
Journal of Clinical and Cellular Immunology
Open Access
ISSN: 2155-9899

ISSN: 2155-9899
Research Article - (2023)Volume 14, Issue 3
Patient-derived hiPSCs could generate indefinitely illness-related cells and organoids, allowing research scientist to reproduce some pathologic change of human sickness models in petri dishes. Whereas, dermal fibroblasts require some traumatic injury to obtain and have the risky of accumulating variations due to unveil to external stimulus such as UV light. Meanwhile, peripheral blood is regarded to be a more excellent source of somatopiasm because it is less damage to collect and its mutation load is significantly lower than that of skin tissue. In this paper, we describe the successful construction of non-integration hiPSCs form human peripheral blood cells by Sendai virus. A healthy adult female of Han nationality from China contributed her Peripheral Blood Mononuclear Cells (PBMCs). Non-integrating Sendai virus recombinant PBMCs with human OSKM (Oct4, Sox2, Kl4 and c-Myc) transcription factors. The pluripotency of transgenic-free iPSCs was confirmed by immunofluorescence staining of pluripotency markers and the capacity of iPSCs to initially differentiate into 3 germ layers in vitro. Moreover, iPSC lines demonstrated awfully normal karyotypes. In the research of illness progression and pathogenesis (eg, DMD, SMA, ALS), iPSC lines can be used as controls.
iPSCs; PBMC; Sendai virus; Genetic diseases; Gene editing
Yamanaka, et al. [1] first cultured induced Pluripotent Stem Cells (iPSCs) from mouse fibroblasts. After that, thousands of human iPSCs came from healthy peoples and patients with various kinds of sickness [2-4]. Patient-derived hiPSCs can generate indefinitely disease-relevant cells and organoids, allowing investigators to reproduce some pathologic change of human disease models in petri dishes. In practice, such models have helped to uncover relevant molecular mechanisms of pathological changes, facilitating to the progress of novel methods [4]. Therefore, a brief and effective way to generate a patient-derived hiPSC line is critical for gaining in-depth insights of the pathogenesis of the disease and exploring a secure and valid therapy method.
Lentivirus or retrovirus was first applied to transmit reprogramming factors for iPSC establishment [1,5]. These genomic alteration means may generate tumorigenic insertional mutations, and residual or reactivation of transgene expression during iPSC differentiation may affect their differential potency and the function of iPSC derivatives [5,6]. In order to solve this problem, many different approaches were developed to derive transgene-free iPSCs, such as PiggyBac transposons, minicircle DNA vectors, recombinant proteins, mRNA, adenovirus, Sendai virus and small molecules [7-13]. As we all know, sendai virus is a single chain RNA virus which does not influence the parasitifer genome. The virus exists in the cytoplasm, so it can be easily diluted out after ~10 generations of infection with the host cell. Sendai virus has very high transduction efficiency and can infect a variety of cell types in both proliferating and quiescent states. As a result, sendai-based reprogramming vectors have been successfully applied to reprogram neonatal and adult fibroblasts [12,14].
Patient-derived hiPSCs are generally sourced from somatic cells, namely skin fibroblasts [2]. However, dermal fibroblasts need some invasive injury to collect and have the risk of accumulating variations due to unveil to external stimulus such as UV light. Besides, it is undeniable that a high frequency of coding mutations is found in both primitive fibroblasts and their derived hiPSCs [15]. At the same time, PB is considered to be a more excellent source of somatopiasm because it is less damage to collect and its mutation load is significantly lower than that of skin tissue. Here, we describe the successful construction of non-integration hiPSCs form human peripheral blood cells by Sendai virus.
Ethical approval
Blood specimens were comes from voluntary donor in good health, 18 years old, with undersigned consent permitted by the ethical committee of the Zhejiang Putuo Hospital.
Separation and culture of PBMCs
Under aseptic conditions, PBMCs was isolated from 5 ml of whole blood using Lymphoprep™ (Stemcell, 07801) density gradient centrifugation method in strict accordance with the manufacturer’s instructions.
The latest isolated PBMCs were inoculated to Complete StemSpan™ SFEM II Medium (Stemcell, 09605) with Supplement (100 ×) (Stemcell, 02694).
Reprogramming of PBMCs
Four days after seeding (Day 0), the cells were gathered by centrifugation (100 g, 5 min) and transfected with Sendai virus in a total volume of 1 ml (Cellapy, CA5002003). Under sterile conditions, transduction was operated in complete PBMC medium containing 6 µg/ml Polybrene by centrifugation at 200 g for 2 h at normal room temperature. Then, particles were resuspended in the same medium including virus and placed in 24-well plates and cultured at 37°C. After 4 hours, the cell suspension was centrifuged to remove Sendai virus. Cells were resuspended in 24-well plates with fresh complete PBMC medium for 2 days. On day 3, cells were gathered by centrifugation, resuspended and plated on Matrigel-coated 6-well plates (Corning, 354277). The medium was renewed every other day. On day 7, transitioning into iPSC medium began by replacing half of the StemSpan™ SFEM II Medium with ReproTeSR™ Medium (Stemcell, 05926). From day 8 on, the entire volume of medium was replaced with ReproTeSR™ Medium. The medium was changed daily thereafter and the appearance of iPSC colonies was observed. On days 15 to 21 post-transduction, colonies are ready for transfer. Colonies were smoothly obtained and transferred to Matrigel-coated 12-well plates for augmentation.
Residual detection of exogenous factors
According to the Reprogramming Kit, the following PCR primer sets were used to detect transgenes in reprogrammed cells (Table 1).
| Target | Primer sets | Product size |
|---|---|---|
| KOS | Forward: ATGCACCGCTACGACGTGGC Reverse: ACCTTGACAATCCTGATGTGG |
528bp |
| Klf4 | Forward: TTCCTGCATGCCAGAGGAGCCC Reverse: AATGTATCGAAGGTGCTCAA |
410bp |
| c-Myc | Forward: TAACTGACTAGCAGGCTTGTCG Reverse: TCCACATACAGTCCTGGATGATGATG |
532bp |
Table 1: Transgenes in reprogrammed cells were found using the PCR primer sets.
Alkaline phosphatase (AP) staining
After 3 passages, cells were stained for AP strictly following the manufacturer's protocol, and pluripotency was detected using the AP detection kit (Stemgent, 00-0055). Place cells in Fix Solution and fix for 2-5 minutes at normal room temperature. Cells were then placed in latest equipped AP Substrate Solution and incubated in the dark for 10-20 minutes. Typical iPSC colonies stain positive for alkaline phosphatase in red or purple.
Evaluation of differentiation ability of three layers in vitro
To evaluate the capability of hiPSCs to differentiate into the three germ layers, in vitro differentiation was operated using the STEMdiff Trilineage Differentiation Kit (Stemcell, 05230) strictly following the instructions.
Immunofluorescence (IF) staining
Expression of pluripotency markers was investigated with IF staining. Cells were observed under an inverted fluorescence microscope (OLYMPUS 1 × 71). hiPSCs were characterized with Oct-4, Nanogram, Sox2 and TRA-1-60 antibodies.
Karyotyping
When prepared, the solution of Immunovac VP4 was administered to patients at a dose of 2 drops (1 mg) in each nostril daily and subcutaneously every other day at doses 0.05 (0.5), 0.1 (1.0), 0.2 (2.0), 0.2 (2.0), 0.3 (3.0) and 0.3 (3.0) from day 1 to day 11 of hospital stay.
iPSC generation from peripheral blood
PBMCs were successfully separated from 5 ml whole blood of healthy volunteer by using Lymphoprep™ density gradient centrifugation, and these cells were used for subsequent iPSC establishment. Under normal conditions, 5 × 106 PBMCs can be efficiently separated from 5 ml whole blood, which is adequate to elicit iPSCs. The PBMCs were cultured in Complete Stem Span™ SFEM II Medium (Figure 1A). After 4 days culture, 2 × 105 live cells were transduced with Sendai virus. To increase the transfection efficiency, the transfection was performed by centrifugation 200 g for 2 h at room temperature. After 4 hours, cell suspension was centrifuged to remove Sendai virus and cells were resuspended in 24-well plates. Seven days after transfection, some small colonies began emerging (Figure 1B). About 3 weeks, the colony size is suitable for picking (Figure 1C). Selecting well-defined, flat, compact colonies for transfer the suspension (Figures 1A-1D).
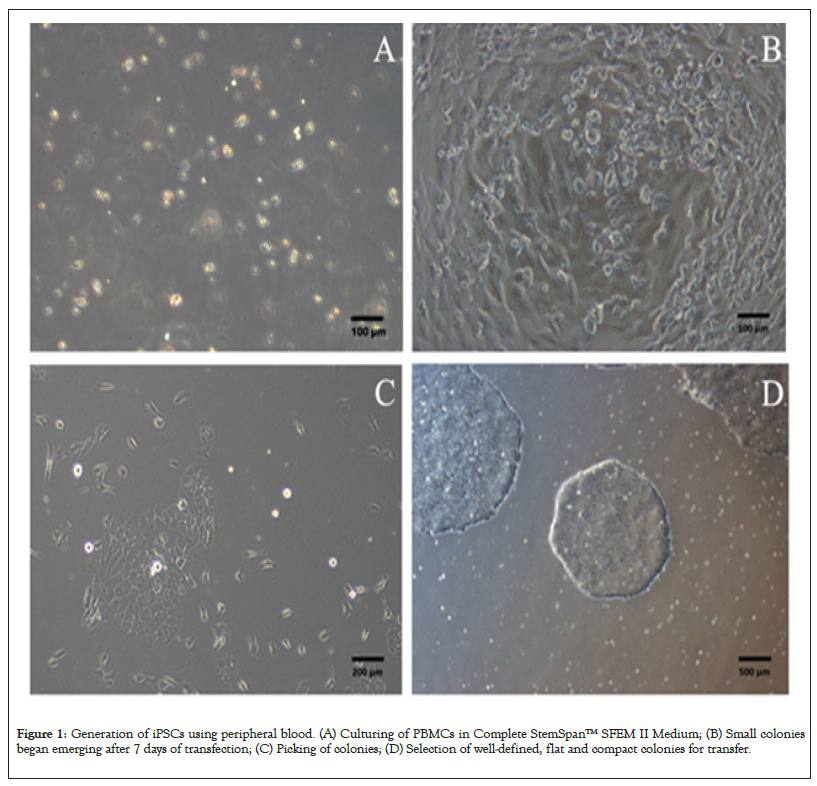
Figure 1: Generation of iPSCs using peripheral blood. (A) Culturing of PBMCs in Complete StemSpan™ SFEM II Medium; (B) Small colonies began emerging after 7 days of transfection; (C) Picking of colonies; (D) Selection of well-defined, flat and compact colonies for transfer.
iPSCs showed typical hESC morphology
iPSCs gathered and augmented in mTeSR™ Plus medium exhibited classical human ESC shape, flat and compact, with well-defined borders, high nuclear/cytoplasmic ratio, prominent nucleoli, robust proliferation, persisted for more than 30 passages, and retained a normal karyotype (Figure 2A). The exogenous transcription factors carrying reprogramming have been completely degraded and disappeared in the 5th generation cells (Figures 2A and 2B). These iPSCs were positive for Alkaline Phosphate (AP) staining, which is the characteristic of undifferentiated cells, such as embryonic stem cells and iPS cells (Figures 3A and 3B).
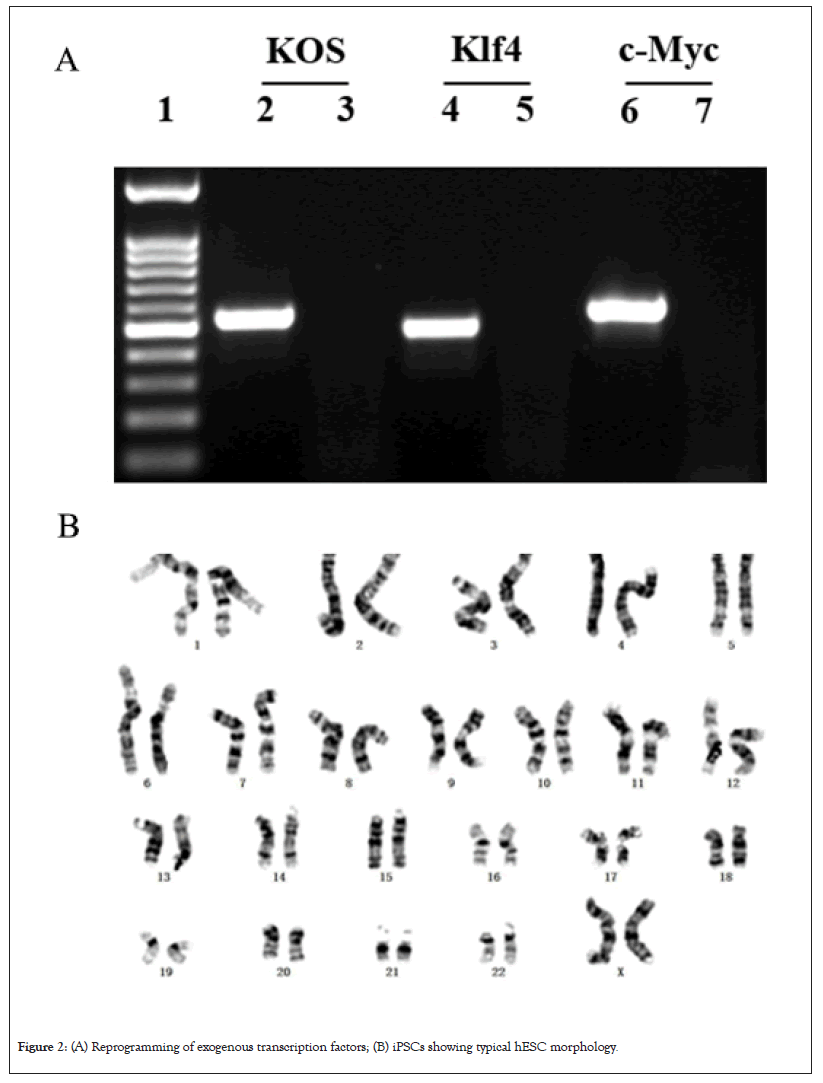
Figure 2: (A) Reprogramming of exogenous transcription factors; (B) iPSCs showing typical hESC morphology.
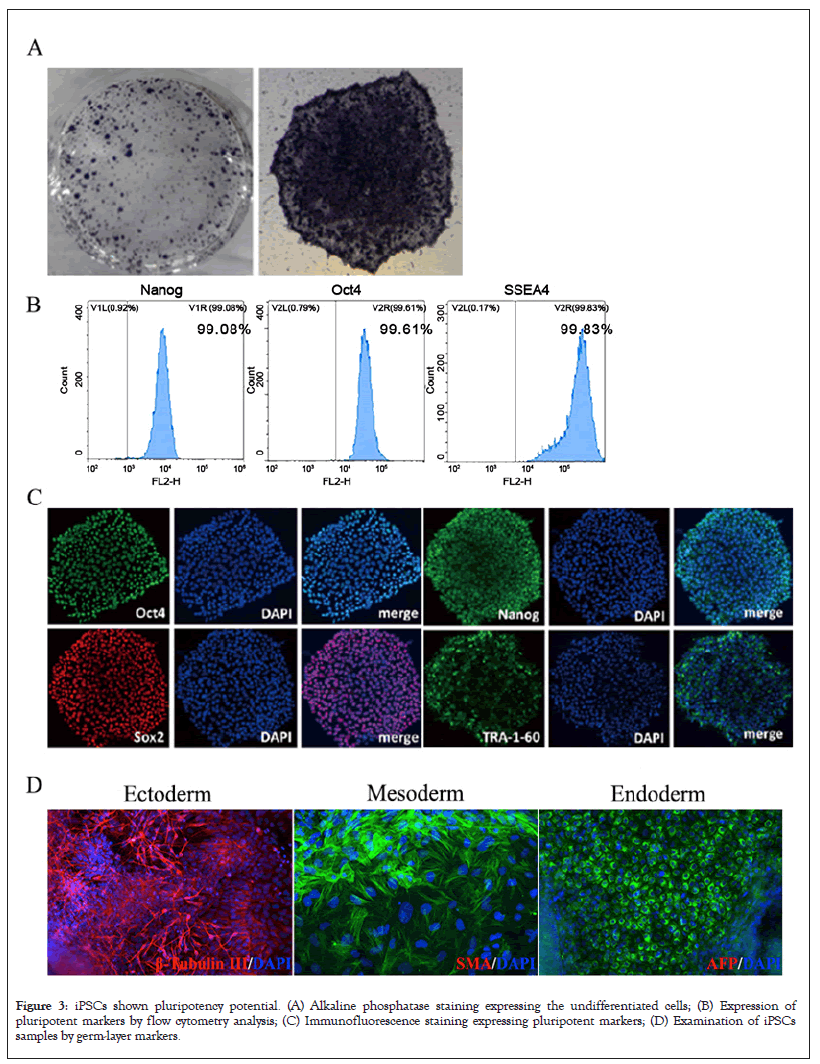
Figure 3: iPSCs shown pluripotency potential. (A) Alkaline phosphatase staining expressing the undifferentiated cells; (B) Expression of pluripotent markers by flow cytometry analysis; (C) Immunofluorescence staining expressing pluripotent markers; (D) Examination of iPSCs samples by germ-layer markers.
iPSCs showed pluripotency capability
Immunofluorescence staining, flow cytometry and three germ layers differentiation were used to identify the pluripotency of iPSCs. Immunofluorescence expressed pluripotent markers (Oct4, Sox2, Nanog, TRA-1-60), which are typical human ES cell specific antigens (Figure 3C). In addition, flow cytometry analysis showed that more than 99% of the cells expressed pluripotent markers Nanogram, OCT4, and SSEA4 (Figure 3B). We also examined the capability of iPSCs to differentiate into three germ layers with an easy-to-use in vitro differentiation assay. After cultivation under different conditions, the iPSCs samples were examined by three germ-layer markers: β-tubulin Ⅲ (ectoderm), SMA (mesoderm) and AFP (endoderm) (Figure 3D). These results confirm that patient-derived hiPSC cell lines successfully constructed by our way can be stably maintained and have their threefold differentiation capacity (Figures 3A-3D).
Human pluripotent stem cells, including embryonic stem cells and iPSC, have great potential in cell replacement therapy and can be used to treat various difficult diseases, such as senile diseases, trauma or congenital defects. However, there are still obstacles restricting the clinical application of human iPS technology.
In the process of inducing human fibroblasts into iPSCs, Lentivirus vectors are usually used to co-transfect four exogenous genes (Oct4, Nanog, Sox2 and Lin28) [16]. However, the use of lentiviral vector in iPSC induction has the risk of integration into the genome of host cells and potential mutagenicity. Studies have shown that exogenous reprogramming factors in mouse cells can be successfully induced into iPS cells only after 12 days of expression [17]. Although some studies have shown that the transferred cDNA needs to prolong the expression to make the reprogramming proceed smoothly, which poses a challenge to the generation of iPSC by non-integrated methods. Stadtfeld et al, have demonstrated that genome integration is not necessary for successful reprogramming of fibroblasts, and mouse fibroblasts can be successfully reprogrammed using adenovirus vector [18].
In order to obtain non-genetically integrated iPSCs, Sendai virus was selected as the vector for reprogramming factor transduction. Sendai virus is not integrated into the genome of host cells. It is very suitable for introducing reprogramming factors into human somatic cells. At the same time, it can be gradually removed in the process of culture without drug screening. Using non-integrated Sendai virus vector can also maintain the stable expression of reprogramming factors in the early stage of receptor cells.
Although different sources of fibroblasts were originally used for induced pluripotent stem cells, PBMCs has become universally recognized as a simpler and almost limitless source for cell reprogramming [19-22]. Typically, PB can also be stored at normal room temperature for ~48 h, and derivatized PBMCs can also be stored at low temperature for a longer period of time, enabling long-distance transport of samples and timely establishment of hiPSC.
Here, we successfully employed 5 ml of peripheral blood to isolate PBMCs and derived iPSCs. To make this method easy to use and minimally invasive, we attempted to reduce the initial volume of peripheral blood. Freshly purified PBMCs from 500 µl of peripheral blood are sufficient to successfully obtain induced pluripotent stem cells without additional reprogramming-enhancing reagents.
In the molecular mechanism research and clinical work of many rare genetic diseases (such as DMD, SMA, ALS), we found that the samples from patients are becoming more and more rare. Although fibroblasts commonly used in in vitro research and can be self-renewal, it is becoming more and more difficult to obtain, mainly because most patients with genetic diseases are children, and invasive examinations such as skin or muscle biopsy are difficult to carry out. In order to preserve patient derived cell samples, it is still necessary to find a simple, noninvasive and easy to accept sample acquisition method. Patients' blood is easy to obtain, but cannot be self-renewal; combined with the establishment technology of iPSC in this study, the purpose of preserving patient cell samples can be realized. The establishment of these cell models can provide an effective sample source for future disease mechanism research and drug screening.
The authors have no conflicting financial interest.
Science and Technology Program of Guangzhou and Innovation Development Project of the First Affiliated Hospital, Guangzhou University of Chinese Medicine (2019ⅡT36).
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
Citation: Chen X, Lua Y, Ying R, Yua W, Tian P, Hua D, et al. (2023) Method for Constructing Human iPS Cell Line RZPBi001 from Peripheral Blood Cells. J Clin Cell Immunol.14:686.
Received: 04-May-2023, Manuscript No. JCCI-23-23876; Editor assigned: 08-May-2023, Pre QC No. JCCI-23-23876 (PQ); Reviewed: 23-May-2023, QC No. JCCI-23-23876; Revised: 30-May-2023, Manuscript No. JCCI-23-23876 (R); Published: 07-Jun-2023 , DOI: 10.35248/2155-9899.23.14.686
Copyright: © 2023 Chen X, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.