Masilungan [4], 5. Annereaux [5], 6. Yamaguti [3], 7. Nagaty [6], 8. Yamaguti [7], 9. Razarihelisoa [8], 10. Velasquez [9], 11. Fischthal and Kuntz [10], 12. Gupta and Miglani [11], 13. Yamani and Nahhas [12], 14. Gupta and Tandon [37], 15. Martens and Moens [38], 16. Geets and Ollevier [13], 17. Sey et al. [14], 18. El-Labadi [15], 19. Al-Zanbagi and Hassan [16], 20. Hassanine et al. [17], 21. Present study.
Re-description: (Based on 18 mature and 7 immature specimens. Morphological features illustrated in Figures 1-3. Measurements, morphometric percentages and morphometric ratios are given in Table 1). Living specimens fleshy of white color and with sluggish movement body dorsoventrally flattened, elongate, stout, with almost straight and smooth margins (in some specimens, body provided with minute spines, especially anteriorly), maximum width at level of mid-body or slightly anterior it. Anterior end tarping (in immature specimens) to rounded (in mature specimens); posterior endless round to tapering possessing a median knob-like protuberance. Tegument smooth in almost specimens but some specimens provided with minute spines, especially anteriorly. Oral sucker absent and replaced by well-developed pharynx. Pharynx spherical, without sacs, ventro-subterminal slightly terminal with conspicuous, oval aperture directed anteroventrally. Ventral sucker absent. Oesophagus moderately long, moderately wide, about 1/10 of body length, very slightly convoluted to straight, without esophageal glands. Esophageal bulb present, very weakly developed, smaller than pharynx and very difficult to be observed in mature specimens. Intestinal bifurcation at the end of anterior forth of body. Ceca 2, simple, straight to very slightly undulating, equal in length (In some specimens, left cecum slightly longer than right one), with the same width along the entire length, terminate anterior to testicular level directly or reach to anterior margins of anterior testis. Ani absent (Figures 1A-1C; Figures 2A-2C).
Testes 2, variable in shape (rounded, elliptical, oval, pyriform), entire, smooth, subequal, with varied position (symmetrical, side-by-side, oblique, obliquely tandem), occupy the anterior 2/3 area of last body fourth, well-separated from the posterior end, separated from each other by small distance or contiguous and sometimes the inner lateral margins overlap each other. Cirrus sac absent. Seminal vesicle sinuous, tubular, often concealed by uterus, winding from some distance posterior to intestinal bifurcation. Distal extremity of the seminal vesicle narrowing anteriorly to insignificant genital atrium, opening into short hermaphroditic duct below halfway between pharynx and intestinal bifurcation. Genital pore pre-equatorial, median (sinistral), at mid-oesophageal level or slightly posterior directly (Figures 1A-1F; Figures 3A-3D).
Ovary entire, spherical to oval, smooth, median or sinistro-submedian, immediately post testicular or separated by short distance, smaller than both testes. Seminal receptacle absent. Mehlis’gland median, well-developed, large, pyriform, postero-lateral to sinistral rim of ovary, just anterior to excretory vesicle. Uterus comes out laterally from Mehlis’gland forming a small uterine seminal receptacle then passes anteriorly between testes and extends in intercecal space forming many convolutions. The distal portion of uterus narrowing at intestinal bifurcation level and unites below mid-oesophageal level with distal portion of seminal vesicle forming hermaphroditic duct. Hermaphroditic duct moderately short, cylindrical and protractible outside body surface (Figures 1A-1F; Figures 3A-3D). Vitellarium follicular; field extends lateral, partly medial, to caeca, distributed in second and third quarters of body, (extends beneath the intestinal bifurcation level by small distance and terminate at cecal ends.), confluent medially, overlaps over intestinal caeca. Follicles numerous, few in number, large, irregularly shaped and arranging themselves roughly in 4 longitudinal rows (Figures 1A-1F; Figures 2A-2C). Eggs numerous, highly dense, oval, moderate in size, non-operculate, thin-shelled, without filaments or knob (Figure 2D).
Excretory vesicle V-shaped with variable sizes; excretory arms short, divided at level of ovary gives off single duct on each side which subsequently divides into three long stems reaching level of oesophagus, excretory pore subterminal (Figures1B-1D and 1F; Figures 2A-2D).
Ultra-structure description (Figures 4 and 5)
SEM examination illustrates that the body dorsoventrally flattened, elongate, stout, with almost straight and smooth margins, maximum width slightly posterior to mid-body level on (Figure 4A). Anterior endless round (Figure 4B), posterior endless round possessing a median, transverse, narrow, slit-like sub-terminal excretory pore (Figure 4D). The enlarged hind part of the body illustrated absence of spines and papillae at this region. Also, it is divided by thin circular grooves. These grooves become crowded towards the posterior extremity (Figure 4D).
Pharynx sub-elliptical, ventro-subterminal or slightly terminal (situated on top of a rather globular cephalic end) with conspicuous, oval, wide, aperture (mouth opening) directed anteroventrally. Cephalic extremity slightly swollen, probably by contraction of muscular pharynx. Body with a slight constriction just posterior to pharynx, and another slight constriction at level of genital pore (Figure 4A and 4B). Inside pharynx, three large domed structures occupy most of internal space of mouth opening; these structures may represent extensions from the muscular layer of pharynx. Also, several, small, sessile, rounded, well-developed, randomly distributed and different sized spherical papillae aggregated on inner tegmental surface of pharynx on (Figure 4C).
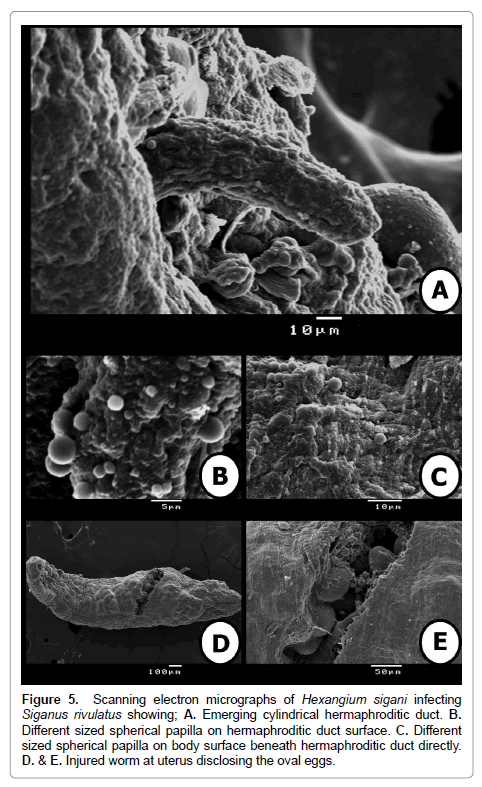
At genital pore level, cylindrical moderately long hermaphroditic duct comes out from the genital pore. Several, small, sessile, rounded, well-developed, randomly distributed and different sized spherical papillae aggregated on outer tegmental surface of hermaphroditic duct on (Figure 5A and 5B). Also, hermaphroditic duct’s surrounding region is represented as a depression in body surface and very crowded by randomly distributed and different sized spherical papillae on (Figure 5C).
Figure 5: Scanning electron micrographs of Hexangium sigani infecting Siganus rivulatus showing; A. Emerging cylindrical hermaphroditic duct. B. Different sized spherical papilla on hermaphroditic duct surface. C. Different sized spherical papilla on body surface beneath hermaphroditic duct directly. D. & E. Injured worm at uterus disclosing the oval eggs.
The worm was incised at middle of the body to examine eggs from its uterus. The eggs are smooth, non-operculate. Another different sized spherical papillae of few numbers and randomly distributed on body surface observed on (Figure 5D and 5E).
Molecular phylogeny (Figure 6)
The genotypes of Hexangium sigani Goto and Ozaki, 1929 (401 nucleotides) recorded in GenBank with accession number KT070706. This sequences aligned with 10 reference sequences representing all the available and appropriate species of the Paramphistomoid (Table 2); 1 species from the Cladorchiidae Fischoeder, 1901, one species from the Mesometridae Poche, 1926, two species from the Microscaphidiidae Looss, 1900 and three species from the Paramphistomidae Fischoeder, 1901, together with four species belongs into the Pronocephaloidea; AY222115 [39] [Labicolidae], AY222114 [40] [Notocotylidae], AY222116 [39] [Opisthotrematidae] and AY222113 [39] [Rhabdiopoeidae], for out-group comparisons.
| Species | Host | Locality | GenBank accession nos. |
References |
|---|---|---|---|---|
| Order; Family | ||||
Hexangium sigani |
Siganus rivulatus Perciformes; Siganidae |
Macady Bay, Red Sea, Egypt | KT070706 | Present study |
| Hexangium sp. | Siganus fuscescens Perciformes; Siganidae |
Not mentioned |
AJ287522 | Cribb et al. [50] |
| Neohexangitrema zebrasomatis | Zebrasoma scopas Perciformes; Acanthuridae |
Not mentioned | AJ287544 | Cribb et al. [50] |
| Solenorchis travassos | Dugong dugon Sirenia: Dugongidae |
Australia | AY222110 | Olson et al. [39] |
| Mesometra sp. | Sarpa salpa Perciformes; Sparidae |
Not mentioned | AJ287537 | Cribb et al. [50] |
| Paramphistomum cervi | Sheep Cetartiodactyla; Bovidae |
China | KJ459937 | Zheng et al. [51] |
| Paramphistomum epiclitum | Bos indicus Cetartiodactyla; Bovidae |
Meghalaya, India | JX678283 | Tandon et al. [52] |
| Paramphistomum sp. | Bos indicus Cetartiodactyla; Bovidae |
Meghalaya, India | JX678226 | Tandon et al. [52] |
| Labicola elongata | Dugong dugon Sirenia: Dugongidae |
Not mentioned | AY222115 | Olson et al. [39] |
| Notocotylus pacifera | Not mentioned | Raleigh, USA | AY245765 | Flowers et al. [40] |
| Lankatrema mannarense | Dugong dugon Sirenia: Dugongidae |
Australia | AY222116 | Olson et al. [39] |
| Rhabdiopoeus taylori | Dugong dugon Sirenia: Dugongidae |
Australia | AY222113 | Olson et al. [39] |
Table 2: Sequence data representing species of the Paramphistomoidea and outgroups determined in the present study, together with key 18S reference sequences (see GenBank accession nos.) and epidemiological information.
All 12 sequences (including out-groups), are aligned over 365 positions (trimmed to parallel the shortest sequence length) and the genetic distance among them. Phylogenetic analysis of this dataset resulted in the Paramphistomidae forming a monophyletic clade (Figure 6) with strong support (BI=100, ML=100, MP=100, NJ=100, ME=100) to the exclusion of out-group taxa.
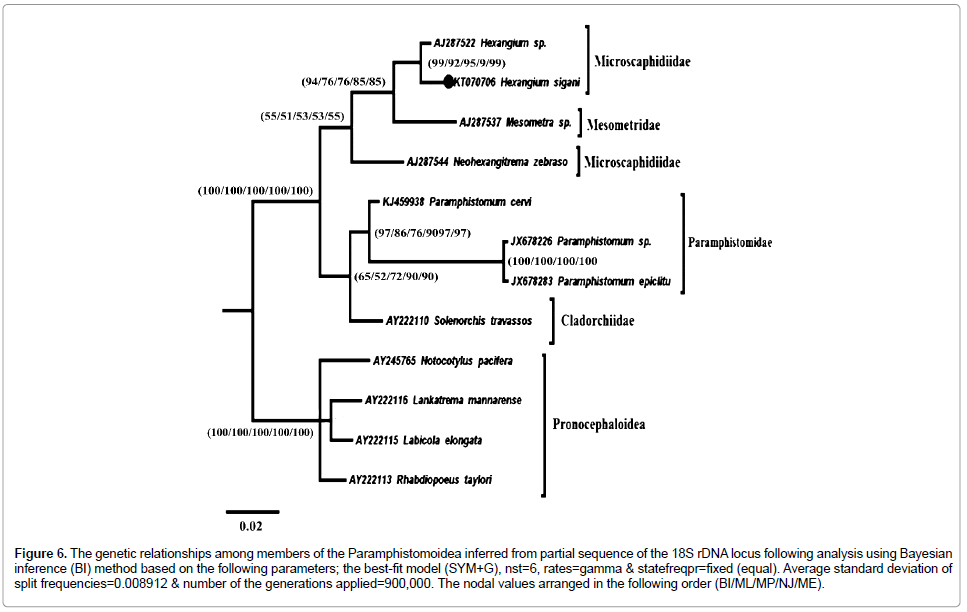
Figure 6: The genetic relationships among members of the Paramphistomoidea inferred from partial sequence of the 18S rDNA locus following analysis using Bayesian inference (BI) method based on the following parameters; the best-fit model (SYM+G), nst=6, rates=gamma & statefreqpr=fixed (equal). Average standard deviation of split frequencies = 0.008912 & number of the generations applied=900,000. The nodal values arranged in the following order (BI/ML/MP/NJ/ME).
The Paramphistomidea divided into two distinct sister clades; Cladorchiidae/Paramphistomidae clade of a differentiated support value (BI=65, ML=51, MP=72, NJ=90, ME=90), and Mesometridae/Microscaphidiidae clade with of weak support (BI=54, ML=51, MP=53, NJ=53, ME=55).
Cladorchiidae clade which represented by only one species Solenorchis travassosi Hilmy, 1949 (Syn. Indosolenorchis hirudinaceus Crusz, 1951), resolved in all resulting trees as basal clade to all species of Paramphistomidae. Paramphistomidae species resolved as a distinct monophyletic clade with strong support (BI=97, ML=86, MP=76, NJ=97, ME=97).
Neohexangitrema zebrasomatis Machida, 1984 resolved as basal to the well-supported Mesometra/Hexangium clade (BI=94, ML=76, MP=76, NJ=85, ME=85). The genus Hexangium resolved as strong supported monophyletic clade (BI=99, ML=92, MP=95, NJ=99, ME=99); Hexangium sigani and Hexangium sp. resolved as two sister clades. In spite of the somewhat strong support and the strong relationship between Hexangium sigani and Hexangium sp., the species H. sigani has a longer branch line than Hexangium sp., which means that the former has number of nucleotides substitutions more than in the later indicating that both species are different.
Discussion
Morphology
Present specimens consistent with Jones criteria which indicate that the newly collected specimens belong to the superfamily Microscaphidiidae Looss, 1900 [32]. These criteria are; excretory pore at posterior extremity, absence of both sucker and cirrus sac, excretory system partly reticulate, metraterm absent, pharynx present and functionally replaces oral sucker. Furthermore, since ventral body-surface not modified as attachment organ, this places present specimens into the family Microscaphidiidae Looss, 1900 [20].
The combined features; vitelline follicles entirely anterior to testes and caeca terminate anteriorly to testes, places present specimens in the genus Hexangium Goto and Ozaki, 1929. Comparison among newly collected specimens and the valid species in the genus Hexangium indicated that present specimens identical to Hexangium sigani Goto and Ozaki, 1929 since sharing in; overall appearance, egg size range, possessing median and genital pore at mid-oesophageal level, alike in vitellarium distribution, ovary position and shape and parasitizing in same host group. In addition, almost of allometric measurements extremely similar and clearly have converging range such as; body width, pharynx length, oesophagus length, oesophageal bulb length, ovary length, testes length and post testicular distance as a percentage of body length. Also, mean testes width/Ovary ratios and pharynx/ oesophageal bulb ratios very identical.
Goto and Ozaki (1929) indicated in the description of H. sigani to the large body size, oblique testes and figured structure resembling a thin-walled cirrus-sac. Both Tubangui & Masilungan and Annereaux stated another two species H. affine and H. secundum, reported from a single specimen and differed from the type species H. sigani in size and arrangement of the testes where; both H. affine and H. secundum and have a smaller body size and symmetrical testes [4,5]. H. secundum characterized from H. affine by larger ovary, larger in dimensions and very inconspicuous cirrus-sac. Present specimens exhibited a wide variability in testes positions [symmetrical, side-by-side, oblique, obliquely tandem] and ovary size, this is consistent with description of H. sigani, H. affine and H. secundum.
Nagaty added a fourth genus in the family Mesometridae Poche, 1926 called Arthurloossia contains one species Arthurloossia loossi [6]. This species appeared to be congeneric with H. sigani with few variations; spiny tegument, especially anteriorly and absence of cirrus-sac. The subsequent study by [7] considered Arthurloossia Nagaty, 1954 a synonym of Hexangium Goto and Ozaki,1929 because Mesometridae characterized by ventral body-surface entirely concave or concave only anteriorly, modified to form accessory attachment organ [19]. The genus Arthurloossia has no such this structure so it reassigned into Microscaphidiidae and subsequentaly Arthurloossia loossi Nagaty, 1954 transferred as Hexangium loossi [6,7].
Within more detailed study on H. sigani, against the other species H. affine, H. secundum and H. loossi, Razarihelisoa presented an overview about the validity of these species and suggested that they might be conspecific [8]. Velasquez specimen of H. sigani indicated to the discernibility of the cirrus sac and variability in testes position and arrangement [9]. Except for the arrangement of the testes H. affine, H. secundum and Yamaguti specimens fall within the range of measurements i.e. Velasquez concluded that H. affine and H. secundum as objective synonyms of H. sigani [3,9]. Fischthal and Kuntz results consist with the previous conclusion by Velasquez with consideration of H. loossi as another objective synonyms of H. sigani with a sign to a presence of cirrus sac with protractible cirrus [9,10]. Only in re-description of H. sigani by Gupta and Miglani, they concluded disagreement with the views of previous authors [11]. Nevertheless, subsequent reports of H. sigani by researchers and present study have continued to accept these synonymies [11-17,39].
The present observations illustrated absence of cirrus sac and this consistent with almost previous observations except [1], [4] and [10]. Al-Jahdali revealed in his description of Hexangium saudii to absence of cirrus sac [23]. On the other hand, Hassanine and Gibson indicated in the description of Hexangium brayi that “cirrus-sac weakly developed, but large, elongate-claviform, extending anteriorly from near middle of body to genital pore containing elongate, saccular seminal vesicle and inconspicuous prostatic complex” [22]. The present worker thinks that presence of weakly developed cirrus-sac but large with absence of illustrations that differentiate between seminal vesicle and cirrus-sac is not convincing and not very logic (Figure 1) [22]. So, we support absence of cirrus sac in all species of the genus Hexangium. Also, male genital system represented only by large elongate seminal vesicle and its distal end joins that of the uterus to form hermaphroditic duct.
The previous comparison among all described forms of H. sigani revealed some morphological variations confined between; absence or presence of tegumental spines, testes arrangement, larger or smaller of body dimensions and ovary size. These variations fall within slight range of variability and not enough on its own here, to indicate lack of conspecificity so, these differences are considered to be of minor importance.
Host-parasite data illustrates that the genus Hexangium parasitizes intestine of marine teleosts (many families) and distributed in tropical and subtropical Indo-Pacific [20]. Present specimens have the same host-parasite data as they reported from marine fish (siganus) and geographically collected from off northern Red Sea region, Egypt. Moreover, present specimens and almost previously described forms of Hexangium sigani reported from several species of siganid fishes; Siganus fuscescens, Siganus sp., Siganus javus, Siganus guttatus, Siganus canaliculatus, Siganus argenteus, Siganus spinus, Siganus vermiculatus, Siganus sutor and Siganus rivulatus, Siganus luridus (present study) [1-6,9-11,13,15-17,39]. Furthermore, Hexangium sigani reported from other fishes belong to other families; Hipposcarus harid [Labridae], Neoniphon samara [Holocentridae], Stolephorus commersonnii [Engraulidae] and Johnius borneensis [Sciaenidae] [6,8,10,11].
As a result of the similarities in host-parasite data of all described forms of H. sigani and present study especially geographic locality, the slight morphological changes and differences in allometric measurements between present study and the previously described forms of H. sigani and between all synonyms can be attributed significantly to host-induced variability where; present specimens reported from three different siganid fishes Siganus rivulatus, Siganus luridus and Siganus sutor. This change of host affect directly on three main measurements which are body length, body width, and suckers width and therefore, any measurement related to the previous measurements may be labile.
Only three digenean trematodes were reported from Siganus luridus; Gyliauchen volubilis Nagaty, 1956 from Red Sea, Egypt [41,42], Hexangium brayi Hassanine & Gibson, 2005 from Sharm El-Sheikh, South Sinai, Egypt [22] and Progyliauchen magnacetabulum Al-Jahdali, 2013 from the coast of Rabigh, Saudi Arabia [23]. No any record of Hexangium sigani reported from Siganus luridus i.e. Siganus luridus represents a new host record of Hexangium sigani Goto and Ozaki, 1929 for the first time.
Ultra-structure description
Present study revealed presence of one main tegumental structure, sensory papillae. These papillae differentiated into three forms; oral papillae, genital papillae and body papillae. Each of these forms exhibited a moderately wide range of variations both in size and in distribution. Hayunga indicates that changes in the microenvironment of helminthes are usually reflected in variations in the structure of the tegument [43].
Presence of different types of sensory papillae on different locations over body tegument of H. sigani may reflect a variation in the functions they performed as following; 1) oral papillae could be involved in contact reception during food detection or feeding as mentioned by [44]. 2) Body papillae might record pressure changes as the tegument stretches as reported by [45]. 3) Genital papillae could be involved in contact reception during fertilization process or could be involved in cross-fertilization between two flukes or in selecting the site of attachment as mentioned by Ashour in his explaining the reason for the abundance of the sensory papillae on lateral sides of ventral surface of body and on the two suckers [46].
Molecular phylogeny
According to the resultant Phylogenetic trees, it was observed that both families Cladorchiidae and Paramphistomidae very close to each other. Also, both families Mesometridae and Microscaphidiidae much related to each other despite of somewhat weak support values. This weak support value can be explained as result of flow of information used in study and more new sequences are needed.
On other hand, Paramphistomidae/Cladorchiidae clade appeared distant from Mesometridae/Microscaphidiidae clade and the well-supported values for each clade sustained this assumption. Another evidence is the host-parasite listed in the previous table (Table 2) where; all species used in the phylogenetic analysis of Cladorchiidae and Paramphistomidae belongs to mammals whereas, all species used in the phylogenetic analysis of Mesometridae and Microscaphidiidae species are from fishes. This consistent exactly with the host-parasite data reported by Jones who indicated that Paramphistomidae are only obtained from the alimentary tract of mammals [47]. Also, mammals represent one of the main groups from which Cladorchiidae are collected [48]. On the other side, host-parasite data of Microscaphidiidae referred that marine and freshwater fishes and turtles are representative hosts to this family [20]. In addition, Mesometridae parasitizes inside intestine of mainly herbivorous marine fishes [19].
Microscaphidiidae clade is paraphyletic, based on the inclusion of a single sequence representing Mesometra sp. from the Mesometridae with strong support values. The previous result matched exactly [37]. Insertion of Mesometra sp. within Microscaphidiids very interesting and further more studies are needed. Mesometridae has similar biological features with the family Microscaphidiidae where all Mesometrids reported from” herbivorous marine fishes (Sparidae, Acanthuridae); off Mediterranean Sea and, rarely, Pacific and Atlantic Oceans” [19]. Furthermore, marine fishes represent one of the major hosts of Microscaphidiids especially (Acanthuridae, Siganidae…..etc.) and Microscaphidiids are “cosmopolitan but probably absent from cold-temperate and polar regions” [20]. Morphologically, the Mesometrids are characterized from Microscaphidiids only by modification of ventral body-surface to form accessory attachment organ [19,49].
The interrelationship among the genera Neohexangitrema, Mesometra and Hexangium can be attributed to several reasons; 1) the great similarities in body structures ‘shape, distribution and position with slight changes represented by; body elongation, absence or presences of spines and their distribution, Pharynx position, Caeca extension in the hind body, variability of genital pore position at oesophageal level, entire testes positioned in the posterior third of body and their position against each other, entire to slightly lobe ovary positioned closely posterior to testes, interracial uterus passes between testes, presence or absence the metraterm and distribution of vitellarium with regard to caeca, arose in association, with an ecological shift. 2) The host parasite data from the previous table indicated that the genera Mesometra Lühe, 1901, Neohexangitrema Machida, 1984 and Hexangium Goto and Ozaki, 1929 parasitized on different families; Sparidae, Acanthuridae and Siganidae (respectively) from the same Order Perciformes. This consistent with data reported by Jones and Blair in which Mesometra obtained from the intestine of Sparidae, and the results of Blair in which Neohexangitrema reported from Acanthuridae and Hexangium reported from many families especially Siganidae [19,20].
Finally, we concluded that reliance on only a single taxon of the most speciose Mesometrids genus are not satisfying enough to clarify this interrelationship between to the two families wherefore incorporation of other sequences of type-taxa in future analyses will give a deeper understanding.
Key to species of Hexangium Goto & Ozaki, 1929
1a. Ecsoma present…. Hexangium ecsomi [16].
1b. Ecsoma absent [2].
2a. Vitelline follicles are arranged in rosette-like groups, caeca distinctly short and more distant from the testes….Hexangium saudii [23].
2b. Vitelline follicles not collected in groups, caeca long and very close to testes [3].
3a. Body shape distinctly pyriform, caeca terminations dilate and saccular, vitelline follicles confined to the intercaecal field…..Hexangium brayi [22].
3b. Body shape distinctly elongate, caeca terminations undifferentiated, vitelline follicles overlapping or lateral to the caeca….. Hexangium sigani [1].
Acknowledgments
We are grateful to Zoology Department, Faculty of Science, South Valley University, Qena, Egypt, for all cooperation, help to us and facilitating required equipment and instruments.
References
- Goto S, Ozaki Y (1929) Brief notes on new trematodes II. Japanese Jour Zool 2: 369-383.
- Yamaguti S (1934) Studies on the helminth fauna of Japan. Part 2, Trematodes of fishes, I. Japanese Journal of Zoology 5: 249-541.
- Yamaguti S (1953) Parasitic worms mainly from Celebes Part 3. Digenetic trematodes of fishes, II. Acta Medica Okayama 8: 257-295.
- Tubangui MA, Masilungan VA (1944) Some trematodes parasites of fishes in the collection of the University of the Philippines. Philipp J Sci 76: 57-67.
- Annereaux RF (1947) Two new trematodes from Philippines fishes. Trans Am Microsc Soc 66: 172-175.
- Nagaty HF (1954) Trematodes of fishes from the Red Sea. Part 5. On Three New Opecoelids and One Mesometrid. J Parasitol 40: 367-371.
- Yamaguti S (1958) Systema Helminthum. Volume 1. The Digenetic Trematodes of Vertebrates. Interscience Publishers Inc., New York.
- Razarihelisoa M (1959) Sur quelques trématodes digènes de poissons de Nossibé (Madagascar). Bulletin de la Société Zoologique de France 84: 421-434.
- Velasquez CC (1961) Some digenetic trematodes of Philippine food fishes. J Parasitol 47: 521-526.
- Fischthal J H, Kuntz RE (1964) Digenetic trematodes of fishes from Palawan Island, Philippines. Part III. Families Hemiuridae and Lepocreadiidae. Proceedings of the Helminthological Society of Washington 31: 109-120.
- Gupta NK, Miglani A (1976) Digenetic trematodes from marine food fishes and wild ducks of Port Blair (Andaman and Nicobar Islands), India. Revista Iberica Parasitologica 36: 219-248.
- Al-Yamani FY, Nahhas FM (1981) Digenetic trematodes of marine fishes from the Kuwaiti coast of the Arabian Gulf. Kuwait Bulletin of Marine Science 3: 1-22.
- Geets A, Ollevier F (1996) Endoparasitic helminthes of the whitespotted rabbitfish (Siganus sutor (Valenciennes, 1835)) of the Kenyan coast: Distribution within the host population and microhabitat use. Belgian J Zool 126: 21-36.
- Sey O, Nahhas FM, Uch S, Vang C (2003) Digenetic trematodes from marine fishes off the coast of Kuwait, Arabian Gulf: Fellodistomidae and some smaller families, new host and geographic records. Acta Zool Acad Sci H 49: 179-200.
- El-Labadi SN, Ismail NS, Khalaf M (2006) Intestinal digenetic trematodes of some fishes from the Gulf of Aqaba, Red Sea. Pakistan J Zool 38: 43-48.
- Al-Zanbagi NA, Hassan A (2013) A multivariate analysis of some Digenean species collected from several Red Sea fishes in Saudi Arabia. Life Science Journal 10: 1244-1255.
- Hassanine RES, Al-Zahrani DA, Touliabah HES, Youssef EM (2016) The life cycle of Hexangium sigani Goto and Ozaki, 1929 (Digenea: Microscaphidiidae) from the Red Sea. J Helminthol 90: 539-546.
- Manter HW (1963) Studies on digenetic trematodes of fishes of Fiji, IV. Families Haploporidae, Angiodictyidae, Monorchiidae, and Bucephalidae. Proceedings of the Helminthological Society of washington 30: 224-232.
- Jones A, Blair D (2005) Keys to the Trematoda. CABI Publishing and the Natural History Museum, London, UK.
- Blair D (2005) Family Microscaphidiidae Looss, 1900: Jones A, Bray RA, Gibson DI: Keys to the Trematoda. CABI Publishing and Natural History Museum, Wallingford.
- Machida M, Uchida A (1990) Trematodes from unicornfishes of Japanese and adjacent waters. Mem Natn Sci Mus 23: 69-81.
- Hassanine RME, Gibson DI (2005) Trematodes of Red Sea fishes: Hexangium brayi n. sp. (Angiodictyidae Looss, 1902) and Siphodera aegyptensis n. sp. (Cryptogonimidae Ward, 1917), with a review of their genera. Syst Parasitol 61: 215-222.
- Al-Jahdali MO (2013) New intestinal trematodes from siganid fishes off the Saudi coast of the Red Sea. Acta Zool Acad Sci H 59: 3-12.
- Cribb TH, Bray RA (2010) Gut wash, body soak, blender and heat-fixation: approaches to the effective collection, fixation and preservation of trematodes of fishes. Syst Parasitol 76: 1-7.
- Justine JL, Briand MJ, Bray RA (2012) A quick and simple method, usable in the field, for collecting parasites in suitable condition for both morphological and molecular studies. Parasitol Res 111: 341-351.
- Garcia LS, Ash LR (1979) Diagnostic Parasitology: Clinical Laboratory Manual. (2ndedn), The C.V. Mosby Company, London.
- Randall JE (1982) The Diver's Guide to Red Sea Reef Fishes. Biblios Pub Distribution Service.
- Lieske E, Myers RF (1994) Coral reef fishes: Caribbean, Indian Ocean, and Pacific Ocean including the Red Sea. London: Harper Collins.
- Lieske E, Myers RF (1996) Coral Reef Fishes: Caribbean, Indian Ocean and Pacific Ocean Including the Red Sea. Princeton, NJ: Princeton University Press.
- Lieske E, Fiedler KE, Myers RF (2004) Coral Reef Guide: Red Sea to Gulf of Aden, South Oman. HarperCollins, London.
- Jones A (2005) Introduction and Key to Superfamilies: Jones A, Bray RA, Gibson DI: Keys to the Trematoda. CABI Publishing and the Natural History Museum, Wallingford.
- Bush AO, Lafferty KD, Lotz JM, Shostak AW (1997) Parasitology meets ecology on its own terms: Margolis et al. revisited. J Parasitol 83: 575-583.
- Lee MR (1993) The petrography, mineralogy and origins of calcium sulphate within the Cold Bokkeveld CM carbonaceous chondrite. Meteoritics 28: 53-62.
- Liesinger K (2011) Microscopic and molecular analyses on digenean trematodes in red deer (Cervus elaphus). Diplomarbeit Pp. 44-46.
- Willey CH (1930) Note on the interpretation and relations of lymph and excretory channels in the trematode Hexangium sigani Goto and Ozaki, 1929. Transactions of the American Microscopical Society 49: 62-65.
- Gupta SP, Tandon VL (1983) On some digenetic trematodes from marine fishes of Puri, Orissa. Indian J Helminthol. 35: 112-136.
- Martens E, Moens J (1995) The metazoan ecto‐and endoparasites of the rabbitfish, Siganus sutor (Cuvier and Valenciennes, 1835) of the Kenyan coast. I. Afr J Ecol 33: 405-416.
- Olson PD, Cribb TH, Tkach VV, Bray RA, Littlewood DTJ (2003) Phylogeny and classification of the Digenea (Platyhelminthes: Trematoda). Int J Parasitol 33: 733-755.
- Flowers JR, Litaker RW, Poore MF, Mullen JE, Paperna I, et al. (2003) Phylogenetic analysis of some aquaculture Pond-Associated digeneans based on SSU rDNA Sequence.
- Abdou NE, Heckmann RA (2001) Fine structural studies on the intestinal trematode, Gyliauchen volubilis Nagaty 1956 from fish Siganus luridus in the Red Sea, Egypt. J Egypt Soc Parasitol 31: 281-294.
- Nagaty HF (1956) Trematodes of fishes from the Red Sea. Part 7. On two gyliauchenids and three allocreadoids, including four new species. J Parasitol 42: 523-527.
- Hayunga EG (1991) Morphological adaptations of intestinal helminths. J Parasitol 77: 865-873.
- Bennett CE (1975) Scanning electron microscopy of Fasciola hepatica L. during growth and maturation in the mouse. J Parasitol 61: 892–898.
- Abdou NE, Ashour AA (2000) Scanning electron microscopy of the tegumental surface of digenetic trematode Stephanostomum egypticum from the Red Sea fishes. J Egypt Soc Parasitol 30: 341-348.
- Ashour AA (1995) Scanning electron microscope observations on Corrigia vitta (Dujardin, 1845) Shtrom, 1940 (Trematoda: Dicrocoeliidae). J Egypt Soc Parasitol 25: 25-30.
- Jones A (2005) Family Paramphistomidae Fischoeder, 1901: Jones A, Bray RA, Gibson DI: Keys to the Trematoda. CABI Publishing and the Natural History Museum, Wallingford, Pp. 229-233.
- Jones A (2005) Family Cladorchiidae Fischoeder, 1901: Jones A, Bray RA, Gibson DI: Keys to the Trematoda. CABI Publishing and the Natural History Museum, Wallingford, Pp. 257-271.
- Jones A, Blair D (2005) Jones A, Bray RA and Gibson DI: Keys to the Trematoda. CABI Publishing and the Natural History Museum, Wallingford, Pp. 189-192.
- Cribb TH, Bray RA, Littlewood DTJ, Pichelin S, Herniou EA (2001) The Digenea. Interrelationships of the Platyhelminthes. Taylor and Francis, London.
- Zheng X, Chang QC, Zhang Y, Tian SQ, Lou Y, et al. (2014) Characterization of the complete nuclear Ribosomal DNA sequences of Paramphistomum cervi. The Scientific World J 2014: 1-11.
- Tandon V, Shylla JA, Ghatani S, Roy B (2012) Direct Submission. Zoology, North-Eastern Hill University, Mawlai, Shillong, Meghalaya 793022, India.
Copyright: © 2018 Khalifa RMA, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.