Fisheries and Aquaculture Journal
Open Access
ISSN: 2150-3508
ISSN: 2150-3508
Research Article - (2016) Volume 7, Issue 2
From February 2012 to February 2013, 406 fish were caught in the Melen fish station, then fixed in 10% formalin and taken to the laboratory to be examined. Mounting of monogeneans carried out using binocular magnifying glass; the determination of the various species was further done using the optical microscope. The colonization of the four pairs of arches by C. thurstonae occurred in the anterio-posterior direction. The other three species showed non-specific model of occupation of the transversal gradient. These different patterns have undergone permanent modifications. The results obtained in this study could be explained based on the heterogeneity of the gill system, the ventilation of current flow, the model of gills colonization by the oncomiracidiums of the Monopisthocotylea. The low diversity values obtained within seasons indicate that this period is harmful for the parasites studied. The species C. halli has exploited the resource space better in all. The populating of arches II and III were the best organized.
Keywords: Distribution, Monopisthocotylea, Fish, Arches, Months
Modern ecology has highly emphasized on the importance of parasites as study models of structure and organization of communities [1]. In most of these works, species diversity was often used to measure the composition of species in a well defined ecosystem or in one of its components. However, the study of diversity could be more revealing if carried out in a spatio-temporal context as the relative abundance of species changes within the same community [2-4]. Moreover, some research works showed not only the heterogeneity of fish gill biotope but also its high complexity with time [5-7]. These authors suggested that, spatio-temporal variability should be taken into consideration for a better understanding of population dynamics and the structure of fish parasites communities. For Hawkins, understanding the determinants of spatial variation in species diversity remains a fundamental problem in modern ecology [8].
The study of niche amplitude and overlap permit not only to know the modalities of community organization, but to also understand the extent of exploitation of the same resource [9]. Such works were carried out in in Finland, Japan and Cameroon on monogeneans gill parasites of different fish species [6,10,11]. It appears therefore that, the consideration of the diversity indexes, the amplitude and the niche overlap might be more promising and complementary for the analysis of population dynamics, enhancing knowledge of the biology of fish gill monogenean parasites guild. The present investigation carried out basically with ecological indexes was aimed to study the monthly colonization of the various gill arches of Oreochromis niloticus (Linné, 1758) by four monopisthocotylea monogenean species.
The host fish were caught from the Melen fish station located at 3°52’N and 11°31’ E. This station consists of 17 interconnected ponds. It is found in the valley which is limited in the North by the campus of University of Yaounde I, in the East by Obili quarter, in the West by the University Hospital Center of Yaounde and in the South by the Cameroon National Herbarium. Samples were collected in the largest pond in which the density of fish was highest. Oreochromis niloticus farming is the predominant activity in this fish station. The vegetation is less diversified. The subequatorial climate in this area is characterized by four well known seasons the: short dry season (from July to August); the long rainy season (from September to mid-November); the long dry season (from mid-November to mid-March) and then a short rainy season (from mid-March to June) [12].
A total of 406 fish were examined with total body length from 25 to 162 mm and weight from 0.3 to 79.6 g. Each month, 30 to 33 fish were caught using gill-nets from February 2012 to February 2013. Once the fish were caught after draining the pond, they were immediately fixed in 10% formalin solution. In the laboratory, the gill arches of each fish side numbered from 1 to 4 in the anterio-posterior direction were removed and each placed in a Petri dish containing tap water for subsequent examination using a stereomicroscope, Wild Heebrugg M50. Parasites observed on gill filaments were colored with eosin and examined under the optical microscope, Olympus CH2, in order to determine the parasite species based on the sclerified parts of the parasites.
Shannon-Weaver (H´) index and Pielou equitability (E) index were used to study the monthly variation of parasite diversity of monogeneans collected. These indexes are calculated as follows: 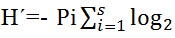 Pi and
Pi and 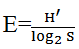 where for a given gill arch, Pi represents the relative abundance of parasite species i and S corresponds to the specific richness. The monthly evolution of niche amplitude of each parasite species was calculated using the standardized index of Levins as follows
where for a given gill arch, Pi represents the relative abundance of parasite species i and S corresponds to the specific richness. The monthly evolution of niche amplitude of each parasite species was calculated using the standardized index of Levins as follows 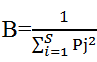 where Pj is the relative abundance of a given parasite species on the gill arch j. The mean monthly abundances of various arches were compared using Kruskall-Wallis test followed by Student t test when necessary. Data analysis was done using excel software and statistix version 2.0.
where Pj is the relative abundance of a given parasite species on the gill arch j. The mean monthly abundances of various arches were compared using Kruskall-Wallis test followed by Student t test when necessary. Data analysis was done using excel software and statistix version 2.0.
Colonization of the four gills arches by Cichlidogyrus thurstonae was mostly done in an anterio-posterior direction without equipartition (Figure 1). This pattern was perturbed in the months of May 2012, when the median arches II and III were more colonized than arch I; July 2012 during which equipartition was observed and August 2012 when arch I was statistically less parasitized. Gill exploitation by C. halli was very unstable and varied from one month to another. Generally, the parasite load of this helminth on arch IV was lower (Figure 2) with no significant difference. During the months of March, June and September, the colonization gradient occurred in an anterio-posterior direction. This pattern was slightly modified in April, October 2012 and February 2013. Thereafter, the colonization showed no fixe pattern except in July 2012 where equipartition was observed. Similarly, the occupation models of gills by C. tilapiae varied from one month to another and latter stabilized in December 2012 and January 2013. In most cases, the parasite load of this species was more important on one of the median gills. This parasite species disappeared from arch II in August and from arch IV in March 2012 (Figure 3). No equipartition was observed during the study period. The colonization of transversal gradient by Scutogyrus longicornis was equally very perturbed and an equipartition was observed in February and November 2012. S. longicornis was not observed in the four pairs of gill arches in March 2012 and on the last three pairs in February 2013 (Figure 4). In June, colonization occurred in an anterio-posterior direction and then was unfixed.
The specific diversity of each gill arch varied from one month to another. The monthly values of Shannon-Weaver were found between 0.76 and 1.53; 1.1 and 1.78; 1.21 and 1.79; 0.44 and 1.78 respectively for arches I, II, III and IV. Thus, this diversity varied in this way: AIV>AIII>AII>AI. The most posterior arch indicated the highest monthly diversity in most cases. The smallest values were observed in September (arch I), June (arches II and IV) and March (arch IV) of 2012.
The equitability profile of each gill arch was similar to that of Shannon-Weaver index. The values of this parameter varied between 0.38 and 0.86 (arch I); 0.54 and 0.90 (arch II); 0.50 and 0.85 (arch III) and 0.44 and 0.89 (arch IV) and the monthly equitability values of the various gill arches were less than 0.80 in 21.15% of cases.
The variation in niche amplitude revealed that, C. halli is the monogenean that used the spatial resource better. This parasite exploited simultaneously the four gill arches of O. niloticus during the months of February-May, July-August 2012 and January 2013. While the species, C. tilapiae, occupied most of the space in June, October and November 2012. The best niche overlap of S. longicornis was observed in February, September and October 2012 while that of C. thurstonae was the most restricted.
During the study period, C. thurstonae showed a precise model of gill occupation. Overall, the colonization of gills by this helminth occurred in the anterio-posterior direction with some perturbations. Various specific models of transversal gradient exploitation by some monogeneans species have previously been mentioned. The gill arches colonization of Hemichromis fasciatus by Onchobdella voltensis and C. longicornis was done in the anterio-posterior direction and by equipartition respectively [13]. Also, the gill occupation model of Barbus martorelli by Dactylogyrus bopeleti and D. insolitus was an equipartition [14]. In this study, the observation of a precise model of gill occupation by C. thurstonae might indicate a low impact of environmental conditions on this species.
Although gill arches colonization by C. tilapiae was perturbed, its monthly number in most cases was higher in one of the median arches and lower on gill IV. The works of Paling, considered as a reference for Teleosteans has shown that the water flux through the gills presents the following gradient: II>III>I>IV [15]. These works allow us to understand firstly that arches II and III which are larger and more ventilated harbor more specimens of C. tilapiae, and secondly that, arch IV which is the smallest and less ventilated harbor less specimens of this species.
Cichlidogyrus halli and S. longicornis showed no precise colonization model of transversal gradient. In fact, the spatial structure of each of these species was perpetually modified. These two species could be more sensible to variations in environmental conditions. However, S. longicornis which disappeared completely from its host in March 2012 and from arches II, III, IV in February 2013 could be more sensitive to perturbations. This observation falls in line with the conclusion made by Dogiel which show that, some fish parasites react highly even to the least physico-chemical modifications of the environment which is considered less perturbed [16]. In August 2012, C. halli was more abundant on arch IV which is the most posterior, the smallest and the less ventilated. This observation was also made with Birgiellus kellensis and Quadriacanthus sp, gill parasites of Clarias camerounensis [17]. According to these authors, the present model can be explained based on the way in which oncomiracidiums of monopisthocotylea colonize fish gills. They first fixe on the body of the host and latter migrate towards the gill in an anterio-posterior direction.
Whatever the gill arch, lower diversity values were observed between close seasons. Therefore, seasonal fluctuations have an impact on the colonization of the various gill arches of O. niloticus of Melen fish station. A seasonal variation of the specific diversity in the community of monogeneans gill parasites of Rutilus rutilus was equally observed in Finland [10]. The same phenomenon was noted in monogenean gill parasites of H. fasciatus in Cameroon [13]. Diversity variation is often explained by the reduction of specific richness during some periods of the year, by pollution or by eutrophication [10,13,18].
Specific diversity calculated by Shannon-Weaver index is a measure of the degree of the community organization; low diversity is synonymous to good organization while high diversity reveals poor organization [7]. It appears therefore that, the communities of the two anterior arches were more organized with time compared to those of the two posterior ones. Monthly equitability of each arch was less than 1 in most cases, implying that monthly arch diversity did not rich the maximal value. Niche amplitude of C. halli appeared to be higher of that of the three other species. Thus, the sensitivity of monogeneans to environmental modifications varies with each species. For Rohde these variations could be more or less perceived according to the selected spatial dimension [19]. This author adds that, it is possible to observe unlike infracommunities between individuals of a same host belonging to two closer localities.