Journal of Women's Health Care
Open Access
ISSN: 2167-0420
ISSN: 2167-0420
Research Article - (2019)Volume 8, Issue 5
Introduction: The voice of an intellectually and physically disabled woman is often forgotten when discussing, investigating and managing endometrial cancer in women with disabilities. This case report explores the need to start strategies for collaborative application of resources to optimize a woman’s experience who is living with disabilities and endometrial cancer.
Case Description: A 41-year old premenopausal woman with a severe intellectual disability and physically debilitating osteogenesis imperfecta presented with a 2-year history of abnormal uterine bleeding (AUB) and unsuccessful hormonal treatment. After two failed hysteroscopies due to her severe bony-pelvic abnormality, limiting access vaginally; the decision for a hysterectomy was made without a histological diagnosis. An Australian Guardianship Tribunal granted permission for both diagnostic and treatment of her AUB and suspicion of endometrial cancer. The ESMO-ESGO-ESTRO-2014 Consensus current recommendations and levels of evidence in management of endometrial cancer are evaluated in context of the case.
Method: Mandatory workup and pathological assessment for diagnosis of endometrial cancer could not be carried out and imaging including: CT scan, transabdominal ultrasound and MRI were relied upon to primarily assess her suspected disease.
An abdominal hysterectomy, bilateral salpingectomy and ovarian conservation were performed. The histopathology post-operatively confirmed stage 1A grade 1 endometrioid adenocarcinoma. The time from initial Gynaecology Oncology referral to final histopathology was 9 months.
Discussion: This is the first report to explore the limitations and challenges of the literature and application of various current diagnostic modalities, surgical approach and outcomes of endometrial cancer in an intellectually and physically disabled woman in Australia.
Endometrial cancer; Osteogenesis imperfecta; Hysterectomy; Abnormal uterine bleeding
There were an estimated 2963 new cases of endometrial cancer diagnosed in 2018 across Australia [1], however, they have been poorly studied in women with intellectual and physical disabilities, which makes designing a policy for managing the 2-4% of women living with a severe impairment in Australia difficult [2]. People with disabilities have the same health needs as non-disabled people for cancer screening, treatment and follow-up.
This case report of a young woman with osteogenesis imperfecta and an intellectual disability diagnosed with endometrial cancer is an example which aims to review our clinical practice in treating both mentally and physically disabled women. There is a potential increased risk for the delayed diagnosis of gynaecologic conditions that require major abdominal or vaginal surgery because thorough and complete pelvic examinations and gynaeoncological workups are often difficult to accomplish in women with disabilities. This narrower margin of health that many women with disability may experience may be exacerbated by poverty and social exclusion, and because they may be vulnerable to secondary conditions, such as post-operative complications, delay in diagnosis and prolonged hospital stay. Evidence suggests that women with disabilities face barriers in accessing the cancer- health services they need in many settings [3,4].
The Osteogenesis Imperfecta (OI) Foundation describes the condition as a rare, heritable condition that affects the body’s production of collagen, which is the major protein in connective tissue, and it is estimated that 6-7 per 100,000 people worldwide are affected. It is characterized by bones that break easily, often from little or no apparent trauma and extra skeletal manifestations. Both dominant and recessive patterns of inheritance are seen, and spontaneous dominant mutations may be responsible for 20-30% of new diagnoses. The number of symptoms, severity of functional impairments and disability of OI vary greatly among patients and range from mild forms with few exterior signs to very severe cases where life expectancy is decreased. Variable features of OI include short stature, hearing loss, scoliosis, dentinogenesis imperfecta, cardiopulmonary issues and obesity. Intelligence is typically normal in OI [5].
The case report by Nishida et al. in 1993, describes the hypothesis by Rosenstock (1968, 1970) and Lynch HT et al. (1966), that osteogenesis imperfecta gave cancer-protection and there were no cases of concurrent cancer and osteogenesis imperfecta. They postulated that a congenital lack of growth-inhibiting agents in procollagen restrained epithelial proliferation and that osteogenesis imperfecta gave biochemical resistance to cancer. Nishida et al. report for the first time the occurrence of ovarian serous carcinoma in a patient with osteogenesis imperfecta [6].
Indeed, although only a handful of cases of osteogenic malignant neoplasms have been reported in the literature: breast cancer (Lyss 1993, Beuzenboc 1995 and Taira 2014) ovarian cancer (Nishida 1993), colon cancer (Fukushima 2001) the association of osteogenesis imperfecta and endometrial cancer is limited [7,8].
As no data is available in the literature with regards to endometrial cancer evaluation in women with both a physical and mental disability, we analyzed a case of a 41-year old premenopausal woman with a severe intellectual disability and osteogenesis imperfecta. She had an intellectual capacity of a 5-year-old, with severe developmental delay and reduced social functioning. Her extreme bony and pelvic abnormalities from previous fractures due to her OI meant she stood no more than 110 cm tall, weighed 50kg (BMI=41) and she was able to walk independently. Her mother and sister were her main caregivers, however they did not have legal guardianship. She presented with a two-year history of abnormal uterine bleeding (AUB), anaemia and multiple presentation for menorrhagia, and after a failed hysteroscopy attempt by a gynaecologist, she was referred to a tertiary gynaecology oncology unit in Australia for further assessment. After unsuccessful hormonal treatment for her bleeding and a second failed hysteroscopy attempt by the gynaecology oncology unit, to establish the cause of her abnormal uterine bleeding; the decision for hysterectomy was made without a histological diag- -nosis of endometrial cancer. An Australian Guardianship application and Tribunal granted permission for both diagnostic and treatment of her AUB and suspicion of endometrial cancer.
The European Society for Medical Oncology (ESMO), European Society for Radiotherapy and Oncology (ESTRO) and European Society of Gynaecological Oncology (ESGO) ESMO-ESGOESTRO December 2014 Consensus recommendations in diagnosis, treatment and follow-up of endometrial cancer were applied to our case to compare standard management recommendations for endometrial cancer to the application of these recommendations in a woman with severe physical and mental disability as with our case [9].
The ESMO-ESGO-ESTRO consensus panel comprised of experts in the management of endometrial cancer. Each panel member was assigned to one of four working groups’ subjects with 12 clinical questions, with a working group chair appointed for each group. Only 3 groups are relevant to our case: 1. Prevention and screening of endometrial cancer, 2. Surgery and 3. Adjuvant treatment. Each working group was responsible for reviewing the relevant literature to draft preliminary recommendations relating to each of their assigned questions. No systematic literature search was undertaken but a vote of agreement was conducted by the expert panel and recommendations from each group were then presented to the entire panel of experts, where they were discussed and modified as required. An adapted version of the consensus was then formulated in our study in the table to define the level of evidence and strength of each recommendation proposed by the group and compared to our case (Table 1). Further modifications and barriers to current recommendations in detecting, diagnosing and treatment of endometrial cancer are evaluated in context of the case.
| ESMO-ESGO-ESTRO December 2014 Consensus recommendation: | Level of evidence Strength of recommendation Consensus – YES % |
Application to case report: | |
|---|---|---|---|
| Which surveillance should be used for asymptomatic women? | There is no evidence for endometrial cancer screening in the general population. | II A 100% |
No screening available. History of menorrhagia for 2 year. |
| What work-up and management scheme should be undertaken for fertility preserving therapy in endometrioid adenocarcinoma grade 1? | Patients with grade 1 EEC requesting fertility-preserving therapy must be referred to specialized center. | V A 100% |
As endometrial sampling could not be performed conservative management with hormones could not be considered. |
| In these patients D&C with/without hysteroscopy must be performed. | IV A 97.3% |
Two failed hysteroscopy attempts. The first hysteroscopy by a gynaecologist was abandoned due to an anaesthetic related tachycardia on the operating table. A year later after referral to the gynaecology oncology unit the second could not be performed due to limited access vaginally due pelvic-bony abnormalities. The cervix could not be reached. |
|
| Grade 1 EEC must be confirmed/diagnosed by a gynaecopathologist | IV A 100% |
Histopathology could not be obtained as limited vagina access. | |
| Pelvic MRI should be performed to exclude overt myometrial invasion. Expert ultrasound as alternative |
III B 100% |
A Pelvic MRI and trans-abdominal ultrasound were performed. Trans vagina ultrasound could not be performed as virgin-intactus. | |
| MPA/MA is the recommended treatment. However, LNG-IUD with/without GnRH can be considered. | IV B 100% |
An LNG-IUG could not be inserted as intra-endometrial access not attainable at time of hysteroscopy. Oral progesterone not considered as no histological diagnosis of cancer/hyperplasia. |
|
| To assess response, D&C, hysteroscopy and imaging at 6 months must be performed. If no response – standard surgical treatment should be performed. | IV B 100% |
No response to hormonal management can be achieved as no hysteroscopy not possible. | |
| After completion of childbearing a hysterectomy and salpingo-oopherectomy should be recommended. Preservations of ovaries can be considered depending on age and genetic factors. | IV B 100% |
Ovarian conservation was carried out. | |
| How does the medical condition influence sugical treatment? | Mandatory work-up must include: family history; general assessment and inventory of comorbidities; clinical examination, including pelvic exam, transvaginal/transrectal ultrasound; and complete pathological assessment (histotype and grade) of an endometrial biopsy or curettage specimen. | V A 100% |
A typical work-up could not be carried out due extreme physical disability and bony deformities, thus histological diagnosis could not be achieved. |
| Extent of surgery should be adapted to the medical condition of the patient. | V A 100% |
A laparotomy, total hysterectomy and bilateral salpingectomy was performed due risk of MIS in an osteogenesis imperfecta patient. | |
| In clinical stage 1, grade 1 and 2: At least one of the three following tools should be used to assess myometrial invasion if, LNG-IUD is considered: Expert ultrasound and/or/MRI and/or intra-operative pathological examination. | IV A 100% |
Intra-operative examination of uterus - bicornuate uterus with polypoidal lesion in both cavities, anterior fibroid. | |
| Other imaging methods (thoracic, abdominal and pelvic CT scan, MRI, PET scan or ultrasound) should be considered to assess ovarian, nodal, peritoneal or metastatic disease. | IV C 94.6% |
No evidence of metastasis or lymphadenopathy. | |
| There is no clinical usefulness of serum markers including CA 125. | IV B 91.9% |
No CA 125 or HE4 taken. | |
| Standard surgery is total hysterectomy with bilateral salpingo-oopherectomy without vaginal cuff. | IV A 100% |
Total abdominal hysterectomy and bilateral salpingectomy with ovarian conservation. | |
| Ovarian preservation can be considered in patients younger than 45 years old with grade 1 EEC with myometrial invasion <50% and no obvious ovarian or other extra-uterine disease. | IV B 100% |
Ovarian preservation performed as patient <45 years and <50% myometrial invasion on intra-operative pathological evaluation. | |
| In case of ovarian preservation salpingectomy is recommended. | IV B 100% |
Bilateral salpingectomy performed. | |
| Minimally invasive surgery is recommended in the surgical management of low-and intermediate risk endometrial cancer. | I A 100% |
Laparotomy was performed due to osteogenesis imperfecta bony abnormalities causing minimal vaginal access and anesthetic risk. | |
| What are the indications for and to what extent is lymphadenectmy indicated in the surgical management of endometrial cancer? | Patients with low-risk endometrioid carcinoma have a low risk of lymph node involvement, therefore, lymphadenectomy is not recommended for these patients. | II A 100% |
No lymphadenectomy done as our patient was pre-menopausal and assumed to have an early cancer/hyperplasia. Consideration that premenopausal women have earlier stage and favorable prognosis. |
| What is the current best definition of risk groups for adjuvant therapy? | In patients with low-risk endometrial cancer, (Stage 1 endometrioid EEC grade 1 or 2, <50% myometrial invasion, LVSI negative) no adjuvant treatment is recommended. | I A 100% |
Stage 1A grade 1 at final histopathology diagnosis and MDT. No adjuvant treatment needed. |
| Note: EEC=endometrioid adenocarcinoma of endometrium. MPA/MA =Medroxyprogesterone acetate/megestrol acetate, LNG-IUD=Levonorgestrel-releasing intrauterine device, LVSI=lymph vascular space invasion. | |||
Table 1: An adapted version of the ESMO-ESGO-ESTRO December 2014 Consensus recommendations formulated to define the level of evidence and strength of each recommendation proposed by the group and compared to our case.
A general assessment including: pelvic examination, transvaginal ultrasound and complete pathology assessment (histology and grade) of an endometrial biopsy or curettage specimen as mandatory workup for diagnosis of endometrial cancer could not be carried out in our case due to her extreme bony abnormalities.
Other imaging methods including abdominal and pelvic CT scan, transabdominal ultrasound and MRI were relied upon to assess ovarian, nodal or peritoneal disease and metastasis and primarily assess the uterus and endometrium in the context of her abnormal bleeding (Figures 1-5). Serum tumour markers, including CA 125 and HE-4 were not carried out in our patient.
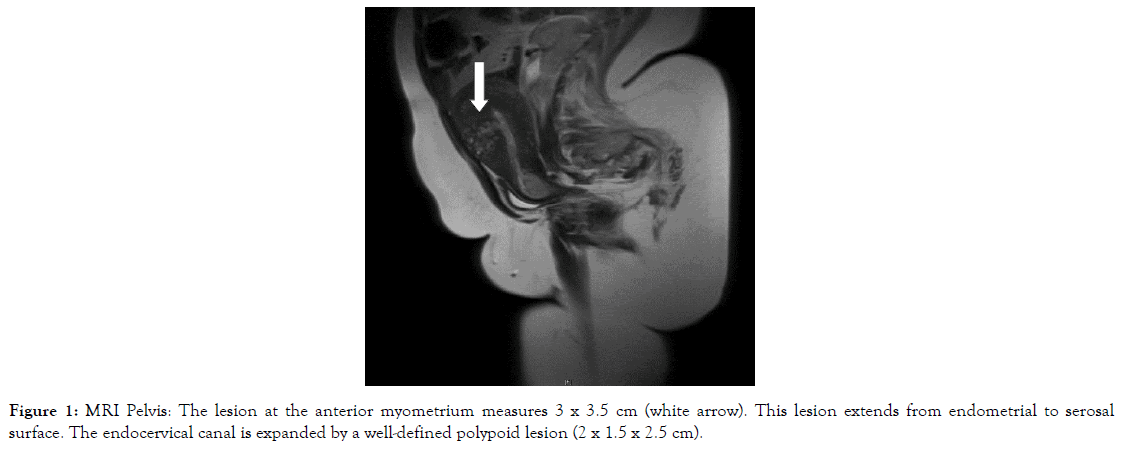
Figure 1. MRI Pelvis: The lesion at the anterior myometrium measures 3 x 3.5 cm (white arrow). This lesion extends from endometrial to serosal surface. The endocervical canal is expanded by a well-defined polypoid lesion (2 x 1.5 x 2.5 cm).
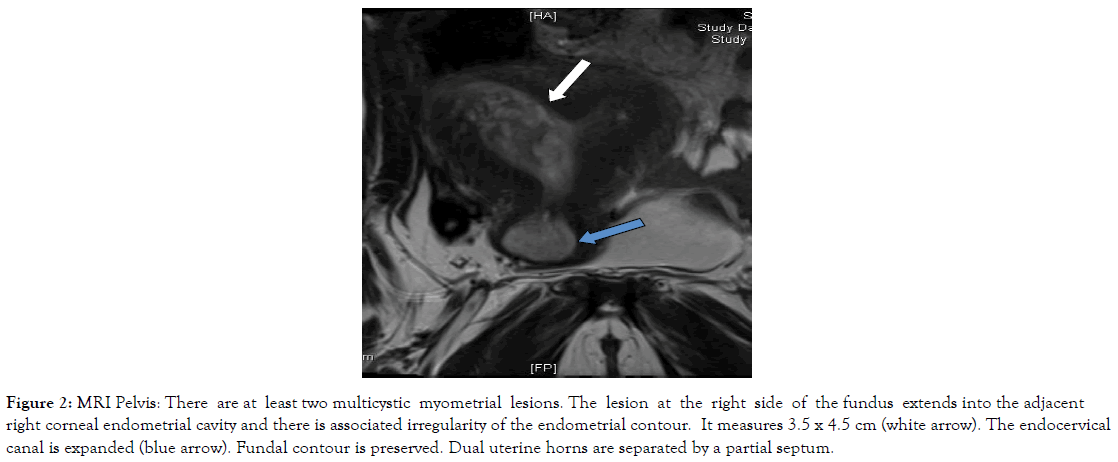
Figure 2. MRI Pelvis: There are at least two multicystic myometrial lesions. The lesion at the right side of the fundus extends into the adjacent right corneal endometrial cavity and there is associated irregularity of the endometrial contour. It measures 3.5 x 4.5 cm (white arrow). The endocervical canal is expanded (blue arrow). Fundal contour is preserved. Dual uterine horns are separated by a partial septum.
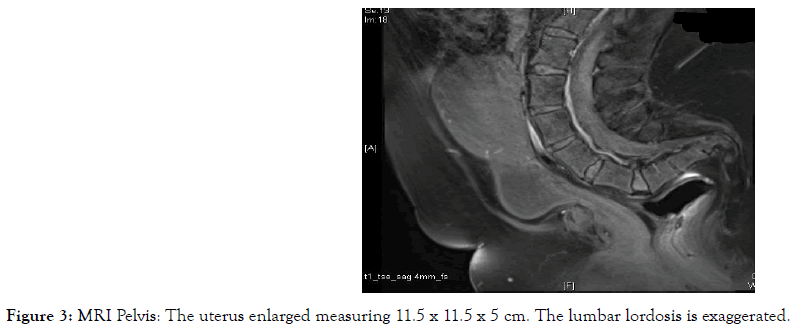
Figure 3. MRI Pelvis: The uterus enlarged measuring 11.5 x 11.5 x 5 cm. The lumbar lordosis is exaggerated.
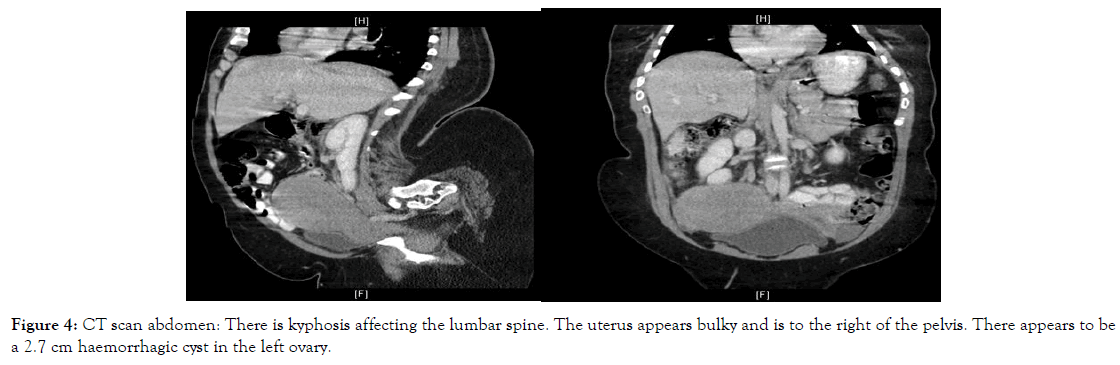
Figure 4. CT scan abdomen: There is kyphosis affecting the lumbar spine. The uterus appears bulky and is to the right of the pelvis. There appears to be a 2.7 cm haemorrhagic cyst in the left ovary.
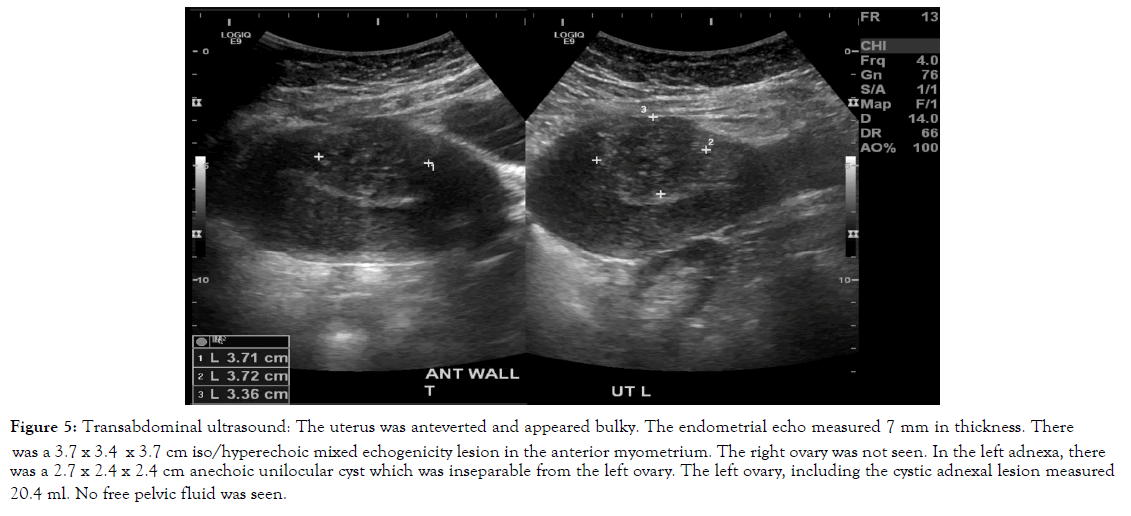
Figure 5. Transabdominal ultrasound: The uterus was anteverted and appeared bulky. The endometrial echo measured 7 mm in thickness. There was a 3.7 x 3.4 x 3.7 cm iso/hyperechoic mixed echogenicity lesion in the anterior myometrium. The right ovary was not seen. In the left adnexa, there was a 2.7 x 2.4 x 2.4 cm anechoic unilocular cyst which was inseparable from the left ovary. The left ovary, including the cystic adnexal lesion measured 20.4 ml. No free pelvic fluid was seen.
The consensus at the multidisciplinary team meeting (MDT) at our tertiary cancer center was to initially perform an examination under anaesthesia and hysteroscopy dilation and curettage with insertion of an LNG-IUD. This was consented for by the ‘person responsible’ which in our case was her mother. Due to the severe bony pelvic abnormality there was no access vaginally to perform a hysteroscopy or obtain an endometrial sample and insertion of an LNG-IUD as the uterine cervix was not accessible. Given her on-going abnormal bleeding and reviewing the imaging at the multidisciplinary team meetings there was consensus of concern for abnormal endometrium.
A New South Wales, Civil and Administrative Tribunal (NCAT) application to the guardianship division to carry out a tribunal for consent for a laparotomy hysterectomy and bilateral salpingectomy was granted after a three-hour hearing.
The management of endometrial cancer including hysterectomy, bilateral salpingectomy with ovarian conservation was performed in our case by a laparotomy as positioning her into lithotomy, as well as obtaining a pneumoperitoneum for a minimally invasive approach could not be carried out due to her osteogenesis imperfecta. This standard surgical approach carries a 5-year survival rate of 91% for stage 1 endometrial cancer, it also results in a permanent loss of reproductive potential [10].
Conservative management of suspected endometrial cancer was considered in our case, however, the assessment of clinical and pathological characteristics of a predicted endometrial malignancy or hyperplasia and selection of the appropriate medical intervention and follow up hysteroscopy could not be conducted.
A decision not to perform a pelvic lymphadenectomy or bilateral oophorectomy was made on the basis that this would increase her post-operative morbidity of potential lymphoedema and surgical menopause symptoms respectively. Clinical evidence shows that ovarian preservation in young women with early-stage endometrioid carcinoma is safe and did not increase the risk of mortality or recurrence [10]. Studies also show that premenopausal women tend to have earlier stage and better prognosis [10,11].
After her abdominal hysterectomy bilateral, she was admitted to ICU post-operatively and developed a clinical small bowel obstruction seven days post-surgery. She was taken back to theatre for a re-look laparotomy which found no evidence of intestinal obstruction. Her total length of stay in hospital was 22 days.
The histopathology confirmed a stage 1A grade 1 endometrioid adenocarcinoma. No adjuvant treatment was required. She will be reviewed every 3-6 months for two years.
The time from initial referral to the Gynaecology Oncology unit to final histopathology was 9 months.
Gynaecological Cancer and Disability
The United Kingdom’s 2009 ‘All Parliamentary Group on Cancer (APPGC)’ emphasizes the inequalities in cancer experienced by those with learning disabilities. Individuals with disability sit along a spectrum and categorization is often achieved through IQ assessment but may also consider the other two factors; social or adaptive dysfunction and early onset, when determining the severity of an individual’s disability [12]. There is no single definition of what constitutes a disability and because of this there are no exact figures. It appears that women with learning disabilities have similar incidence rates to the general population for most cancers. Research indicates that those with learning disabilities have high rates of diseases that are not being properly treated, and that they often have poor access to preventative services and lower uptake of cancer screening [13]. As in our case, a histopathology diagnosis could not be obtained preoperatively and from the initial gynaecology oncology consultation to final histopathology, staging was nine months.
Communication difficulties among those with severe learning disabilities make the occurrence of incorrect diagnosis or ‘diagnostic overshadowing’ (where symptoms are attributed to the learning disability without appropriate investigation) more likely. At the same time, women from this group are at an increased risk of suffering from multiple health conditions, such as having a learning disability and a physical disability. This further highlights the need for the tailoring of cancer information and services if the needs of this group are going to be met [14]. Health care providers must ensure adjustments to policies and processes to ensure that women with disabilities have equal access to gynaecological and oncological services in Australia.
To understand the health needs of those with learning disabilities and provide services to meet these needs, it is important that health professionals should be asked about their confidence when communicating with and treating those with disabilities and further training in this area offered where appropriate. Although there is some evidence that those with learning disabilities have lower uptake rates of cancer services, such evidence is often from small-scale studies. Further research needs to be undertaken to get a better picture of service use, and experiences of service use, among this group [15,16].
Osteogenesis Imperfecta, Disability, Obesity and Endometrial Cancer
The general health needs of women with OI are the same as in unaffected adults as with all women living with disability. Maintaining a healthy weight should be a priority. Being overweight not only increases the risk for many health problems, such as diabetes and cardiovascular conditions, but also puts additional stress on the skeleton, which is particularly unhealthy for people with OI [5].
More recently, interest in studying the relationship between obesity, endometrial cancer incidence and outcomes has grown in the general population, but these studies are still limited in the disabled population. This case report supports the hypothesis.
Nonetheless, the clinical implications of OI as with other physical and mental disabilities are associated with increased duration of hospital stay, higher costs of care, higher risk of nosocomial infections, and decreased survival in both non-malignant and malignant conditions [4]. OI has also been shown to have a negative impact on patients undergoing surgery and as a significant predictor of chemotherapy toxicity. Despite this growing body of literature, the impact of osteogenesis imperfecta in gynaecologic oncology patients has yet to be elucidated.
People with mobility limitations and intellectual or learning disabilities are at greatest risk for obesity and the obesity rates amongst adults with disabilities are 53% higher than adults without disabilities [17]. People with disabilities can find it more difficult to eat healthy, control their weight, and be physically active. This is often multifactorial and may include: physical limitations that can reduce a person’s ability to exercise, pain, a lack of accessible environments that can enable exercise and a lack of resources [17-19].
The obesity epidemic has also had a dramatic impact on endometrial cancer incidence. Oncologists are seeing more young obese women affected by endometrial cancer who desire maintaining future fertility. Among older patients with endometrial cancer, the severity of obesity is becoming worse, with many women having a BMI>40 kg/m2 and multiple medical comorbidities. The physically and intellectually disabled population is no exception. There is a critical need to refine options for conservative, nonsurgical management for both groups of women. Additional strategies should continue to be explored [20]. According to Emerson and Baines (2010), certain groups are also at risk for obesity due to living in restrictive environments or having particular syndromes that are associated with obesity [21].
Most patients with endometrial cancer have an identifiable source of excess estrogen and typically display a characteristic clinical profile comprising a high body mass index (BMI). According to a recent meta-analysis involving six studies and 3,132 cancer cases, relative risk (RR) for developing endometrial cancer in women with metabolic syndrome is 1.89(95% confidence interval[CI] 1.34- 2.67, p ≤ 0.001). According to individual components of metabolic syndrome, obesity is associated with the greatest increase in RR of 2.21 (p ≤ 0.001) [22]. A high BMI does however correlate with good prognostic features of endometrial cancer, including low tumour grade, endometrioid histology and presentation at early stage [23].
OI is a lifelong condition. Respiratory failure is the most frequent cause of death for people with OI, followed by accidental trauma. With good medical management and supportive care, many people who have OI will lead healthy, productive lives and can expect an average life span. It is probable that the incidence of endometrial cancer and osteogenesis imperfecta is no greater than in the general population. But, because of the lack of literature, this cannot be confirmed and before the premise is accepted, the relationship with obesity in osteogenesis imperfecta must be seen to be like that of spontaneously occurring tumours and etiological factors of significance must be dismissed.
Evidence of the growing obesity epidemic, with no exceptions in the both physically and intellectually disabled women, has been well documented; however, impact of the increase in endometrial cancer in these populations has not been studied. In our case, we highlighted that standard investigations, treatment and management of endometrial cancer could not be carried out due to the patient’s severe intellectual and physical disability. Urgent increase in gynaecology oncology research on the impact of endometrial cancer in the disabled population needs to be undertaken and especially within the context of the increasing obesity epidemic. Health care and safe, evidenced-based adjustments to standard practice and protocols needs to be accessible to both the health care providers and the physically and intellectually disabled woman.
Citation: Weishaupt J (2019) ‘Endometrial Cancer: Access Denied’ A Review of the Forgotten Voice of Mental and Physical Disability in Gynaecology Oncology in Australia. J Women's Health Care 8:476. doi: 10.35248/2167-0420.19.8.476.
Received: 02-Jul-2019 Accepted: 23-Oct-2019 Published: 30-Oct-2019
Copyright: © 2019 Weishaupt J. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original work is properly cited.