Journal of Clinical & Experimental Dermatology Research
Open Access
ISSN: 2155-9554
ISSN: 2155-9554
Research Article - (2019)Volume 10, Issue 4
Introduction: Actinic keratosis (AKs) are part of the cancerization field, a region adjacent to AKs containing subclinical and histologically abnormal epidermal tissue due to Ultraviolet (UV)-induced DNA damage. The photoproducts, “as consequence of DNA damage induced by UV”, are mainly cyclobutane pyrimidine dimers (CPDs). Fernblock® demonstrated in previous studies significant reduction of the number of CPDs induced by UV radiation. Photolyases are a specific group of enzymes that remove the major UV-induced DNA lesions by a mechanism called photo-reactivation.
Methods: A monocentric, prospective, controlled, and double blind interventional study was performed to evaluate the effect of a new medical device (NMD) containing a DNA-repair enzyme complex (photolyases, endonucleases and glycosilases), a combination of UV-filters, and Fernblock® in the treatment of the cancerization field in 30 AK patients after photodynamic therapy (PDT). Patients were randomized into two groups: patients receiving a standard sunscreen (SS) and patients receiving the NMD. Clinical, dermoscopic, reflectance confocal microscopy (RCM) and histological evaluations were performed.
Results: An increase of AKs was noted in all groups after three months of PDT without significant differences between them (p=0.476). A significant increase in the number of AKs was observed in SS group after six (p=0.026) and twelve months of PDT (p=0.038); however, this increase did not reach statistical significance in the NMD group. Regarding RCM evaluation, honeycomb pattern assessment after twelve months of PDT showed significant differences in the extension and grade of the atypia in the NMD group compared to SS group (p=0.030 and p=0.026, respectively). Concerning histopathological evaluation, keratinocyte atypia grade improved from baseline to six months after PDT in all the groups, with no statistically significant differences between the groups. Twelve months after PDT, p53 expression was significantly lower in the NMD group compared to SS group (p=0.028). The product was well-tolerated, with no serious adverse events reported.
Conclusion: Our results provide evidence of the utility of this NMD in the improvement of the cancerization field and in the prevention of the development of new AKs.
Actinic keratosis; Cancerization field; Repair enzyme; Photolyases; Treatment; Prevention; Reflectance confocal microscopy
Actinic keratosis (AK) is a common skin tumor caused by chronic exposure to ultraviolet (UV) radiation. Recent studies have demonstrated that AKs are part of the cancerization field, a region adjacent to AKs containing subclinical and histologically abnormal epidermal tissue [1,2]. It has been reported that AK progression to squamous cell carcinoma (SCC) may range from 0.025% to 16% [3]. There is increasing evidence that all types of AK lesions may progress to SCC, regardless of keratinocyte atypia thickness [4]. Thus, early recognition and treatment of the cancerization field is highly recommended in order to prevent the appearance of SCC.
Cancerization field pathogenesis include UV-induced DNA damage by the formation of a high number of cyclobutane pyrimidine dimers (CPDs) and pyrimidine (6-4) photoproducts (6-4 PPs), which are the main cause of non-melanoma skin cancer [5]. Fernblock® has demonstrated in several studies efficacy in the prevention and repair of DNA damage by reducing the number of CPDs induced by UV radiation [6-9]. Photolyases are specific photorepair enzymes that are present in algae and some non-placental mamals [10]. Their function is to recognize and remove the UV-induced DNA lesions by a mechanism called photo-reactivation [11]. A growing number of clinical and experimental studies have recently demonstrated the efficacy of photolyase-based novel approaches on cancerization field; however, most of these reports include a limited number of patients, a short follow-up period, and are not double-blind studies [12-26]. Additionally, other repair enzymes such as glycosylases and endonucleases have been described and they do not need light to be activated [27,28].
A new medical device (NMD) containing a complete DNArepair enzyme complex including photolyases, glycosyla26ses and endonucleases, a combination of filters with full spectrum sun protection, and an extract of Polypodium leucotomos leaves (Fernblock®) has been recently developed (Heliocare 360°AK). To our knowledge, this is the first MD containing DNA-repair enzymes and Fernblock® that has well-known antioxidant, photoprotective, and immune-modulatory activities [29-31].The aim of this study was to demonstrate by clinical, dermoscopic, confocal microscopy and histological evaluation the improvement in the field of cancerization, and the prevention of the appearance of new AKs, with this NMD compared to a standard sunscreen (SS) in patients with AK after photodynamic therapy (PDT) treatment.
Design and population
This is a monocentric, prospective, controlled, and double-blind interventional study to evaluate the effect of a NMD containing a DNA-repair enzyme complex, a combination of UV-filters, and Fernblock® (Heliocare 360AK) in the treatment of the cancerization field in 30 AK patients. Duration of the study was 12 months, with a total of six visits. An area of 25 cm2 on the scalp or face was selected for evaluation in all patients. One PDT session was performed at the baseline visit (TB) on the target area, and cryotherapy was performed during follow-up visits if residual and/or new AK lesions were observed. Topical application of the sunscreen started one week after PDT. Subjects were instructed to self-apply the product three times a day on the pre-specified area.
A total of 30 patients with AK lesions on the scalp or face were enrolled and they were randomized into two groups: 15 patients receiving a standard sunscreen (SS) and 15 patients receiving the NMD. SS was exactly the same sunscreen as the NMD (SPF 100) but without the DNA-repair enzyme complex and Fernblock®. In addition, patients were also randomized into two other groups: patients receiving treatment for a total of 6 months and a subsequent follow-up visit a 12 months (10 SS and 10 NMD), and patients receiving treatment for a total of 12 months (5 SS and 5 NMD).
Written informed consent was obtained from all patients after having read and understood the information approved by the ethics committee. The study was approved by the institutional research board and was conducted according to the Declaration of Helsinki Principles. Eligibility criteria included AK patients older than 18 years of age, the presence of at least four AKs in an area of 25 cm2 on the scalp or face, and absence of previous treatments in the target area during the previous three month period. Patients were excluded from the study for pregnancy, other skin diseases requiring systemic treatment, neoplastic lesions (melanoma or non-melanoma skin cancer) in the specified area, or sensitization to one or more components of the product.
Methods and evaluation
Clinical, dermoscopy and RCM evaluations were performed at TB before PDT and during all follow-up visits (one week post- PDT-T0, one month post-PDT-T1, three months post-PDT-T3, six months post-PDT-T6, one year post-PDT-T12). Additionally, Investigator Global Assessment (IGA), Patient Global Assessment (PGA) and Actinic Keratosis Quality of Life (AKQoL) were also evaluated.A biopsy was taken for histopathological assessment at TB and at the end of the treatment period (T6 and T12).
Clinical and dermoscopic evaluation
An area of 25 cm2 on the scalp or face was selected for evaluation at basal visit, and subsequently revised in each followup using a plastic wrap. At baseline this area was subdivided in four quadrants and AKs of each of the quadrants were annotated. Number, size (mm) and grade (I/II/III) of the AK lesions were collected at all visits. Clinical and dermoscopic pictures were taken at each follow-up visit.
Reflectance confocal microscopy evaluation
Reflectance confocal microscopy (RCM) images were obtained using the VivaScope 1500® device (MAVIG Gmbh, Munich, Germany). All RCM images were obtained and evaluated by the same investigator. A specific spot in the pre-specified area was marked in the plastic wrap in order to perform RCM assessments at the same site in all visits. RCM uses a low-power 830-nm laser beam that generates horizontal sections of the skin of 1.0 μm lateral resolution up to approximately 200 μm in depth. Instruments and acquisition procedures have been previously described [32]. At each spot, 50 images of 500 × 500 μm were acquired starting above the stratum corneum until papillar dermis (termed “vivastack”). Moreover, a minimum of 4 mosaics (termed as “vivablock”), with a maximum area of 8 × 8 mm, were obtained per lesion, one in the stratum corneum level, one in the epidermis (stratum granulosum/spinosum), one at the dermoepidermal junction (DEJ), and one in papillary dermis. Videos at dermal level were acquired to evaluate enlarged vessels. The same investigator without regard to patient ’ s data retrospectively evaluated all recorded confocal images. All RCM evaluated parameters are specified in Table 1.
| Location | Features | Scoring |
|---|---|---|
| Epidermis | Parakeratosis | 0= absent; 1 ≤ 10%; 2=10-25%; 3 ≥ 25% |
| Atipical honeycomb pattern(extension) | 0= absent; 1 ≤ 10%; 2=10-25%; 3 ≥ 25% | |
| Atipical honeycomb pattern (grade) | 0=absent; 1=slightly atypical; 2=moderate atypia; 3=severe atypia | |
| Mottled pigmentation | 0= absent; 1 ≤ 10%; 2=10-25%; 3 ≥ 25% | |
| Inflammatory Infiltrate | 0= absent; 1 ≤ 10%; 2=10-25%; 3 ≥ 25% | |
| DEJ | Polycyclic papillary contours | 0= absent; 1 ≤ 10%; 2=10-25%; 3 ≥ 25% |
| Dermis | Inflammatory Infiltrate | 0= absent; 1 ≤ 10%; 2=10-25%; 3 ≥ 25% |
| Round vessels | 0= absent; 1 ≤ 10%; 2=10-25%; 3 ≥ 25% | |
| Solar elastosis | 0=absent; 1=slight solar elastosis; 2=moderate-severe solar elastosis |
Table 1: Parameters for RCM evaluation.
Histopathology evaluation
Two 3 mm-punch biopsies were performed in all patients at TB and T6 visits in two sites of cancerization field (without visible AK lesions). Additionally, in the subgroup of patients with treatment until T12 (5 SS and 5 NMD) a third biopsy was done in another pre-specified site of the precancerous field. All specimens were processed using the standard histopathologic method of paraffin embedding sectioning and hematoxylineosin (HE) staining. Evaluated histopathological characteristics are specified in Table 2. Additionally, histochemical and immunohistochemical (IHQ) studies for p53, Ki67 and orcein were conducted on all biopsies. Criteria used for evaluation of immunohistochemistry evaluation are specified in Table 3.
| Location | Features | Scoring |
|---|---|---|
| Epidermis | Epidermis thickness | Evaluated as a qualitative variable (mm) |
| KIN | 0= absent; 1=I (atypical basal and suprabasal cells); 2=II (two-thirds thickness); 3=III (all layers) | |
| Dermis | Dermis thickness | Evaluated as a qualitative variable (mm) |
| Inflammatory Infiltrate | 0=absent; 1=focal; 2=diffuse | |
| Solar elastosis | 0=absent; 1=slight solar elastosis; 2=moderate-severe solar elastosis |
Table 2: Evaluated histopathological characteristics.
| IHQ | Scoring |
|---|---|
| Orcein | Evaluated in dermis as a qualitative variable (0=absent; 1=focal; 2=diffuse) |
| Ki67 | Evaluated in epidermis as a quantitative variable |
| p53 | Evaluated in epidermis as a quantitative variable |
Table 3: Evaluated immunohistochemical studies.
Statistical analysis
Quantitative variables were summarized by their mean and standard deviation (SD), and categorical variables by relative and absolute frequencies. The relationship between the categorical variables was evaluated using χ2 test, and for quantitative variables Kruskal –Wallis test was used. P values <0.05 were considered statistically significant. All computations were performed using the SPSSv24 statistical package (Chicago, IL).
Demographic features of the patients
A total of 30 patients were included in the study, and all but one concluded the complete study period. The baseline characteristics of the patients in both treatment groups did not differ significantly (Table 4). Twenty-two (73.4%) were males and 8 (26.6%) females. The age ranged from 59 to 91, with a mean age of 75 years (± 7.3). With regard to skin phototype, 70% (21/30) were phototype II, 26.7% (8/30) phototype III, and one patient (3.3%) phototype IV.
| Variable | Total (n=30) N̊ (%) | |
|---|---|---|
| Epidemiological features | ||
| Age [Mean (± SD)] | 75.3 (± 6.7) | |
| Sex [N(%)] | ||
| Male | 22 (73.4) | |
| Female | 8 (26.6) | |
| Family history of Cancer [(N%)] | ||
| Yes | 5 (16.7) | |
| No | 25 (83.3) | |
| Personal history of Cancer [(N%)] | ||
| Yes | 3 (10.0) | |
| No | 27 (90.0) | |
| Clinical features | ||
| Cutneous photype [(N%)] | ||
| 1 | 0(0) | |
| 2 | 21 (70) | |
| 3 | 8 (26.7) | |
| 4 | 1 (3.3) | |
| Actinic Keratosis Number [Mean (± SD)] | 6.5 (± 1.7) | |
| Actinic keratosis grade [Mean (± SD)] | 1.6 (± 0.6) | |
| Mean Actinic keratosis diameter [Mean (± SD)] | 7.2 (± 3.4) | |
Table 4: Baseline characterstics of the patients.
Clinical evaluation
At baseline evaluation (TB), the total number of AKs ranged from 4 to 13, without significant differences between groups (Figures 1 and 2). One week after PDT (T0) clinical clearance of 95% of all AKs was noted. From a dermoscopic point of view, an improvement in scaling, erythema, pigmentation and the presence of follicular plugs was observed in all the patients. A slight increase in AKs was noted in both groups at T3 without significant differences between them (p=0.476). Regarding T6, a significant increase in the number of AKs compared to T0 was observed in the SS group (p=0.026), but there was no statistically significant increase in the NMD group group. In addition, at T6 the number of AKs was lower compared to T3 in the NMD group but was higher in the SS group (Figures 1 and 2). A significant increase in AK number was identified in SS group at T12 compared to T0 (p=0.038). Conversely, in the NMD group the increase was not-significant (p=0.083). None of the evaluated patients presented any malignant neoplasm during the follow-up of the study. The NMD was well-tolerated, with just ocular itching reported in 3.3% (1/30) of the patients. No serious adverse events were reported.
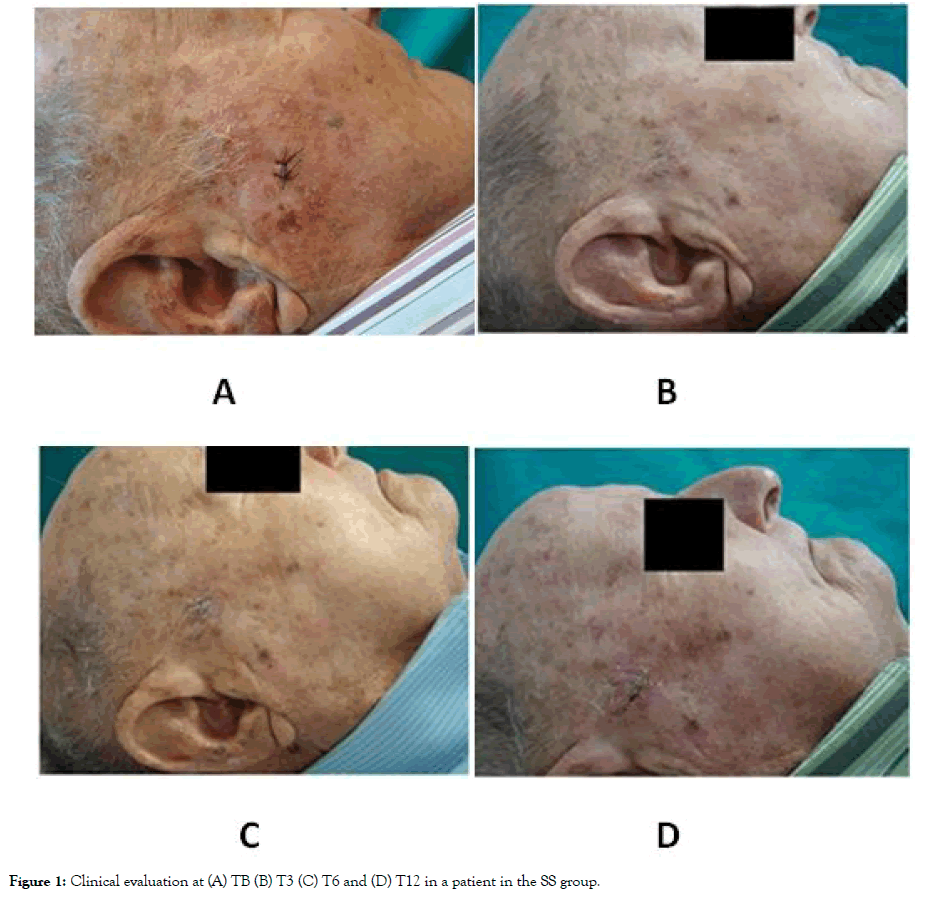
Figure 1: Clinical evaluation at (A) TB (B) T3 (C) T6 and (D) T12 in a patient in the SS group.
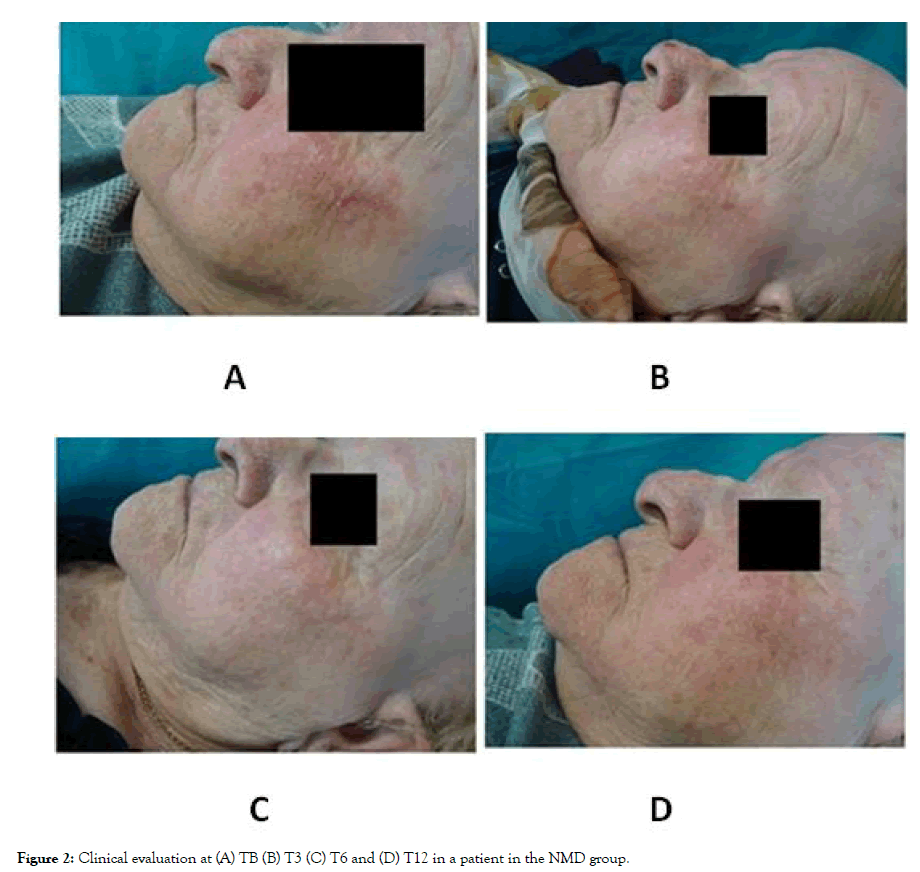
Figure 2: Clinical evaluation at (A) TB (B) T3 (C) T6 and (D) T12 in a patient in the NMD group.
Regarding the subjective clinical evaluation, PGA score (intense improvement) was higher in the NMD group as compared to the SS one at T6 (80% in NMD group vs 64% in SS) and T12 (60% vs 33%). Concerning IGA, there were no significant differences at T3 and T6, but a higher score in the NMD group was observed at T12 (100% vs 83%). No differences were identified regarding AKQoL.
Confocal microscopy evaluation
At baseline (TB), no statistically significant differences were identified in any of the evaluated parameters between study groups (Table 5). Atypia in the honeycomb pattern was mild in 40% (12/30), moderate in 53.3% (16/30), and severe in 6.7% (2/30) of patients (Figure 3). An improvement in atypical honeycomb pattern was observed one month after PDT (T1) in both groups (p=0.003), with absence of atypia in 20% (6/30), mild atypia in 66.6% (20/30), moderate atypia in 10% (3/30), and severe atypia in 3.3% (1/30) of patients. Although no statistically significant differences between the groups were detected at T1, we observed a trend (p=0.076) with a predominance of mild or absent atypia in the NMD group compared to the SS group, in which more cases of moderate atypia and one case of severe atypia were identified. No statistically significant differences in the honeycomb pattern were identified at T3 and T6 visits. Conversely, honeycomb pattern assessment at T12 showed significant differences in the extension and grade of the atypia in the NMD group compared to the SS group (p=0.030 and p=0.026, respectively) (Figure 4). Honeycomb pattern score was also significantly higher in the SS group compared to the NMD one at T12 (p=0.01). Furthermore, a significant reduction in the honeycomb pattern score at T12 compared to T0 was seen in the NMD group (p=0.034), but not in the SS group (p=0.276).
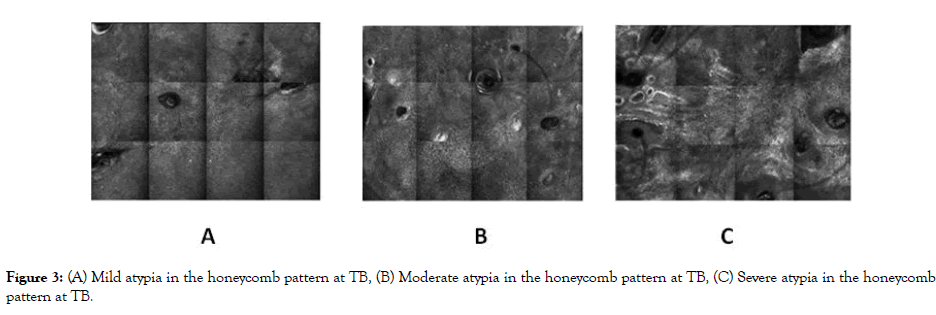
Figure 3: (A) Mild atypia in the honeycomb pattern at TB, (B) Moderate atypia in the honeycomb pattern at TB, (C) Severe atypia in the honeycomb pattern at TB.
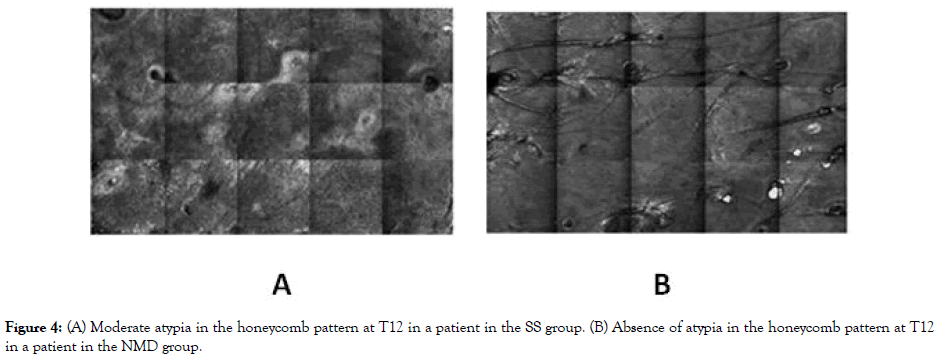
Figure 4: (A) Moderate atypia in the honeycomb pattern at T12 in a patient in the SS group. (B) Absence of atypia in the honeycomb pattern at T12 in a patient in the NMD group.
| Features | N (%) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TB | p value | T1 | p value | T3 | p value | T6 | p value | T12 | p value | ||||||
| SS | NMD | SS | NMD | SS | NMD | SS | NMD | SS | NMD | ||||||
| Epidermis | |||||||||||||||
| Parakeratosis | |||||||||||||||
| None | 5 (55.6) | 4 (44.4) | 0.174 | 7 (58.3) | 5 (41.7) | 0.355 | 3 (60) | 2 (40) | 0.674 | 4 (66.7) | 2 (33.3) | 0.403 | 1 (20) | 2 (40) | 0.49 |
| <10% | 4 (44.4) | 5 (55.6) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (100) | 0 (0) | 0 (0) | |||||
| 10-25% | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |||||
| >25% | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |||||
| Atipical HP (extension) | |||||||||||||||
| None | 0 (0) | 0 (0) | 0.097 | 4 (66.7) | 2 (33,3) | 0.512 | 4 (50) | 4 (50) | 0.589 | 3 (50) | 3 (50) | 0.991 | 0 (0) | 4 (80) | 0.03 |
| <10% | 5 (33,3) | 10 (66,7) | 8 (42.1) | 11 (57.9) | 6 (42.9) | 8 (57.1) | 9 (47.4) | 10 (52.6) | 3 (60) | 1 (20) | |||||
| 10-25% | 9 (64.3) | 5 (35.7) | 3 (60 | 2 (20) | 4 (66.7) | 2 (33.3) | 2 (50) | 2 (50) | 2 (40) | 0 (0) | |||||
| >25% | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |||||
| Atipical HP (grade) | |||||||||||||||
| None | 0 (0) | 0 (0) | 0.311 | 4 (66.7) | 2 (33,3) | 0.091 | 4 (50) | 4 (50) | 0.809 | 3 (50) | 3 (50) | 0.472 | 0 (0) | 4 (80) | 0,026 |
| Mild atipia | 4 (33.3) | 8 (66.7) | 7 (35.0) | 13 (65.0) | 8 (50) | 8 (50) | 9 (56.3) | 7 (43.8) | 2 (40) | 1 (20) | |||||
| Moderate atipia | 10 (62.5) | 6 (37.5) | 3 (100) | 0 (0) | 2 (50) | 2 (50) | 2 (28.6) | 5 (71.4) | 3 (60) | 0 (0) | |||||
| Severe atipia | 1 (50) | 1 (50) | 1 (100) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |||||
| Mottled pigmentation | |||||||||||||||
| None | 0 (0) | 2 (100) | 0.339 | 1 (25) | 3 (75) | 0.041 | 0 (0) | 2 (100) | 0.014 | 0 (0) | 1 (100) | 0.034 | 0 (0) | 0 (0) | 0,117 |
| <10% | 6 (46.2) | 7 (53.8) | 7 (38.9) | 11 (61.1) | 4 (25) | 9 (75) | 2 (18.2) | 9 (81.8) | 2 (40) | 5 (100) | |||||
| 10-25% | 8 (57.1) | 6 (42.9) | 7 (87.5) | 1 (12.5) | 11 (78.6) | 3 (21.4) | 11 (73,3) | 4 (26,7) | 2 (40) | 0 (0) | |||||
| >25% | 1 (100) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (50) | 1 (50) | 1 (20) | 0 (0) | |||||
| Inflammatory infiltrate | |||||||||||||||
| None | 9 (50) | 9 (50) | 0.135 | 9 (47.4) | 10 (52.6) | 0.917 | 14 (56) | 11 (44) | 0.228 | 12 (50) | 12 (50) | 0.535 | 5 (100) | 5 (100) | NS |
| <10% | 3 (33.3) | 6 (67.6) | 2 (50) | 2 (50) | 0 (0) | 2 (100) | 2 (40) | 3 (60) | 0 (0) | 0 (0) | |||||
| 10-25% | 3 (100) | 0 (0) | 4 (57.1) | 3 (42.9) | 0 (0) | 1 (100) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |||||
| >25% | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |||||
| DE junction | |||||||||||||||
| Polycyclic papillary contours | |||||||||||||||
| None | 7 (43.8) | 9 (56.3) | 0.747 | 8 (42.1) | 11 (57.9) | 0.215 | 6 (40) | 9 (60) | 0.187 | 7 (46.7) | 8 (53.3) | 0.759 | 1 (20) | 5 (100) | 0,024 |
| <10% | 7 (58.3) | 5 (41.7) | 7 70.0) | 3 (30.0) | 7 (70) | 3 (30) | 3 (50) | 3 (50) | 4 (80) | 0 (0) | |||||
| 10-25% | 1 (50) | 1 (50) | 0 (0) | 1 (100) | 1 (100) | 0 (0) | 4 (57.1) | 3 (42.9) | 0 (0) | 0 (0) | |||||
| >25% | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 2 (100) | 0 (0) | 0 (100) | 0 (0) | 0 (0) | |||||
| Dermis | |||||||||||||||
| Inflammatory Infiltrate | |||||||||||||||
| None | 6 (42.9) | 8 (57.1) | 0.519 | 7 (53.8) | 6 (46.2) | 0.706 | 7 (43.8) | 9 (56.3) | 0.511 | 12 (50) | 12 (50) | 0.291 | 2 (40) | 5 (100) | 0,083 |
| <10% | 8 (61.5) | 5 (38.5) | 6 (42.9) | 8 (57.1) | 6 (54.5) | 5 (45.5) | 1 (33.3) | 2 (66.7) | 3 (60) | 0 (0) | |||||
| 10-25% | 1 (33.3) | 2 (67.7) | 2 (66.7) | 1 (33.3) | 1 (100) | 0 (0) | 1 (50) | 1 (50) | 0 (0) | 0 (0) | |||||
| >25% | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |||||
| Round vessels | |||||||||||||||
| None | 12 (52.2) | 10 (47.8) | 0.385 | 12 (52.2) | 11 (47.8) | 0.224 | 12 (50) | 12 (50) | 0.512 | 12 (80) | 12 (80) | 0.861 | 5 (100) | 4 (80) | 0,292 |
| <10% | 2 (50) | 2 (50) | 0 (0) | 3 (100) | 1 (33.3) | 2 (66.7) | 1 (6,7) | 2 (13,3) | 0 (0) | 1 (20) | |||||
| 10-25% | 0 (0) | 2 (100) | 2(66.7) | 1 (33.3) | 1 (100) | 0 (0) | 1 (6,7) | 1 (6,7) | 0 (0) | 0 (0) | |||||
| >25% | 1 (6,7) | 0 (0) | 1 (100) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |||||
Table 5: Confocal microscopy evaluation.
In addition, at T12 we evaluated the atypical honeycomb pattern in SS and NMD patients who received treatment for 12 months as compared to those who received it for 6 months only. Both extension and grade of atypia were significantly higher in those in the NMD group that received just 6 months of treatment compared to 12 months treatment (p=0.004 and p=0.004, respectively), but these differences were not observed in the SS group (p=0.651 and p=0.592, respectively).
With respect to other parameters, we observed an increase in the epidermal and dermal inflammatory infiltrate at T0 due to the PDT treatment which declined progressively at T3 and T6 to even lower levels at TB. Moreover, at T12 there was a trend regarding dermal inflammatory infiltrate that was absent in the NMD group compared to the SS group (p=0.083). Additionally, a higher prevalence of polycyclic papillary contours was detected at T12 in the SS group compared to the NMD group (p=0.024). No statistically significant differences were identified in the assessment of the stratum corneum and the presence of round blood vessels.
Histopathology evaluation
At baseline, hyperkeratosis with parakeratosis, pleomorphism of keratinocyte nuclei, mild to moderate inflammatory infiltrate and solar elastosis were observed in all patients. Of all the cohort, 77.5% (31/40) showed KIN I, 22.5% (9/40) KIN II, and none showed KIN III. KIN grade did not show any statistically significant differences between the groups at TB (p=0.413) or T6 (p=0.831).Although not significant, keratinocyte atypia grade improved from the TB visit to T6 in both groups (Figure 5). Regarding T12, no significant differences between the KIN grade of SS and the NMD group (p=0.738) were noted. Epidermal and dermal thickness evaluation did not showed any statistically significant differences.
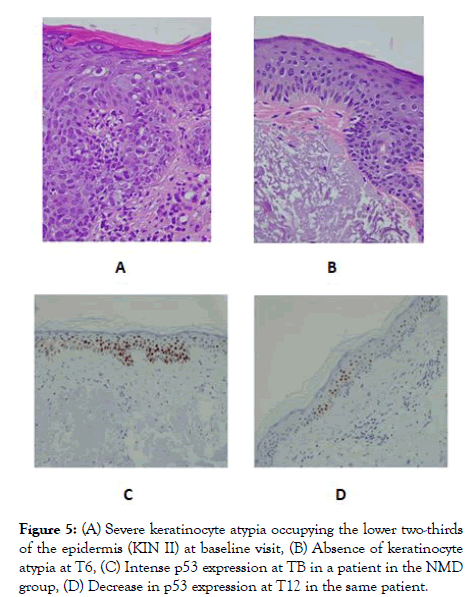
Figure 5: (A) Severe keratinocyte atypia occupying the lower two-thirds of the epidermis (KIN II) at baseline visit, (B) Absence of keratinocyte atypia at T6, (C) Intense p53 expression at TB in a patient in the NMD group, (D) Decrease in p53 expression at T12 in the same patient.
Concerning histochemistry and immunohistochemistry, no statistically significant differences were identified in orcein and Ki67 proliferation index at TB, T6 and T12, however an improvement was noted in Ki67 proliferation index in both groups. Although there was a tendency towards decreased expression of P53 with respect to TB, no significant differences were observed at T6 (p =0.611). However, p53 expression was significantly lower in the NMD group as compared to SS (p=0.028) at T12 (Figure 5).
AK lesions are premalignant skin conditions that represent one of the most frequent dermatological consultations. Several fielddirected treatments are available with high clearance rates [33], although relapse in patients with chronic exposure to UV radiation are very frequent.
In recent years, several studies have demonstrated the utility of photolyase-based sunscreens in the treatment of the cancerization field [12-26]. Consistent with these studies, we have demonstrated the efficacy of a NMD with a complete DNA-repair enzyme complex and Fernblock® in the prevention of the appearance of new AK lesions. Results from our cohort demonstrate a lower number of AK lesions in patients treated in the NMD group as compared to SS after six and twelve months. In this regard, Eibenshutz et al. also performed a randomized and parallel-group study with a photolyase-based device and demonstrated a significant reduction of new AK lesions, however their trial was not double-blind [34]. In addition, Moscarella et al. recently conducted a randomized, double-blind and parallel-group study, and describeda significantly lower percentage of patients who presented new lesions, but just taking into account in the analysis the subgroup of patients with <10 AK lesions [26]. Of note, during the twelve-month treatment period, none of the patients in our cohort developed any skin neoplasms in the study area.
In addition to clinical and dermoscopic evaluation, we performed RCM assessment as a novel noninvasive technique that provides in vivo evaluation of the epidermis and superficial dermis at a cellular resolution in real time. RCM enables repeated imaging of the same lesion over time, which can be used for monitoring efficacy of non-invasive treatments. It has demonstrated to be helpful not only in identifying and grading AK lesions, but also in monitoring AK treatment outcomes [35-38].In this regard, several studies have been performed with RCM to evaluate the efficacy of field-directed treatments such as 5-fluorouracil [39], ingenol mebutate [40], PDT [41], diclofenac [42], imiquimod [43], and surgery [44]. However, just two studies have used RCM to evaluate improvement in cancerization field by other MD with DNA-repair enzymes [19,26]. Puig et al. [18] demonstrated a decreased in stratum corneum alterations, and an improvement in atypical honeycomb pattern four weeks after topical application of a photolyase-based sunscreen, however they studied a small number of patients (n=13), and they did not compare with a SS group.
Consistent with our study, Moscarella et al. described similar results regarding RCM parameters in both groups at one, three and six months of follow-up [26]. Similarly, in our cohort we did not identify significant differences at T1, T3, and T6, however honeycomb pattern assessment at T12 showed significant differences in the extension and grade of atypia, as well as in honeycomb pattern score in the NMD group with respect to the SS group. Thus, our results provide evidence for the benefit to honeycomb pattern after long-term use of our NMD. Strikingly, we also identified by RCM a decrease in the inflammatory infiltrate after treatment, with absence of dermal inflammatory infiltrate in the NMD group, and persistence in the SS group. To our knowledge, none of these previous studies have detected an improvement in this parameter that also represents an improvement in the cancerization field.
Concerning histopathological evaluation, we demonstrated an improvement in keratinocyte atypia grade after treatment, although no differences were detected between groups. In addition, our results provide further support for the utility of DNA-repair enzymes in p53 expression improvement. There is strong evidence for the role of p53 in UV-induced skin carcinogenesis. P53 is a tumor suppressor gene that regulates DNA replication in response to DNA damage from UV exposure. Several reports have demonstrated that there is a significant increase in p53 expression levels in AKs and SCCs [45]. Furthermore, reduction in p53 expression levels has been reported after photolyase-based approaches [46]. In our study, a tendency to reduced p53 expression was noted at T6 and T12 as compared to TB visit.In addition, p53 expression was significantly lower in the NMD group as compared to SS at T12. In this regard, Puig et al. [18] did not find any significant changes in p53 expression after one-month treatment, but they suggested the necessity of long-term follow-up studies. As previously reported, a high level of expression of Ki67 was identified in our cohort at baseline, however, our study failed to demonstrate significant improvement in this proliferation index after treatment with the NMD.
In conclusion, our results show the utility of the NMD in the improvement of the cancerization field and in the prevention of the development of new AKs. Early treatment of the cancerization field with the present NMD (Heliocare 360° AK), could be useful to prevent the development of AK lesions and SCC. Long-term studies with a larger number of patients are needed to confirm the efficacy of these findings.
Citation: De Unamuno BB, Aguilera NC, García IA, Andrino AC, Ros ML, Rodrigo R, et al. (2019) Long-Term Efficacy of a New Medical Device Containing Fernblock® and DNA Repair Enzyme Complex in the Treatment and Prevention of Cancerization Field in Patients with Actinic Keratosis. J Clin Exp Dermatol Res. 10:499. doi: 10.35248/2155-9554.19.10.499
Received: 17-Jun-2019 Accepted: 24-Jun-2019 Published: 30-Jun-2019 , DOI: 10.35248/2155-9554.19.10.499
Copyright: © 2019 De Unamuno BB, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.