Journal of Clinical & Experimental Dermatology Research
Open Access
ISSN: 2155-9554
ISSN: 2155-9554
Case Report - (2022)Volume 13, Issue 4
Melanoma is the most dangerous type of skin cancer. Melanoma diagnosed during pregnancy or within 1 year of delivery is defined as pregnancy-associated melanoma. Approximately 35% of women diagnosed with melanoma are in childbearing age. Strong evidence for potential association between In-Vitro Fertilization (IVF) treatment and hormone-related cancers is unavailable. We present a case of a 27 years old pregnant female who was diagnosed with melanoma at 22 weeks of gestation after IVF treatment.
Melanoma; Pregnancy Associated Melanoma (PSM); IVF treatment; Fitzpatrick phenotype II
Melanoma is the most serious type of skin cancer. The incidence of melanoma is increasing globally [1]. It represent some of the most frequent malignancies diagnosed in women during pregnancy and approximately 35% of women diagnosed with melanoma are in the childbearing age [1]. The frequency of pregnancy-associated melanoma could continue to increase due to the trend of delayed childbearing [2]. Evidence regarding association of melanoma and pregnancy by In-Vitro Fertilization (IVF) is limited. We present a case of female patient diagnosed with melanoma during 22 weeks of IVF pregnancy.
A 27 years old pregnant woman with Fitzpatrick skin type II, blond hair and blue eyes with 22 weeks of gestation post IVF presented with bleeding mole. The lesion changed in size during last years and more so especially during last month. There was no history of recent trauma on the lesion. She provided history of extensive sun exposure and no history of Skin Protection Factor (SPF) containing creams during childhood. There was positive history of familial melanoma. On clinical examination of her back, a dark nodular lesion on top of a flat lesion, larger than two centimeters arising on an irregular base was noticed.
Different colors (black, brown, dark brown, light brown) and irregularity on the borders was evident (Figure 1).
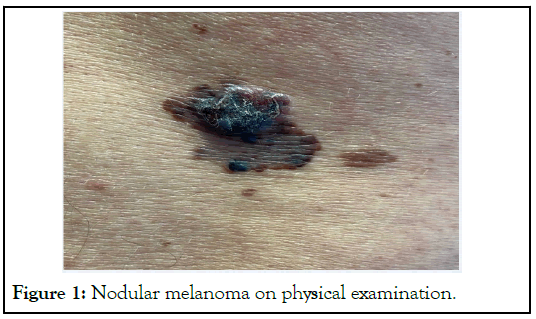
Figure 1: Nodular melanoma on physical examination.
Dermoscopy examination showed an asymmetric nodular lesion, with multiple colors, irregular borders on the base of the lesion and white-blue veil (Figure 2). The lesion was excised under local anesthesia with lidocaine in the onco-dermatology unit. Biopsy of the lesion confirmed presence of the nodular melanoma. Histopathologic examination showed 2.8 mm infiltration of the neoplastic melanocytes into reticular derma, light lymphocytic intratumoral infiltration and mitotic index of two on ten on high power fields.
Figure 2: Nodular melanoma, dermoscopy view.
On immunohistochemical staining, atypical cells were Melan A strongly positive, HMB45 positive without gradient, ki67 15%, p16 negative and there was no evidence of vascular CD31 invasion or lymphatic D2-40 invasion. After the second day of biopsy, the patient had a miscarriage of twin babies. After the biopsy result, a re-excision with wide margin was done. The sentinel node biopsy was negative. Melanoma was developed in a preexisting dysplasic nevus. Complete follow up with Positron Emission Tomography (PET) scan, echography and skin examination with dermoscopy continue to be performed. Follow-up based on the guidelines for the first five years was recommended. During the first three years of follow-up, there was no evidence of melanoma recurrence.
Genetic predisposition and environmental factors are implicated in pathogenesis of melanoma. Ultraviolet (UV) rays are the only preventable risk factor for developing malignant melanoma. Our patient reported extensive history of sun exposure during her childhood period. However, in our case it is difficult to certainly associate the occurrence of melanoma due to UV ray exposure. Melanoma in women is most frequently seen during their reproductive years. A Norwegian study found that 31 percent of all malignancy occurs during pregnancy and melanoma is the most frequent malignancy [3]. Increased melanocytes activity during pregnancy due to hormonal stimulation is seen in the form of melasma, linea nigra, genital and areolar darkening [4]. The effect of pregnancy and hormones during pregnancy in malignant melanoma is not well understood [5]. In our patient, the size of lesion increased during pregnancy.
Mortality in Pregnancy-Associate Melanoma (PAM) is reported to be 17% higher than melanoma diagnosed in nonpregnant women [6]. Many hypothetical mechanisms for melanoma progression have been postulated. Delayed diagnosis may contribute to the worse outcomes in PAM than melanoma in non-pregnant women [7]. Increased lymphangiogenesis during pregnancy has also been claimed to be responsible for increased melanoma metastases [8,9]. Secondly, the growth factors associated with pregnancy could be implicated in enhancement of tumor growth [10] due to stimulation of neoangiogenesis and tissue invasion [11]. Moreover, pregnancy-associated metalloproteinase produced by the placenta is also implicated in increased invasiveness, progression and migration of melanoma [12]. Breslow thickness is increased in pregnancy-associated melanomas than other melanomas diagnosed in females of similar age [1].
Melanoma was traditionally considered to be a non-hormone- associated neoplasm [13]. The role of the sex hormones on melanoma progression is currently unclear [14], but immunomodulatory effects of sex hormones are known. Human chorionic gonadotropin, estrogen, progesterone, and others contribute to the induction of immunologic tolerance and angiogenesis at the beginning of gestation to protect the developing fetus [15]. All malignancies exploit variety of mechanisms that induce immune tolerance providing cells implantation and evading immune attack in a manner similar to the process of placentation. Sex hormones are also known to be implicated in stimulation of melanocytes and melanin production. The decreased activation of estrogen receptor β enhances melanoma growth and progression [13] according to recent researches. While, progesterone inhibits melanoma cell growth through stimulation of autophagy, but this effect is not mediated via progesterone receptors [16].
IVF has history of more than four decades. Supraphysiological levels of estrogen and progesterone are used during the process. Clomiphene citrate or gonadotropins are used for ovarian stimulation or both. Chorionic gonadotropin or progesterone is used to stimulate luteal phase and during the first trimester of pregnancy sometimes progesterone treatment is followed. Melanocytes are sensitive to sex- hormones and there is a presence of these hormonal receptors in them (estrogen receptor β) [17]. However, the definitive mechanism of estrogen-associated melanoma carcinogenesis remains to be established. It could be possible that excess levels of estrogen during pregnancy induce down regulation of estrogen receptor β which may enhance melanoma growth [17]. Studies have examined the association between IVF treatment and melanoma [18,19]. One study involving 12 cases of melanoma in women with IVF did not find an association between the two [18]. A larger study involving 55 cases of melanoma in women with IVF treatment with an average of 17 years of follow-up reported 3.6-fold higher risk of melanoma in those who gave birth compared with women with IVF but did not give birth [19]. The risk is not evident in women with non-IVF infertility treatment [20]. In our case, we believe increase in activity of melanoma was probably due to IVF treatment. Unfortunately, the patient lost twin pregnancy after biopsy of lesion. The cause of this outcome is unknown. Incidences of other malignancies including breast, cervical, colon, and thyroid cancers have not been reported to be higher in women undergoing IVF treatment [21,22]. Clomiphene citrate has been reported to increase the risk of melanoma [17]. However, it still remains to be determined if IVF process is a potential risk of melanoma or is actually a coincidental event-for these women.
In our case, melanoma was seen in a pregnant woman who went through IVF treatment. There is not enough evidence to suggest that there is an association between fertility medication use and melanoma or is actually a coincidental event-for these women. Additional studies with longer follow-up are needed to identify whether women following fertility treatment have an increased risk of developing melanoma. Although, IVF treatments have not definitively been linked to melanoma, we recommend addressing melanoma screening and prevention strategies especially in patient with high risk during infertility consultation.
RM, LB, JT and MF were involved in the conceptualization of the article. RM, LB, JT, MF, AP and MG were involved literature search, review of the draft and revision of the draft. All authors approved the manuscript.
Written informed consent was obtained from the patient to publish this report.
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
Citation: Mala R, Berdica L, Thimja J, Fida M, Patil A, Goldust M (2022) Melanoma after In-Vitro Fertilization: A Risk Factor or Just a Coincidence? J Clin Exp Dermatol Res. 13:614.
Received: 25-Jul-2022, Manuscript No. JCEDR-22-18080; Editor assigned: 29-Jul-2022, Pre QC No. JCEDR-22-18080 (PQ); Reviewed: 12-Aug-2022, QC No. JCEDR-22-18080; Revised: 19-Aug-2022, Manuscript No. JCEDR-22-18080 (R); Published: 30-Aug-2022 , DOI: 10.35248 / 2155-9554.22.13.614
Copyright: © 2022 Mala R, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.