Pancreatic Disorders & Therapy
Open Access
ISSN: 2165-7092
ISSN: 2165-7092
Research Article - (2025)Volume 15, Issue 1
Introduction: Chronic pancreatitis is a crippling, progressive inflammatory disease of the pancreas associated with excruciating abdominal pain and exocrine and endocrine insufficiency, leading to poor quality of life, emotional stress and financial burden. The main indication for surgical intervention is intractable pain. Frey’s procedure is the most preferred procedure.
Objectives: The purpose of this study is to assess long-term pain management in patients with chronic pancreatitis who underwent Frey’s procedure using a validated pain score, as well as exocrine and endocrine insufficiencies and quality of life.
Methods: This is a prospectively analyzed retrospective study done at KIMS-Sunshine Hospital, Secunderabad. 48 patients who underwent Frey’s procedure between 2016 and 2021 with a minimum 1-year follow-up were analyzed using validated pain score used by Izbicki pain score, exocrine and endocrine funtions, quality of life (SF-6) and nutritional status was analyzed in terms of steatorrhea, glycaemic control and weight gain.
Results: The total number of patients enrolled was 52. Four patients were identified intraoperatively as having malignancy by frozen section and were therefore converted to the Whipple procedure. So, 48 (30=male, 18=female, mean age was 35.2 years) patients were analyzed in this study. 6 patients underwent hepaticojejunostomy along with Frey’s procedure in view of benign biliary stricture. 18 (37.5%) out of 48 patients had previously undergone pancreatic duct stenting multiple times elsewhere.
In the study of 48 patients with a preoperative mean overall pain score of 66.67, in comparison, the postoperative mean overall pain score at 3 months, 1 year, 3 years and at 5 years were found to be 6.27, 6.37, 8.26 and 8.42 respectively, here demonstrating an unequivocal and statistically significant (p<0.05) pain reduction in both short term and long-term.
In the 18 patients who had previous stenting, the pain scores were compared prior to and after surgery using propensity matching.
In the first 3 months following surgery, there was weight gain in most of the patients (65%). though some patients showed mild weight loss subsequently on long-term follow-up, it was not statistically significant (p-value-0.041).
Steatorrhea which was seen in 25 (52%) patients preoperatively was persistent following surgery. Additionally, 5 new patients developed steatorrhea on follow-up
During the disease, there was worsening of DM (preoperatively 16 (32%) pts had DM of them 6 were on insulin and remaining on OHD) and new onset DM was seen in 12 (24%) patients postoperatively. We can thus infer that surgery has no role over glycaemic control (P-value<0.05).
The SF 36 quality of life questionnaire revealed generally increased quality of life in the whole group, owing mostly to pain reduction. Before surgery, the mean Physical and Mental Component Scores (PCS and MCS) were 25.37.67 and 26.289.94, respectively, which improved to 57.7815.56 and 48.3026.82 with a p-value of <0.05 at 3 years.
Grade A pancreatic leak was seen in 4 pts, 2 pts underwent re-exploration for bleeding without mortality. Over a mean follow-up period of 36 months, there was no incidence of malignancy in our study population.
Conclusion: Frey’s procedure is safe and effective in relieving pain in CCP with improved quality of life and seems to be better than PD stenting. However, it has no role in the control of exocrine and endocrine pancreatic insufficiency.
Frey’s procedure; Chronic calcific pancreatitis
Chronic pancreatitis is a progressive inflammatory disorder of the pancreas characterized by intractable pain and irreversible destruction of pancreatic parenchyma resulting in exocrine and endocrine insufficiency [1]. Unrelenting pancreatic pain is the most dominant and disabling symptom that leads to narcotic addiction, dietary restriction, lifestyle modification and repeated hospital admissions. More than exocrine and endocrine insufficiency, pancreatic pain is the primary clinical variable that can impair all aspects of quality of life. As there is no specific treatment to counteract the primary disease process, treatment is mainly directed against symptoms and complications. Hence, the primary target therapeutic options need to target pain management and evaluate the efficacy of the newer methods for the same [2]. Although medical management and endoscopic interventions are primarily offered to the patients, surgery is needed in about 40% to 75% and it is more effective than endoscopic interventions in terms of rapid, effective and sustained pain relief [3].
The main indication for surgery is intractable abdominal pain not relieved with medication, development of jaundice due to peri choledochal inflammation and suspicion of malignancy. The choice of surgical procedure depends upon the presence and extent of ductal dilatation, glandular morphology and the presence of inflammation in the head. An enlarged pancreatic head is considered to be the ‘pacemaker’ of the pain and its resection is deemed necessary to relieve pain [4]. Pancreaticoduodenectomy may provide good pain relief, but there has been an increasing enthusiasm for duodenumpreserving pancreatic head resection along with ductal drainage procedures [5]. Two such procedures have gained importance; they are Beger’s procedure and Frey’s procedure. Although the long-term results of Frey and Beger procedures are equivalent, FP has shorter operation time and lower morbidity as compared with the Beger procedure Frey’s procedure is more popular among pancreatic surgeons as it avoids pancreatic neck resection and requires single anastomosis [6]. This novel technique was first described by Frey and Smith in 1987 which consists of local pancreatic head resection combined with Longitudinal Pancreatico-Jejunostomy (LR-LPJ). The crucial triangle for pain genesis in chronic pancreatitis lies between the distal common bile duct, the duct of wirsung and the superior mesenteric portal vein. This triangle should be the target for local resection of the pancreatic head in Frey’s procedure.
The aim of this study is to evaluate preoperative and postoperative pain control in patients with chronic pancreatitis undergoing Frey’s procedure using a validated pain score, nutritional status, exocrine, endocrine status and quality of life.
This is a prospectively analyzed retrospective study conducted at KIMS-Sunshine hospitals comprising 48 patients (n=48) between 2016 and 2021 with at least 1-year follow-up.
Ethical clearance from the institutional review board was obtained and informed, written consent was secured from all patients involved in the study.
Chronic pancreatitis was defined using the marseilles criteria [7]. The diagnosis was based on clinical symptoms and pancreatic morphological changes detected in imaging studies.
CT pancreatic protocol was performed on all patients. A precontrast study was performed, followed by a biphasic postcontrast study, one at 30 to 40 sec in the pancreatic parenchymal phase and at 70 seconds in the portal venous phase.
The pancreatic parenchyma was observed for the presence of any mass lesion. The pancreatic duct diameter was assessed and intraductal calculi were documented for presence and extent. The pancreatic duct was dilated if its maximum diameter was greater than or equal to 6 mm (Figure 1), CECT abdomen in pancreatic protocol in a patient with chronic pancreatitis. Axial image at the level of body of the pancreas (a) and coronal reformatted image at the level of uncinate process (b) reveal diffuse volume loss in the pancreas with dilatation of the pancreatic duct).
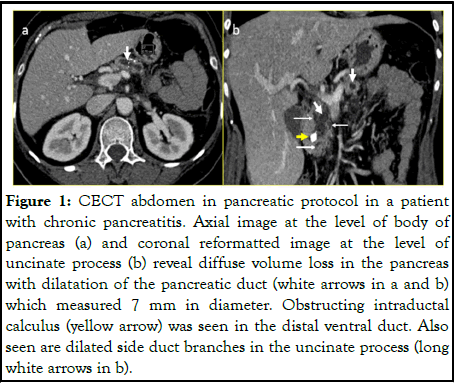
Figure 1: CECT abdomen in pancreatic protocol in a patient with chronic pancreatitis. Axial image at the level of body of pancreas (a) and coronal reformatted image at the level of uncinate process (b) reveal diffuse volume loss in the pancreas with dilatation of the pancreatic duct (white arrows in a and b) which measured 7 mm in diameter. Obstructing intraductal calculus (yellow arrow) was seen in the distal ventral duct. Also seen are dilated side duct branches in the uncinate process (long white arrows in b).
Magnetic Resonance Cholangiopancreatography (MRCP) was additionally performed in patients who were found to have dilated CBD with dilated IHBR. MRCP images in these patients were additionally assessed for the presence of CBD Stricture and to rule out other causes of biliary obstruction such as choledocholithiasis and neoplasm. Upper GI endoscopy and serum CA 19.9 is performed in every case. Assessment of exocrine, endocrine and nutritional function, work status, analgesic intake and pain severity were done. Izbicki pain score was used (Table 1) for the assessment of pain severity [8]. Exocrine (Table 2), endocrine, quality of life and nutritional status were analyzed in terms of steatorrhea, glycaemic control, weight gain and the SF-36 questionnaire [9].
| Frequency of pain | Points |
|---|---|
| Daily | 100 |
| Several times a week | 75 |
| Several times a month | 50 |
| Several times a year | 25 |
| None | 0 |
| Analgesics medication | Points |
| Morphine | 100 |
| Buprenorphine | 80 |
| Pethidine | 20 |
| Tramadol | 15 |
| Paracetamol | 5 |
| Disease related inability to work | Points |
| Permanent | 100 |
| <1 year | 75 |
| <1 months | 50 |
| <1 week | 25 |
| No inability in last year | 0 |
Table 1: Pain score model.
| Frequency points | Frequency points |
|---|---|
| Daily | 100 |
| Several times a week | 75 |
| Several times a month | 50 |
| Several times a year | 25 |
| None | 0 |
Table 2: Scoring system for diarrhea and bloating sensation.
Inclusion criteria
All age groups of patients with diagnosed CCP have the following:
•Intractable pain.
•Main duct disease with intraductal calculi.
•Ductal dilatation >/ 6 mm.
Of the 48 patients, who fit into the above criteria, 18 pts also had CCP with biliary stricture; they underwent Frey’s with hepatico-jejunostomy.
Exclusion criteria
•Suspected malignancy.
•Severe portal hypertension.
Operative technique
In our unit, we perform the Frey’s procedure with bilateral subcostal incision. After thorough exploration and exclusion of unsuspected pancreatic cancer, we start our dissection by doing extensive kocherisation. Failure to do this critical step will make head coring impossible. Lesser sac is then entered to expose the body and the tail of the pancreas. The next step is to ligate and divide the right gastroepiploic vessels to complete the exposure of the pancreas. The pancreatic duct is then accessed by aspiration with a 22 G needle or by palpation. In the majority of the cases, since the pancreatic duct is dilated, simple palpation or a blind vertical incision with electrocautery at the midpoint of the superior and inferior border of the pancreas in the body region will allow entering the pancreatic duct. The duct is exposed and opened throughout its length. The duct is opened to within 1 cm of the tail distally and into the pancreatic head proximally. Extensive coring of the pancreatic head is done with a thin rim of pancreatic tissue over the portal vein (Figure 2), Head coring with lay opened duct). Rouxeny pancreatojejunostomy was performed in a single layer using interrupted non-absorbable sutures.
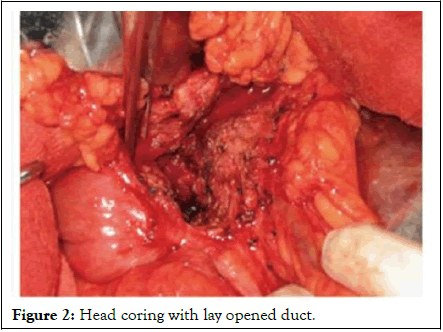
Figure 2: Head coring with lay opened duct.
Postoperatively, the patients are followed up at every 3 months, 1 year, 3 years and 5 years after surgery. In our study population, all the patients had a minimum followup upto 1 year.
Statistical analysis
Continuous data are presented as median (i.q.r.). Student’s t-test was used for the analysis of normally distributed and the Wilcoxon test for non-normally distributed continuous data. The χ2 test or Fisher’s exact test was used, as appropriate, for categorical data. The cumulative probability of remaining completely free from pain after surgery was calculated by the Kaplan-Meier method. An Odds Ratio (OR) with a 95 percent confidence interval was estimated using the final regression model. P ≤ 0.050 was considered statistically significant. All reported P values are two-tailed and were not adjusted for multiple testing. Statistical analysis was performed using SPSS version 15.0 (SPSS Chicago, Illinois, USA).
The total number of patients enrolled was 52. Four patients were identified intra-operatively as having malignancy by frozen section and were therefore converted to the Whipple procedure. So, 48 (30=male, 18=female, mean age was 35.2 years) patients were analyzed in this study. Our cohort stretched from the youngest patient, a tender 6-year-old, to the most seasoned among us, a venerable soul at 75 years of age. Six patients underwent hepatico-jejunostomy along with Frey’s procedure in view of benign biliary stricture. 18 (37.5%) of 48 patients had previously undergone pancreatic duct stenting multiple times elsewhere.
Pain
In this study, there was a significant improvement in the overall pain score (pain scores at preoperative and post-operative) (Table 3 and Figure 3).
| Pre-operative (mean) |
Postoperative (mean) immediate 3 months | Postoperative (mean) immediate 1 year | Postoperative (mean) 36 months | Postoperative (mean) 60 months | p value | |
|---|---|---|---|---|---|---|
| VAS | 85.14 | 5.45 | 5.30 | 5.45 | 5.54 | <0.05 |
| Frequency of pain | 90.57 | 6.29 | 6.20 | 6.32 | 6.32 | <0.05 |
| Analgesia requirement | 20 | 8.10 | 8.00 | 8.5 | 8.5 | <0.05 |
| Inability to work | 90.95 | 10.90 | 10.95 | 12.1 | 13.2 | <0.05 |
| Mean overall pain score | 66.67 | 6.27 | 6.37 | 8.26 | <0.05 |
Table 3: Comparison of preoperative and postoperative VAS pain score, frequency of pain, analgesia required and inability to work.
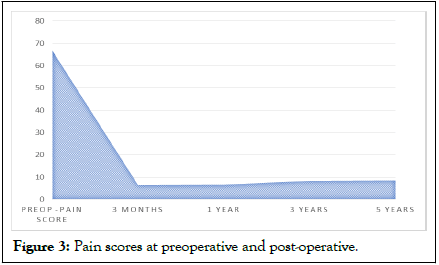
Figure 3: Pain scores at preoperative and post-operative.
Stent vs. surgery
After propensity score matching, in the 18 pts who had pancreatic duct stenting the mean overall pain scoring prior to surgery was 54.24 and the overall pain score was 6.27 postsurgery. We can hence infer that pain relief from stenting is much less when compared to that achieved by Frey’s procedure. However, in view of the small sample size of our study population, more studies with greater numbers are recommended to establish this observation (Table 4).
| Mean overall pain score | Pain score in stent group (6 months) | Pain score in surgery group (6 months) | P<0.05 |
| 54.24 | 6.27 |
Table 4: Scores in the stent group and surgery group.
Weight
Most patients (n=31, 65%) showed weight gain post-surgery (mean=48.6, std. deviation=-7.02) on short-term follow-up. However, some (n=17, 35%) showed mild weight loss on followup though, this number was not statistically significant (p-value 0.041). The short-term weight gain was considered to be due to the release of ductal obstruction but the long-term assessment of weight analysis post-procedure requires further studies (Table 5).
| Weight | Mean | Std. deviation | p-value |
|---|---|---|---|
| Pre-operative | 46.7 | 7.02 | 0.041 |
| Immediate post-op | 48.6 | 7.56 | |
| Postoperative 3 years | 42.3 | 6.220 |
Table 5: Preoperative vs. postoperative weight analysis.
Steatorrhoea
Steatorrhea (passing bulky, oily, foul-smelling stools floating on the pan) was seen in 25 pts preoperatively and persisted following surgery, 5 new patients developed post-surgery. It shows that there was ongoing fibrosis (Table 6).
| Steatorrhoea | |
|---|---|
| Pre-operative | 25 |
| Post-operative after 3 months | 30 (including the 25 preop-onset) |
| Post-operative after 1 year | 36 |
| Post-operative after 3 years | 39 |
Table 6: Steatorrhoea in preop vs. post-operative.
Glycaemic status
During the course of the disease there was worsening of DM (preoperatively 16 (32%) pts had DM, of them 6 were on insulin and remaining on OHD). New onset DM was seen in 12 (24%) patients within the span of 1 year post-surgery. The need for insulin was increased in the preoperative group. We can thus, infer surgery has no role in glycaemic control, however, studies with larger samples are required to establish this (Table 7).
| Pre-operative | Postoperative | Total | P value<0.05 (pre-sx vs. post-insulin requirement) | |||
|---|---|---|---|---|---|---|
| 16 | 12 (New onset) | 28 (24 months follow-up) | ||||
| OHA | Insulin | OHA | Insulin | OHA | Insulin | |
| 10 | 6 | 11 | 1 | 16 | 12 | |
Table 7: Glycemic control in preop vs. postoperative group.
Quality of life
The SF 36 quality of life questionnaire revealed generally increased quality of life in the whole trial group, owing mostly to pain reduction. Before surgery, the mean Physical and Mental Component Scores (PCS and MCS) were 18.3 ± 7.26 and 22.28 ± 9.4 respectively, which improved to 56.7 ± 8.56 and 42.3 ± 16.82 with p value<0.05 (Figure 4).
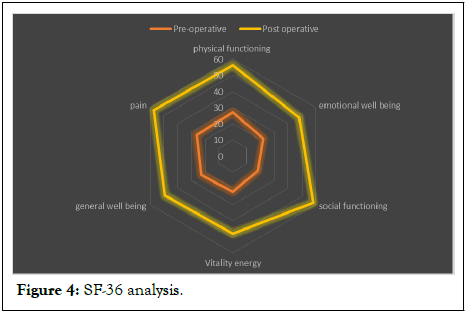
Figure 4: SF-36 analysis.
Post OP complications
Grade A pancreatic leak was seen in 4 pts, 2 pts underwent reexplanation for bleeding with zero mortality. Our data infers that safe procedure to undergo surgery.
Although various explanations for pain genesis in chronic pancreatitis have been postulated, the two most popular hypotheses are ductal hypertension and perineural inflammation [10]. Based on these, ductal drainage and pancreatic head resection have emerged as the two main options for the surgical management of chronic pancreatitis. In keeping with recent reports on pain relief after surgery for chronic pancreatitis, this study has shown that Frey’s procedure led to significant and sustained pain relief over a median follow-up of 3 years [11,12].
As concluded in similar previous studies conducted by Gestic, et al., Fanconi, et al. and Masaki Tanaka, et al. our study also concluded that the short-term and long-term outcome of overall pain score following Frey’s procedure was better [13,14].
Dhruba Narayan Sah, et al. have concluded that Frey’s procedure can be a safe option for patients with chronic pancreatitis with a sample of 26 patients. In our study we included a larger population of 48 patients and allowed the same conclusion [15].
A multicentre randomized trial from Holland compared 19 endotherapy cases with 20 surgical drainage cases. Four patients in the endotherapy group were converted to surgery. The pain relief was 32% following endotherapy and 75% following surgery. The Izbcick scores and SF 36 quality of life scores were also superior in the surgery group [16]. Our study too concluded the same.
In a meta-analysis, done by Yanming Zhou, et al. which included 23 studies, Frey’s procedure resulted in weight gain in 60.5% of patients and also noted that insulin requirement decreased or no insulin required after surgery in some patients, the exocrine function of their patients slightly improved one year after Frey procedure, but in over study, there was no improvement in exocrine/endocrine function [17].
Frey, Falconi, Roch, Rajendra and their colleagues noted that the insulin requirement was decreased or no insulin was required after surgery in some patients with pre-existing insulindependent diabetes mellitus, probably because they improved their dietary habits and quit alcohol consumption. However, in our study, we noticed deterioration of endocrine function.
Studies conducted by L Soundarya Rajan, et al. and Satyajithrath, et al. concluded that quality of life increased in every domain following Frey’s procedure, this was proven with statistical significance in our study also [18,19].
A Brazilian review article by Gestic, et al. concluded that the morbidity rate associated with Frey’s procedure was 28.7% (most common being infections: Pneumonia, SSI, UTI) with no mortality. In our study, the rate is 12.5%, with no mortality.
Frey’s procedure is safe and effective in relieving pain in CCP with improved quality of life and seems to be better than PD stenting. However, it has no role in the control of exocrine and endocrine pancreatic insufficiency.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Eppa VR, Musham R, Senapathy G, Ganta V (2025) Long-Term Outcome Following Frey’s Procedure-A Single-Centre Experience. Pancreat Disord Ther. 15:341.
Received: 17-Dec-2023, Manuscript No. PDT-23-28524; Editor assigned: 20-Dec-2023, Pre QC No. PDT-23-28524 (PQ); Reviewed: 04-Jan-2024, QC No. PDT-23-28524; Revised: 13-Feb-2025, Manuscript No. PDT-23-28524 (R); Published: 20-Feb-2025 , DOI: 10.35248/2165-7092.25.15.341
Copyright: © 2025 Eppa VR, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.