Journal of Antivirals & Antiretrovirals
Open Access
ISSN: 1948-5964
ISSN: 1948-5964
Research Article - (2021)
Background: It is necessary to use an effective vaccine to end the COVID-19 pandemic. CoronaVac vaccine is used in our country and we aimed to examine the level of antibody development after the first dose.
Methods: This is a retrospective, cross-sectional research. The data of the people, who applied to a university hospital between January and February 2021,were analyzed. Those who had SARS-CoV-2 IgG and IgM measurement in the previous two weeks before the CoronoVac vaccine, and those who were both found negative and who had SARS-CoV-2 IgG and IgM measurement after the first dose of CoronaVac vaccine were included in the research. SARS-CoV-2 IgG/IgM were measured by VIDAS® (BioMérieux, Marcy-l'Etoile, France) device for the detection of spike protein specific IgG/IgM of SARS-CoV-2 in human serum with ELFA (Enzyme Linked Fluorescent Assay) technique.
Results: 30 people were included in this research. It was found that the individuals had SARS-CoV-2 IgG and IgM measurements between 14 and 21 days after the first dose of CoronaVac vaccine. It was observed that 30% (n=9) of the cases had a history of COVID-19.The rate of positivity for SARS CoV-2 IgG level after vaccination was 40% (n=12/30) and it was 77.8% (n=7/9) in cases with a history of COVID-19 and it was significantly higher than those without a history of COVID-19 (p=0.013).
Conclusions: A single dose of CoronaVac vaccine is not enough, but perhaps a single dose of vaccination may be sufficient for those who have had COVID-19.
COVID-19; CoronaVac vaccine; SARS-CoV-2 IgG
A new strain of coronavirus was identified as the cause of a series of pneumonia cases in Wuhan, a city in Hubei province of China, at the end of 2019. The virus spread rapidly all over the world, causing a global pandemic and the pandemic still continues. The name of the virus was defined as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), and the World Health Organization (WHO) defined the disease as COVID-19, which stands for 2019 coronavirus disease, in February 2020 [1].
To prevent SARS-CoV-2 infection, vaccine developement is considered as the most promising approach to control the pandemic and is followed by the whole world. By the end of 2020, several vaccines were ready to use in different parts of the world with emergency approval [2].
CoronaVac 600 SU/0.5 ml (Sinovac Life Sciences, Beijing, China) vaccine, which is an inactive COVID-19 vaccine has been used after obtaining emergency approval in our country [3]. As of January 14, 2021, vaccination has been initiated for our population starting with healthcare workers [4]. The vaccine, which was administered intramuscularly as 2 doses in 28 days, was shown to be safe and immunogenic in Phase 1 and 2 trials [5,6].
In this research, it was aimed to evaluate the effectiveness of the CoronaVac vaccine after the first dose by examining the results of people who had SARS-CoV-2 immunoglobulin G (IgG) and immunoglobulin M (IgM) measurements in our hospital.
This is a retrospective, cross-sectional research and was conducted with the informed consent of all participants. Ministry of Health approval has been obtained. Erzincan Binali Yildirim University Ethics committee approval was obtained (Date: 22.03.2021 and Decision no: 05/27).
The data of the people who applied to the COVID-19 Antibody Polyclinic of Erzincan Binali Yildirim University (EBYU) Mengücek Gazi Training and Research Hospital between January and February 2021 were retrospectively analyzed; the people who met the inclusion criteria were detected and their data were collected. Accordingly, those who had SARS-CoV-2 IgG and IgM measurement in the previous two weeks before the CoronoVac vaccine, and those who were both found negative and who had SARS-CoV-2 IgG and IgM measurement after the first dose of CoronaVac vaccine were included in the research. People who had negative antibody results before vaccination but were not vaccinated and those who did not have a measurement of antibody levels after vaccination were not included in the research. The demographic data of the individuals (age, gender, presence of comorbidities, medications, etc.), whether they had COVID-19 and how long ago they had COVID-19 were evaluated. For the participants having a history of COVID-19; Complete Blood Count (CBC), serum ferritin, C-reactive Protein (CRP) and D-dimer levels’ results were also collected.
30 people were included in this research. It was found that the individuals had SARS-CoV-2 IgG and IgM measurements between 14 and 21 days after the first dose of CoronaVac vaccine.
SARS-CoV-2 IgG and IgM measurements
SARS-CoV-2 IgG was measured by VIDAS (BioMérieux, Marcy- l'Etoile, France) device for the detection of spike protein specific IgG of SARS-CoV-2 in human serum with ELFA (Enzyme Linked Fluorescent Assay) technique.
Interpretation of the results
The interpretation of the results according to the test value is as: <1,00 (negative) and >1.00 (positive) [7].
CBC was measured by the Sysmex XN-1000 Hematology System (SysmexCorporation, Kobe, Japan) automated blood counter. Serum ferritin level was measured by chemiluminescence immunoassay (Centaur XP, Siemens Healthcare, Germany). CRP was measured by the BNTM II System device by the nepholometric method (Siemens, Munich, Germany). D-dimer level was measured from whole blood by the AQT90 flex Radiometer (Bronshoj, Denmark) device.
Statistical analysis
NCSS (NumberCruncher Statistical System) program was used for statistical analysis. Descriptive statistical methods (mean, standard deviation, median, frequency, percentage, minimum, maximum) were used while evaluating the study data. The suitability of quantitative datawith normal distribution was tested by Shapiro-Wilk test and graphical analysis. Mann-Whitney U test was used for the comparison of quantitative variables that did not show normal distribution between two groups. Pearson's chi-square test and Fisher's exact test were used to compare qualitative data. Spearman correlation analysis was used to evaluate the relationships between quantitative variables. Statistical significance was accepted as p<0.05.
The research was carried out in EBYU Mengücek Gazi Training and Research Hospital between the dates of 01.01.2021-08.02.2021. 43.3% (n=13) of the cases were female and 56.7% (n=17) were male. The ages of the cases ranged from 23 to 54, with a mean age of 37.13 ± 7.89.
It was observed that 30% (n=9) of the cases had a history of COVID-19. The duration of having COVID-19 ranges from 2 to 8 months before the vaccination, with an average of 4.28 ± 2.28 months.
In 40% of the cases (n=12), it was observed that SARS CoV-2 IgG result was positive after the first dose of vaccine. The IgG levels of the cases ranged from 0.04 to 44.76, and the mean value was found to be 3.47 ± 9.40 (Table 1).
| Descriptive features | ||
|---|---|---|
| Age (year) | Min-max (median) | 23-54 (36) |
| Mean ± SD | 37.13 ± 7.89 | |
| Gender | Female | 13 (43.3%) |
| Male | 17 (56.7%) | |
| History of COVID-19 | No | 21 (70%) |
| Yes | 9 (30%) | |
| The duration of having COVID-19 (month) | Min-max (median) | 2-8 ay (3 ay) |
| Mean ± SD | 4.28 ± 2.28 | |
| SARS-CoV-2 IgG result after 1st dose vaccine | Negative | 18 (60%) |
| Positive | 12 (40%) | |
| Min-max (median) | 0.04-44.76 (0.67) | |
| Mean ± SD | 3.47 ± 9.40 | |
Table 1: Distribution of descriptive features.
The leukocyte counts of people having a history of COVID-19 during the disease period varied between 4.300 and 9.800/mm3 , with an average of 7055.56 ± 1636.39/mm3 ; their platelet counts between 197.000 and 342.000/μL, with an average of 248000.00 ± 49272.20/μL; hemoglobin levels varied between 11.1 and 17.3 g/ dL, with an average of 14.70 ± 1.95 g/dL; lymphocyte counts varied between 510 and 3880/μL, with an average of 1897.78 ± 1066.84/ μL; neutrophil counts variedbetween 1650 and 6220/μL, with an average of 4213.33 ± 1481.07/μL; serum CRP levels varied between 3 and 23 mg/L, with an average of 6.63 ± 7.33 mg/L; D-Dimer levels varied between 128 and 690 μg/L, with an average of 345.22 ± 175.68 μg/Land ferritin levels ranged from 6 to 150 ng/mL, with an average of 70.44 ± 48.47 ng/mL (Table 2).
| Laboratory results | ||
|---|---|---|
| Leukocyte counts (/mm3) | Min-max (median) | 4300-9800 (6800) |
| Mean ± SD | 7055.56 ± 1636.39 | |
| Platelet counts (/µL) | Min-max (median) | 197000-342000 (226000) |
| Mean ± SD | 248000.00 ± 49272.20 | |
| Hemoglobin levels (g/dL) | Min-max (median) | 11,1-17.3 (15.1) |
| Mean ± SD | 14.70 ± 1.95 | |
| Lymphocyte counts (/µL) | Min-max (median) | 510-3880 (1640) |
| Mean ± SD | 1897.78 ± 1066.84 | |
| Neutrophil counts (/µL) | Min-max (median) | 1650-6220 (4610) |
| Mean ± SD | 4213.33 ± 1481.07 | |
| CRP levels ( 0-5 mg/L) | Min-max (median) | 3-23 (3) |
| Mean ± SD | 6.63 ± 7.33 | |
| D-Dimer levels (μg/L) | Min-max (median) | 128-690 (342) |
| Mean ± SD | 345.22 ± 175.68 | |
| Ferritin levels (ng/mL) | Min-max (median) | 6-150 (50) |
| Mean ± SD | 70.44 ± 48.47 | |
Note: CRP: C-Reactive Protein
Table 2: Distribution of laboratory results of cases with history of COVID-19.
The age and gender distributions of the cases according to the SARS CoV-2 IgG levels after vaccination did not show a statistically significant difference (p>0.05). The rate of positivity for SARS CoV- 2 IgG level after vaccination in cases with a history of COVID-19 was found to be statistically significantly higher than those without a history of COVID-19 (p=0.013; p<0.05) (Table 3, Figure 1).
| Post vaccine SARS CoV-2 IgG level | ||||
|---|---|---|---|---|
| Negative | Positive | p | ||
| Age | Min-max (median) | 25-54 (37.5) | 23-48 (32.5) | a0.181 |
| Mean ± SD | 38.72 ± 7.77 | 34.75 ± 7.77 | ||
| Gender | Female | 7 (53.8) | 6 (46.2) | b0.547 |
| Male | 11 (64.7) | 6 (35.3) | ||
| History of COVID-19 | No | 16 (76.2) | 5 (23.8) | c0.013* |
| Yes | 2 (22.2) | 7 (77.8) | ||
| CRP | N | 2 | 6 | - |
| Min-max (median) | 3-23 (13) | 3-12 (3) | ||
| Mean ± SD | 13.00 ± 14.14 | 4.50 ± 3.67 | ||
Note: aMann Whitney U Test; bPearson Chi-Square Test; cFisher’s Exact Test *p<0.05; CRP: C-Reactive Protein
Table 3: Comparisons by post vaccine SARS CoV-2 IgG results.
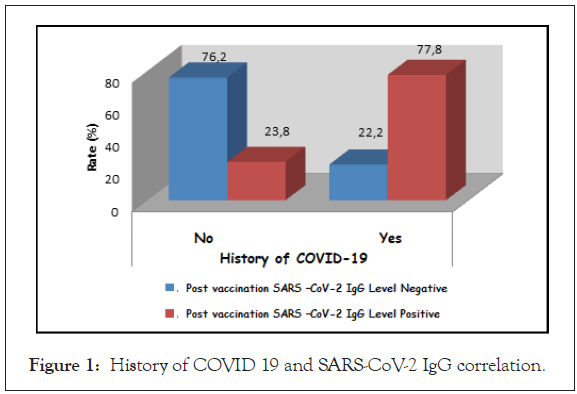
Figure 1: History of COVID 19 and SARS-CoV-2 IgG correlation.
No statistically significant difference was found between the post-vaccination SARS CoV-2 IgG results of the cases, according to gender and COVID-19 history (p>0.05). There was also no statistically significant relationship between age and CRP levels and post-vaccination SARS CoV-2 IgG results (p>0.05) (Table 4).
| Post vaccine SARS CoV-2 IgG Levels | ||||
|---|---|---|---|---|
| Min-max (median) | Mean ± SD | p | ||
| Gender | Female (n=13) | 0.04-44.76 (0.81) | 4.52 ± 12.14 | a0,586 |
| Male (n=17) | 0.06-29 (0.39) | 2.68 ± 6.92 | ||
| History of COVID-19 | No (n=21) | 0.04-44,76 (0.36) | 2.80 ± 9.64 | a0,067 |
| Yes (n=9) | 0.12-29 (1.65) | 5.06 ± 9.14 | ||
| r | p | |||
| Age | -0.279 | 0.135 | ||
| CRP | -0.078 | 0.854 | ||
Note: aMannWhitney U Test; r=Spearman’s Correlation Coefficient; CRP: C-ReactiveProtein
Table 4: Comparisons by post vaccine SARS CoV-2 IgG levels.
One of the important ways to control the COVID-19 pandemic is to produce an effective vaccine. Currently, various vaccines generated by different methods are being used all over the world with emergency use approval [2]. When the effectiveness of vaccines in use are examined; Pfizer/BioNTech, Gamaleya, Moderna and AstraZeneca announced the vaccine efficiency as 95%, 92%, 94.5%, 70%, respectively [8-10]. For the CoronaVac vaccine, efficacy statements have been received from different countries at different rates (50.4%, 65.3%, 78%, 91.25%), and these data have not been published yet [11,12]. SARS-CoV-2 vaccines have been developed very rapidly and because of this they have brought some concerns in terms of long-term side effect profile. Anaphylaxis is one of the important life-threatening side effects and there are concerns that it may be more likely to be seen in mRNA vaccines [12-14].
CoronaVac 600 SU/0.5 ml (Sinovac Life Sciences, Beijing, China) vaccine, which is an inactive COVID-19 vaccine isbeing used after obtaining emergency use approval in our country [3]. The vaccine is administered intramuscularly in 2 doses, 28 days apart. When the literature was examined, it was found that real-life data on CoronaVac vaccine was not shared before. The data in our research is the first real-life data of CoronoVac vaccine and is important in this regard.
According to the data of our research, the rate of antibody development after the first dose of vaccine in all individuals was 40%, and this rate was found to be 77.8% in those who had the diseasebefore. The rate of antibody development after vaccination in those who had COVID-19 before was found to be significantly higher than those who did not (p=0.013; p<0.05). It has been shown that cellular immunity continues even after 6 months in people who have had COVID-19 in the research conducted by Breton et al. [15]. Another research that was conducted by Schwarzkopf et al. showed that cellular immunity occurs in people with COVID-19 even though antibody response did not occur [16]. With the effect of cellular immunity in people who have previously encountered with SARS-CoV-2, a higher antibody response may have been obtained after a single dose of vaccine. In the light of these data, perhaps a single dose of vaccine will provide sufficient immunity in people having a history of COVID-19 without a significant antibody response. Thus, in countries with limited access to vaccines, resources can be used more efficiently by administering a single dose of vaccine to this group. Nevertheless, studies involving large numbers of people are needed.
Another issue is how protective the antibodies against SARS-CoV-2 are. In the study conducted by Lumley et al., itwas shown that the incidence of COVID-19 in healthcare workers with SARS-CoV-2 IgG positivity was significantly lower. Antibodies against spike protein and/or nucleocapsid have been shown to be protective [17]. In the study conducted by Borgonovo et al., itwas shown that IgG formed against spike protein continued for 7 months and symptomatic COVID-19 infection did not develop in these people [18]. In another study by Zhang et al., it was shown that B lymphocyte response developed in the first 14 days after a single dose of CoronaVac vaccine, but T lymphocyte response developed after the second dose [19]. In the light of these data, the fact that 77.8% of SARS-CoV-2 IgG positivity was detected after a single dose of CoronaVac vaccine in people who had COVID-19 previously and were found to be SARS-CoV-2 IgG negative, makes us think that the administration of a single dose of CoronaVac vaccine in this group could be effective.
There are some limitations in our study. One of them is being conducted in a single center with a small number of people. In addition, cellular immunity was not evaluated and protection was measured only by the antibody level. In this context, multi-centered studies consisting of larger groups evaluating antibody and cellular immune response level after first and second dose of vaccination are needed.
In conclusion, antibody development level was found to be 40% after a single dose of CoronaVac vaccine. This rate is below the desired protection rateand this finding is showing that a single dose of CoronaVac vaccine is not sufficient.
There is no special thanks.
The authors declare that they have no conflict of interest.
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008. Ministry of Health approval has been obtained. Erzincan Binali Yildirim University Ethics committee approval was obtained (Date: 22.03.2021 and Decision no: 05/27).
Informed consent was obtained from all patients for being included in the study.
Study conception and design
Umut Devrim Binay, Faruk Karakecili, Orcun Barkay, Ozlem Gul, Cuma Mertoglu.
Data collection
Umut Devrim Binay, Ozlem Gul.
Data analysis and interpretation
Orcun Barkay, Cuma Mertoglu.
Drafting of the article
Umut Devrim Binay, Faruk Karakecili, Ozlem Gul.
Critical revision of the article
Cuma Mertoglu, Orcun Barkay, Umut Devrim Binay.
Citation: Binay UD, Karakecili F, Barkay O, Gul O, Mertoglu C (2021) Level of SARS-CoV-2 IgG Antibodies after a Single Dose CoronaVac Vaccine: Primarily Report. J Antivir Antiretrovir. S18:004.
Received: 22-Apr-2021 Accepted: 06-May-2021 Published: 13-May-2021 , DOI: 10.35248/1948-5964.21.s18.004
Copyright: © 2021 Binay UD, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.