Journal of Drug Metabolism & Toxicology
Open Access
ISSN: 2157-7609
ISSN: 2157-7609
Market Analysis - (2021)
Pharmaceutical market research analysis (PMRA) can validate a product's potential reach and market penetration capabilities. Investors use it to put a value on drug-making companies and their product pipelines. Those companies use it to monitor the competition, identify potential unmet needs and value their products. Donors and grant providers use PMRA to look for drugs being developed to treat specific diseases and the likelihood of providing a cure for the disease they represent.
Pharmaceutical companies need to weigh the potential of a product, which can often depend on the number of patients with the indication the drug is approved to treat. If existing products are already approved to treat that indication, a new product must represent a significant improvement to justify its development cost. Pharma firms must constantly evaluate their products as research progresses and more investment is needed to fund drug trials. Many Pharma firms will initially test a product to treat a rare indication with no current approved treatments in order to avoid having to compete against the results from existing treatments.
The pharmaceutical industry is of great interest to investors looking for stocks that offer high returns on investment despite having higher than average risk. They use PMRA to determine the potential revenues a pharmaceutical company might realize if all its products in development receive Food and Drug Administration (FDA) approval. That potential to generate revenue is then weighed against the company's current stock price and market capitalization. For this revenue potential to be realized the products must first pass FDA scrutiny and then gain the confidence of doctors.
Graphical representation:
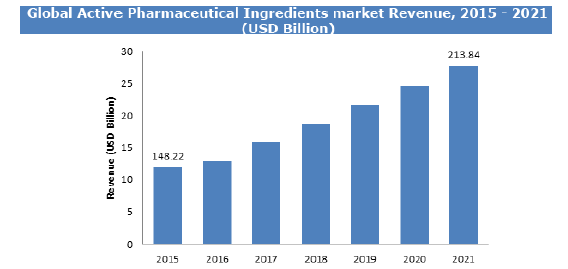
Grant providers and charitable non-profit groups seeking ways to treat a specific disease can use PMRA to look for appropriate products and companies to support. PMRA can also be used to evaluate where there are unmet needs for treatment and to identify products that can provide potential cures. Grant providers use this research to determine how many patients can be treated with a drug under development, how effective the results appear to be, and how close it is to reaching the market. They can also determine whether it can be tested on patients with the type of indication they represent.
The global pharmaceuticals market was worth $934.8 billion in 2017 and will reach $1170 billion in 2021, growing at 5.8%, according to a recent Pharma market research report by The Business Research Company. This is an accelerated pace compared to 5.2% for the years before 2017, but is slower than the other two large healthcare segments, medical equipment and healthcare services. Healthcare as a whole is growing at over 7% year on year. Part of the explanation for the relatively slow current growth of the Pharma market is that the launch of major new products has slowed and that companies are restricting their R&D investment. For example, despite the huge potential for any effective and safe new drug for treating Alzheimer’s disease, Pfizer has ended its Alzheimer’s research program while AstraZeneca and GSK have cut back. High failure rates, the $2 billion average cost of developing a new drug, and falling returns on investment down from 10.1% a year in 2010 to 3.2% in 2017 for the big Pharma companies, according to Deloitte’s are restraining the launch of high-priced new breakthrough drugs such as those that boosted the market in earlier years. Most pharmaceutical industry growth now is coming instead from the increased size of the global aging population, which boosts demand for long - term treatments
Copyright: This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.